Abstract
The development of new materials and technologies for effective treatment of liquid radioactive waste is an urgent task. In present work, the novel Ti-Ca-Mg phosphate sorbents were developed. Sorbents were characterized by X-ray diffraction (XRD), Fourier transmittance infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDX), and N2 adsorption-desorption techniques. The influence of the chemical composition of mixed Ca-Mg-Ti phosphate sorbents and solution pH and the nature of the radionuclide on the sorption efficiency of 137Cs, 85Sr, and 60Co radionuclides were determined. The obtained materials demonstrated excellent affinity towards 137Cs, 85Sr, and 60Co radionuclides (Kd reached up to 105 mL g−1). For all Ti-Ca-Mg phosphates, an increase in the sorption efficiency of 137Cs with an increase in the titanium content was observed. It was shown that the samples of Ti-Ca-Mg phosphates containing of 33–60 wt% titanium effectively removed 137Cs, 90Sr, and 60Co radionuclides at pH 8.0–11.0. The prepared Ti-Ca-Mg phosphate sorbents are promising for one-stage treatment of aqueous solutions of complex composition from 137Cs, 85Sr, and 60Co radionuclides and could be used for development of advanced technology for liquid radioactive waste treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One of the most important and urgent tasks in the nuclear power industry is the safe processing of liquid radioactive waste of low and medium level of activity (Management of low and intermediate level radioactive wastes with regard to their chemical toxicity 2002). In environmental monitoring campaigns for anthropogenic radionuclides released in the course of the Fukushima nuclear accident (2011), most focus had been on gamma-emitting radionuclides. Long-lived radionuclides 137Cs (τ1/2 = 30.2 years), 90Sr (τ1/2 = 28.8 years), and 60Co (τ1/2 = 5.3 years) are the most dangerous for humans and the environment. Some of the understudied radionuclides are of radiological concern, others are promising tracers for environmental, geochemical processes such as oceanic mixing (Steinhauser 2014; Merz et al. 2013). In practice, it is important not only to minimize the amount of radioactive waste processed but also to simplify the treatment technology. This can be achieved by using broad-spectrum sorbents characterized by high affinity to long-lived radionuclides 137Cs, 90Sr, and 60Co. A large number of sorbents of different chemical composition are known with high sorption characteristics towards certain radionuclides. At the same time, information on broad-spectrum sorbents is very limited (Handley-Sidhu et al. 2014; Nie et al. 2017; Feng et al. 2018).
Thus, transition metal ferrycyanide, both in individual form and supported on various high-porous matrices, polymer-organic nanoparticles, has been used for selective removal of 137Cs radionuclide (Pshinko et al. 2016; Ivanets et al. 2014a; Zhang and Chai 2012; Zaunbrecher et al. 2015; Kakehi et al. 2015; Bratskaya et al. 2014; Groenewold et al. 2004). Titanosilicates and manganese oxides are characterized by high values of the distribution coefficient (Kd) at 90Sr adsorption from aqueous solutions with high salinity (liquid radioactive waste with high hardness, sea water, etc.) (Oleksiienko et al. 2017; Ivanets et al. 2016; Wallace et al. 2013; Rashad et al. 2016). There are given the large number of works devoted to the sorption study of stable Co2+ ions and 60Co radionuclide by various natural sorbents (Rashad et al. 2016; Mahmoud et al. 2017), activated carbon (Granados et al. 2006; Hamed et al. 2016), materials based on industrial and agricultural wastes (Milenkovic et al. 2016; Ahmadpour et al. 2009), oxides and hydroxides of metals (Borai et al. 2015; Guo et al. 2017; Zhao et al. 2013), and also modified natural materials (Ivanets et al. 2014b; Ivanets et al. 2018), and synthetic ion-exchange resins (Luca et al. 2012) from aqueous solutions.
Composite sorption materials based on mixed phosphates Ti-Ca-Mg have great prospects in the development of sorbents for removal of various radionuclides. Ion exchangers based on Ti phosphates are characterized by very low solubility and a high degree of treatment from toxic metal ions, in particular radionuclides 90Sr and 137Cs (Luca et al. 2012; Komarneni et al. 1994). Ca and Mg phosphates are characterized by high affinity to 90Sr and 60Co radionuclides, but practically do not have sorption activity towards 137Cs (Nilchi et al. 2004; Maslova et al. 2008; Kitikova et al. 2017). The sorbents based on composite Ca-Mg-Ti phosphates are expected to have the advantages of individual Ti phosphates and Ca and Mg phosphates which were synthesized by heterogeneous precipitation (Ivanets et al. 2019) and mechanic-chemical methods (Maslova et al. 2019). In current work, we described a one-step method for obtaining Ti-Ca-Mg phosphates by interacting of phosphatized dolomite of different chemical composition (PD-1 and PD-2) with an aqueous solution of titanyl-diammonium sulfate.
As far as we know, earlier studies of the sorption properties of complex Ti-Ca-Mg phosphates towards aqueous solutions of 137Cs, 90Sr, and 60Co radionuclides were not carried out. Data on simultaneous removal of stable Cs+, Sr2+, and Co2+ ions by natural and synthetic sorbents were presented in a few papers. There are limited data on sorbents showing high efficiency of purification of aqueous solutions of complex radionuclide composition (137Cs + 85Sr + 60Co) (Ozeki and Aoki 2016; Smiciklas et al. 2007; Park et al. 2010; Hamed et al. 2014).
The objective of the present study is to: (i) prepare novel inexpensive and sustainable Ti-Ca-Mg phosphates adsorbents, which can be used for 137Cs, 85Sr, and 60Co radionuclides purification with low cost and high capacity and (ii) study the sorption properties of these adsorbents depending on their phase and chemical composition, the pH of the solution towards 137Cs, 85Sr, and 60Co radionuclides from model multicomponent liquid radioactive waste.
2 Experimental
2.1 Synthesis of Ti-Ca-Mg Phosphates
The samples of phosphated dolomite Ca0.7Mg0.3HPO4∙2H2O (PD-1) and Ca2.65Mg3(NH4)1.3(PO4)4(CO3)0.3·6H2O (PD-2) (Kitikova et al. 2017) and 3.85 wt% aqueous solution of titanyl-diammonium sulfate (NH4)2TiO(SO4)2 H2O obtained from the waste products of apatite nepheline ores (Gerasimova et al. 2013) were used as starting materials for Ti-Ca-Mg composite phosphates preparation. The given quantities of PD-1 or PD-2 were suspended in the solution with the specified ratio of V/m under constant stirring at a temperature of 25 °C for 24 h. Ti-Ca-Mg phosphate samples were obtained with a ratio of Ti/(Ca + Mg) in the reaction mixture of 10, 20, 33, and 60 wt%, designated as PD-1-1, PD-1-2, PD-1-3, PD-1-4 and PD-2-1, PD-2-2, PD-2-3, and PD-2-4, respectively (Ivanets et al. 2019).
2.2 Methods of Sorbent Characterization
XRD phase analysis of sorbents was performed at the ADVANCED8 facility (Brucker, Germany) using CuKα radiation. Phase identification was performed using the base of radiographic powder standards “JCPDS PDF2.” Surface morphology and elemental analysis were performed using a scanning electron microscope JSM-5610 LV with the chemical analysis prefix JED-2201 (JEOL, Japan). The adsorption properties and texture of the samples were estimated from the isotherms of low-temperature (− 196 °C) physical adsorption-desorption of nitrogen on the surface area and porosity analyzer ASAP 2020 MP (Micromeritics, USA). Specific surface area was calculated by BET (ABET) and single-point BET (Asp) methods; pore volume was calculated by single-point adsorption method (Vsp.ads) and desorption (Vsp.des) isotherm branches. According to the obtained results, the average diameter of pores for adsorption (Dsp.ads) and desorption (Dsp.des) isotherm branches according to the equation 4V/A was calculated. Before analysis, the samples were vacuumed for 1 h at a temperature of 150 °C and a residual pressure of 133.3 × 10−3 Pa.
2.3 Sorption Experiment
Sorption of 137Cs, 85Sr, and 60Co radionuclides from aqueous solutions was studied at their joint presence under static conditions at room temperature; the ratio of the solid and liquid phase V/m was 500 mL/g, the contact time of 24 h. The pH of the aqueous solutions varied from 2.0 to 11.0 and was measured with pH-meter I-160 (ZIP, Belarus). The initial activity of 137Cs, 85Sr and 60Co in model solutions was in the range of (1.5–3.0) × 105 Bq L−1. The radionuclide activity in the solution before (Аinit, kBq L−1) and after sorption (Аeq, kBq L−1) was measured by MKSАТ1315 γ,β-spectrometer (Atomtex, Belarus).
The removal efficiency (S, %) and the distribution coefficient (Kd, mL g−1) were calculated by following equations:
where V is the volume of solution, mL; m—the mass of the sorbent, g.
3 Results and Discussion
3.1 Ti-Ca-Mg Phosphate Sorbent Characterization
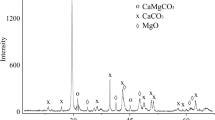
XRD and IR spectroscopy data (Fig. 1) indicate that the products of the interaction of PD-1 and PD-2 with titanyl ions were amorphous mixed of Ti-Ca-Mg phosphate, the chemical composition of which depends on the composition of the initial phosphated dolomite and the synthesis conditions. Thus, the interaction with PD-1 proceeded by the mechanism of dissolution-deposition sequentially with magnesium hydrophosphate, then with calcium hydrophosphate. For PD-2, the interaction proceeded by the mechanism of heterogeneous exchange reaction simultaneously with tertiary calcium and magnesium phosphates and magnesium-ammonium phosphate.
According to data of nitrogen adsorption-desorption (Fig. 2a), when PD-1 and PD-2 interacted with titanyl ions, a mesoporous Ti-Ca-Mg mixed phosphate was formed with a high specific surface area and pore volume. Samples obtained on the basis of PD-1 had a specific surface area of 220–230 m2 g−1, pore volume – 0.32-0.37 cm3 g−1 with an average diameter of 5.7–6.7 nm. The corresponding characteristics for samples based on PD-2 were of 140–144 m2 g−1, 0.27–0.34 cm3 g−1, and 7.6–9.6 nm. Interaction of PD-1 and PD-2 samples with titanyl ions was accompanied by a significant change in the morphology of the obtained Ti-Ca-Mg phosphates. Therefore, the initial samples were represented by polygonal prism with pronounced sharp edges, while samples PD-1-4 and PD-2-4 contained primarily the fine phase which was presented by amorphous phosphates (Fig. 2b).
Thus, the synthesized sorbents had different chemical and phase composition. The morphology and texture characteristics of mixed Ti-Ca-Mg phosphates significantly depend on the initial Ca-Mg phosphates (Fig. 2). It was obvious that the determined chemical and structural-phase transformations should have an effect on the sorption characteristics of the samples.
3.2 Effect of pH on Sorption of 137Cs, 85Sr, and 60Co Radionuclides
The efficiency of radionuclide sorption had a complex dependence on the pH of the aqueous solution. The highest sorption efficiency of 137Cs for samples based on PD-1 was observed at pH 2.0 and 8.0–11.0. For all Ti-containing samples, Kd reached 103–104 cm3 g−1 and a removal efficiency of 70–95%. A noticeable decrease in 137Cs sorption occurred at pH 5.0 (Fig. 3, Table 1). According to the diagram of the state of phosphate ions, pH 5.0 corresponds to the protonation of hydrophosphate ion and the transition to the form dihydrophosphate ion, which may adversely effect on the efficiency of ion exchange removal of 137Cs, due to an increase in the positive charge of the surface.
The effect of pH on the sorption efficiency of 137Cs for series of PD-2 samples differed significantly from that of PD-1 based sorbents. With an increase in pH from 2.0 to 5.0, the distribution coefficient and the removal efficiency of 137Cs increased, and with a further increase in pH in the range of 5.0–11.0 had sufficiently close values. At the same time, the highest removal efficiency of 137Cs was observed for Ti-containing sorbents regardless of pH solutions. The increase in sorption activity of the obtained Ti-Ca-Mg phosphates with an increase in the titanium content was due to the well-known high affinity of Ti phosphates towards Cs+ ions.
According Fig. 4 and Table 2, the sorption efficiency of 85Sr for Ti-Ca-Mg phosphate sorbents based on PD-1 and PD-2 samples increased with the change of the pH solution from 2.0 to 5.0 and practically did not change with further increase in pH up to 11.0. In acidic solutions at pH 2.0, the distribution coefficient of 90Sr for PD-1 series samples was 102–104 mL g−1, and the removal efficiency varied from 35 to 98%. The samples of Ti-Ca-Mg the phosphates based on PD-2 were characterized by significantly higher efficiency towards 90Sr in comparison with the PD-1 series throughout the studied pH range. At the pH range of 5.0–11.0 for PD-2 series the distribution coefficient reached 104–105 mL g−1 and the removal efficiency was not less than 95%. Meanwhile, all samples of the PD-2 series, regardless of the Ti content, were characterized by comparable sorption characteristics.
The dependence of 60Co sorption by Ti-Ca-Mg mixed phosphates was quite similar to the above described results for 85Sr: (i) low sorption at pH 2.0; (ii) for samples of PD-1 series, the dependence of the efficiency of sorption from the content of Ti was extreme; (iii) for all samples based on PD-2, sorption was comparable; and (iv) sorbents based on PD-2 were more effective compared with samples of PD-1series (Fig. 5, Tables 3).
For the sorbent PD-1-3 in the pH range of 5.0–11.0, the value of the 60Co distribution coefficient reached 6 × 103 mL g−1, and the removal efficiency was 95%. For sorbents of PD-2 series under similar conditions, the 60Co distribution coefficient was in the range of (2.0–6.0) × 104 mL g−1, and the removal efficiency reached 99%. Summarizing the data on the effect of pH on the sorption efficiency of 137Cs, 90Sr, and 60Co by Ti-Ca-Mg mixed phosphates, it can be concluded that the highest efficiency was achieved for sorbents based on PD-2 at pH 5.0–11.0.
3.3 The Influence of the Sorbent Composition of the Sorption Efficiency
Figure 6 shows the graphs of dependence of lgKd on the sorbent composition at pH 8.0. The composition of sorbents had a significant influence on the removal efficiency of 137Cs, 85Sr, and 60Co radionuclides from aqueous solutions. Depending on the nature of radionuclides, the sorption efficiency for Ti-Ca-Mg mixed phosphates was different. The initial PD-1 sample had minimum values of the distribution coefficient 137Cs (120 mL g−1). The increase in the Ti content in PD-1 series samples led to a consistent increase in the values of distribution coefficient 137Cs radionuclide in the pH range 2.0–11.0, reaching a maximum for the PD-1-4 sample 7.4 × 103 mL g−1. For PD-2 series, an increase in the distribution coefficient of 137Cs from 68 to 7.0 × 103 mL g−1 with an increase in the content of Ti was also observed.
The dependence of Kd85Sr and 60Co radionuclides on the composition of mixed sorbents of the PD-1 series was extreme. With the introduction of Ti into the sorbent, the sorption efficiency firstly increased, and then decreased. The sorbent PD-1-3 showed the highest efficiency for which the distribution coefficient of 85Sr and 60Co was 9.9 × 103 and 4.6 × 103 mL g−1, respectively.
The introduction of Ti into PD-2 led to a consistent increase in the 85Sr distribution coefficient from 7.1 × 103 to 2.0 × 104 mL g−1 for PD-2 and PD-2-4 samples, respectively. A completely different dependence on the composition was observed for 60Co sorption by PD-2 series samples. The introduction of Ti up to 20% into the PD-2 (PD-2-1 and PD-2-2 samples) had no significant effect on the sorption efficiency of 60Co, while the distribution coefficient remained up to (8.0–8.7) × 104 mL g−1. A further increase in the Ti content in the samples led to a slight decrease in the distribution coefficient to (2.5–5.0) × 104 mL g−1.
The introduction of Ti in the composition of the initial Ca-Mg phosphate led to the high affinity for the radionuclide 137Cs reaching the maximum distribution coefficient value of 7.4 × 103 mL g−1 for the highest Ti content in sorbent. All the data obtained indicate PD-1-3 and PD-2-3 samples effectively remove 137Сѕ, 85Sr, and 60Co radionuclides at their joint presence in aqueous solutions in the pH range of 8.0–11.0.
4 Conclusions
New sorbents based on Ti-Ca-Mg mixed phosphates were obtained using phosphated dolomite and industrial waste from apatite nepheline ore processing and characterized by means XRD, FT-IR, DTA-TG, SEM-EDX, and N2 adsorption-desorption methods. The sorption properties of Ti-Ca-Mg phosphates significantly depend on the composition of sorbents, the pH of the medium, and the nature of the radionuclide. Introduction of Ti into composition of Ca-Mg phosphates significantly increased sorption affinity towards 137Cs radionuclide. The sorption properties towards 85Sr and 60Co had a complex dependence on composition of Ti-Ca-Mg phosphates. The maximum simultaneous sorption of 137Cs, 85Sr, and 60Co radionuclides (Kd 103–104 mL g−1) was observed for PD-1(PD-2)-3 and PD-1(PD-2)-4 samples with a Ti content of 33–60 wt% (TiO2) in the pH range of 8.0–11.0. It was shown that the obtained sorbents are promising materials for one-stage treatment of liquid radioactive waste with complex radionuclide composition (137Cs + 85Sr + 60Co).
References
Ahmadpour, A., Tahmasbi, M., Bastami, T. R., & Besharati, J. A. (2009). Rapid removal of cobalt ion from aqueous solutions by almond green hull. Journal of Hazardous Materials, 166, 925–930.
Borai, E. H., Breky, M. M. E., Sayed, M. S., & Abo-Aly, M. M. (2015). Synthesis, characterization and application of titanium oxide nanocomposites for removal of radioactive cesium, cobalt and europium ions. Journal of Colloid and Interface Science, 450, 17–25.
Bratskaya, S., Musyanovych, A., Zheleznov, V., Synytska, A., Marinin, D., Simon, F., & Avramenko, V. (2014). Polymer-inorganic coatings containing nanosized sorbents selective to radionuclides. 1. Latex/Cobalt Hexacyanoferrate(II) Composites for Cesium Fixation. ACS Applied Materials & Interfaces, 6, 16769–16776.
Feng, M.-L., Sarma, D., Gao, Y.-J., Qi, X.-H., Li, W.-A., Huang, X.-Y., & Kanatzidis, M. G. (2018). Efficient removal of [UO2]2+, Cs+, and Sr2+ ions by radiation-resistant gallium thioantimonates. Journal of the American Chemical Society, 140, 11133–11140.
Gerasimova, L. G., Maslova, M. V., & Nikolaev, A. I. (2013). Synthesis of the new nano-porous titanosilicates using ammonium oxysulphotitanite. Glass Physics and Chemistry, 39, 602–608.
Granados, F., Bertin, V., Bulbulian, S., & Solache-Rios, M. (2006). 60Co aqueous speciation and pH effect on the adsorption behavior on inorganic materials. Applied Radiation and Isotopes, 64, 291–297.
Groenewold, G. S., Avci, R., Karahan, C., Lefebre, K., Fox, R. V., Cortez, M. M., Gianotto, A. K., Sunner, J., & Manner, W. L. (2004). Characterization of interlayer Cs+ in clay samples using secondary ion mass spectrometry with laser sample modification. Analytical Chemistry, 76, 2893–2901.
Guo, Z., Ling, Q., Zhou, Y., Wei, L., Zhou, R., Niu, H., Li, Y., & Xu, J. (2017). Synthesis of three-dimensional flower-like α-Fe2O3microspheres for high efficient removal of radiocobalt. Journal of Radioanalytical and Nuclear Chemistry, 2017(314), 1897–1904.
Hamed, M. M., Attallah, M. F., & Metwally, S. S. (2014). Simultaneous solid phase extraction of cobalt, strontium and cesium from liquid radioactive waste using microcrystalline naphthalene. Radiochimica Acta, 102, 1017–1024.
Hamed, M. M., Ali, M. M. S., & Holiel, M. (2016). Preparation of activated carbon from doum stone and its application on adsorption of 60Co and 152+154Eu: equilibrium, kinetic and thermodynamic studies. Journal of Environmental Radioactivity, 164, 113–124.
Handley-Sidhu, S., Hriljac, J. A., Cuthbert, M. O., Renshaw, J. C., Pattrick, R. A. D., Charnock, J. M., Stolpe, B., Lead, J. R., Baker, S., & Macaskie, L. E. (2014). Bacterially produced calcium phosphate nanobiominerals: sorption capacity, site preferences, and stability of captured radionuclides. Environmental Science & Technology, 48, 6891–6898.
Ivanets, A. I., Shashkova, I. L., Drozdova, N. V., Davydov, D. Y., & Radkevich, A. V. (2014a). Recovery of cesium ions from aqueous solutions with composite sorbents based on tripolite and copper (II) and nickel (II) ferrocyanides. Radiochemistry, 56, 524–528.
Ivanets, A. I., Shashkova, I. L., Kitikova, N. V., & Drozdova, N. V. (2014b). Extraction of Co (II) ions from aqueous solutions with thermally activated dolomite. Russian Journal of Applied Chemistry, 87, 270–275.
Ivanets, A. I., Prozorovich, V. G., Kouznetsova, T. F., Radkevich, A. V., & Zarubo, A. M. (2016). Mesoporous manganese oxides prepared by sol-gel method: synthesis, characterization and sorption properties towards strontium ions. Environmental Nanotechnology, Monitoring & Management, 6, 261–269.
Ivanets, A. I., Kitikova, N. V., Shashkova, I. L., Radkevich, A., Shemet, L., & Sillanpää, M. (2018). Effective removal of 60Co from high-salinity water by Ca-Mg phosphate sorbents. Journal of Radioanalytical and Nuclear Chemistry, 318, 2341–2347.
Ivanets, A. I., Shashkova, I. L., Kitikova, N. V., Maslova, M. V., & Mudruk, N. V. (2019). New heterogeneous synthesis of mixed Ti-Ca-Mg phosphates as efficient sorbents of 137Cs, 90Sr and 60Co radionuclides. Journal of the Taiwan Institute of Chemical Engineers, 104, 151–159.
Kakehi, J., Kamio, E., Takagi, R., & Matsuyama, H. (2015). Cs+ rejection behavior of polyamide RO membranes for feed solutions with extremely low salt concentrations. Industrial & Engineering Chemistry Research, 54, 8782–8788.
Kitikova, N. V., Ivanets, A. I., Shashkova, I. L., Radkevich, A. V., Shemet, L. V., Kul’bitskaya, L. V., & Sillanpää, M. (2017). Batch study of 85Sr adsorption from synthetic seawater solutions using phosphate sorbents. Journal of Radioanalytical and Nuclear Chemistry, 314, 2437–2447.
Komarneni, S., Li, Q. H., & Roy, R. (1994). Microwave-hydrothermal processing for synthesis of layered and network phosphates. Materials Chemistry, 4, 1903–1906.
Luca, V., Bianchi, H. L., & Manzini, A. C. (2012). Cation immobilization in pyrolyzed simulated spent ion exchange resins. Journal of Nuclear Materials, 424, 1–11.
Mahmoud, M. R., Rashad, G. M., Metwally, E., Saad, E. A., & Elewa, A. M. (2017). Adsorptive removal of 134Cs+, 60Co2+ and 152+154Eu3+ radionuclides from aqueous solutions using sepiolite: single and multi-component systems. Applied Clay Science, 141, 72–80.
Management of low and intermediate level radioactive wastes with regard to their chemical toxicity, IAEA-TECDOC-1325, Vienna, Austria, 2002.
Maslova, M. V., Rusanova, D., Naydenov, V., Antzutkin, O. N., & Gerasimova, L. G. (2008). Synthesis, characterization, and sorption properties of amorphous titanium phosphate and silica-modified titanium phosphates. Inorganic Chemistry, 47, 11351–11360.
Maslova, M. V., Mudruk, N. V., Ivanets, A. I., et al. (2019). A novel sorbent based on Ti-Ca-Mg phosphates: synthesis, characterization and sorption properties, Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-019-06949-3.
Merz, S., Steinhauser, G., & Hamada, N. (2013). Anthropogenic radionuclides in Japanese food: environmental and legal implications. Environmental Science & Technology, 47, 1248–1256.
Milenkovic, A. S., Smiciklas, I. D., Sljivic-Ivanovic, M. Z., Zivkovic, L. S., & Vukelic, N. S. (2016). Effect of experimental variables onto Co2+ and Sr2+ sorption behavior in red mud-water suspensions. Journal of Environmental Science and Health, Part A, 51, 679–690.
Nie, Z., Finck, N., Heberling, F., Pruessmann, T., Liu, C., & Lützenkirchen, J. (2017). Adsorption of selenium and strontium on goethite: EXAFS study and surface complexation modeling of the ternary systems. Environmental Science & Technology, 51, 3751–3758.
Nilchi, A., Maragheh, M. G., & Khanchi, A. (2004). Synthesis and ion-exchange properties of crystalline titanium and zirconium phosphates. Journal of Radioanalytical and Nuclear Chemistry, 261, 393–400.
Oleksiienko, O., Wolkersdorfer, C., & Sillanpää, M. (2017). Titanosilicates in cation adsorption and cation exchange – a review. Chemical Engineering Journal, 317, 570–585.
Ozeki, K., & Aoki, H. (2016). Evaluation of the adsorptive behavior of cesium and strontium on hydroxyapatite and zeolite for decontamination of radioactive substances. Bio-medical Materials and Engineering, 27, 227–236.
Park, Y., Lee, Y.-C., Shin, W. S., & Choi, S.-J. (2010). Removal of cobalt, strontium and cesium from radioactive laundry wastewater by ammonium molybdophosphate–polyacrylonitrile (AMP–PAN). Chemical Engineering Journal, 162, 685–695.
Pshinko, G. N., Puzyrnaya, L. N., Shunkov, V. S., et al. (2016). Removal of cesium and strontium radionuclides from aqueous media by sorption onto magnetic potassium zinc hexacyanoferrate (II). Radiochemistry, 58, 491–497.
Rashad, G. M., Mahmoud, M. R., Elewa, A. M., Metwally, E., & Saad, E. A. (2016). Removal of radiocobalt from aqueous solutions by adsorption onto low-cost adsorbents. Journal of Radioanalytical and Nuclear Chemistry, 2016(309), 1065–1076.
Smiciklas, I., Dimovic, S., & Plecas, I. (2007). Removal of Cs1+, Sr2+ and Co2+ from aqueous solutions by adsorption on natural clinoptilolite. Applied Clay Science, 35, 139–144.
Steinhauser, G. (2014). Fukushima’s forgotten radionuclides: a review of the understudied radioactive emissions. Environmental Science & Technology, 48, 4649–4663.
Wallace, S. H., Shaw, S., Morris, K., Small, J. S., & Burke, I. T. (2013). Alteration of sediments by hyperalkaline K-rich cement leachate: implications for strontium adsorption and incorporation. Environmental Science & Technology, 47, 3694–3700.
Zaunbrecher, L. K., Cygan, R. T., & Elliott, W. C. (2015). Molecular models of cesium and rubidium adsorption on weathered micaceous minerals. The Journal of Physical Chemistry A, 119, 5691–5700.
Zhang, A., & Chai, Z. (2012). Adsorption property of cesium onto modified macroporous silica–calix[4]arene-crown based supramolecular recognition materials. Industrial & Engineering Chemistry Research, 51, 6196–6204.
Zhao, D., Wang, Y., Xuan, H., Chen, Y., & Cao, T. (2013). Removal of radiocobalt from aqueous solution by Mg2Al layered double hydroxide. Journal of Radioanalytical and Nuclear Chemistry, 295, 1251–1259.
Funding
This work was financially supported by the Belarusian Republican Foundation for Fundamental Research (grant #X18P-026) and the Russian Foundation for Fundamental Research Bel-a according to the research project N18-53-00003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ivanets, A., Kitikova, N., Shashkova, I. et al. One-Stage Adsorption Treatment of Liquid Radioactive Wastes with Complex Radionuclide Composition. Water Air Soil Pollut 231, 144 (2020). https://doi.org/10.1007/s11270-020-04529-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04529-7