Abstract
Comparing with the traditionally immiscible two-phase extraction, the homogeneous liquid–liquid extraction technique shows potential in industrial separation engineering due to nearly infinite contact interface. In this work the ionic liquid (IL) compounds such as N-(carboxymethyl)-N,N-dimethylethanaminium bis-trifluoromethane-sulfonimide ([DHbet][Tf2N]) and N-(carboxyethyl)-trimethylammonium bistrifluoromethane-sulfonimide ([THbet][Tf2N]) were synthesized. The homogeneous extraction behaviors of europium with two ILs were studied as functions of solution pH, ionic strength, contact time, and initial europium concentration. The results indicated that both homogeneous extractions were dependent on pH and independent on ionic strength. The extraction capacities for [DHbet][Tf2N] and [THbet][Tf2N] were 3.29 mmol/L and 3.16 mmol/L, respectively. ILs could be recovered using 1.0 M hydrochloric acid. The mole-ratio method indicated the formation of a mononuclear complex between the europium ion and IL. Total europium extraction efficiencies of more than 91% for [DHbet][Tf2N] and more than 90% for [THbet][Tf2N] were obtained by quadruple-stage countercurrent extraction. The result proves the feasibility of the homogeneous liquid–liquid extraction technique as an alternative option for europium separation from aquatic solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nuclear electricity power station generates high density energy with nearly greenhouse gas zero emission [1,2,3]. Nevertheless the resultant spent fuel contains more than 300 kinds of radioactive nuclides including fission products, activation elements, ultra uranium nuclides and unutilized uranium [4], which in most cases is processed to recover uranium and plutonium with the well-known PUREX extraction process [5, 6]. The residual wastewater as a result of the process retains major radioactivity and a large proportion of nuclides. The treatment and ultimate disposal of the wastewater is thus a challenging problem. The radioactive metal americium-241 (241Am) is significant on account of high radioactivity (specific activity of 3.43 Ci/g), heavy metal toxicity and a long half-life of 432.2 years [7]. Exposure to 241Am would cause the lesion of tissues and cancer [8]. With the aim of diminishing the adverse effect, the separation of 241Am from aquatic waste is being attempted with adsorption [9, 10], solvent extraction [11,12,13], and chemical precipitation [14]. It is worth noting that the solvent extraction technique shows promising with advantages of large handling capacity, continuous operation and high enrichment factor based on the multi-stage operation [15,16,17,18,19,20,21]. Although being powerful, efficient and reliable, the traditionally immiscible two-phase extraction technique deploys too much not friendly organic solvent of volatilization, hazard and toxicity [13, 16, 22]. Replacing solvent and altering extraction way would promote the applicability of solvent extraction.
Ionic liquids (ILs) refer to fluid salts made of ions and possess unique physicochemical properties such as low-volatilization, low melting point, high cohesive energy and non-combustibility compared with molecular liquids [23]. Over the past decades ILs has been extensively studied in the fields of catalysis [24], synthesis [25], separation [26], transformation and utilization of biomass [27]. The traditionally immiscible two-phase extraction processes utilize ILs as diluents, which do not participate coordination reaction. However the viscosity of ILs restricts the mass transfer area, increases the equilibrium time, and limits the separation performance [25]. Recently the hydro-soluble ILs at a certain temperature shows attractivity. It means changing the temperature to interconvert two-phases and homogeneous phase. The phase interconversion phenomenon based on temperature is thermomorphic behavior, based on which the homogeneous liquid–liquid extraction (HLLE) technique is established. Several prominent advantages associated with the technique include infinite contact interface and fast kinetics. For example 1-hexyl-3-methylimidazolium tetrafluoroborate [C6mim][BF4] could separate silver metal from 4,4-bis-(dimethylamino)-thiobenzophenone solution by HLLE, in which the critical thermomorphic temperature was at 323 K [28]. Onghena and Binnemans [29] proved the binary phase of betainium bis(trifluoromethylsulfonyl)imide with water could form a homogeneous phase at ≥ 328 K to extract metals. Based on the reported references, it is convinced that the HLLE technique based on a specific IL can be applied in the domain of Am extraction.
In the present work, we synthesized two novel ionic liquids named N-(carboxymethyl)-N,N-dimethylethanaminium bistrifluoromethanesulfonimide ([DHbet][Tf2N]) and N-(carboxyethyl)-trimethylammonium bistrifluoromethane- sulfonimide ([THbet][Tf2N]) (shown in Fig. 1), which could form homogeneous phase with water at 358.15 K, 343.15 K, respectively. Effects of pH, contact time and ionic strength on the homogeneous extraction of europium were studied, in which europium worked as a nonradioactive surrogate for Am due to same oxidation state, similar ionic radius and coordination chemistry [30]. The complex formations and dissociation constants were discussed using the mole-ratio method. The stripping experiment was conducted to study the recovery performance. Multistage counter-current extraction was simulated.
Reagents and instruments
Reagents
All chemical reagents used in the study were of analytical grade and directly used without purification unless otherwise specified. Europium nitrate hexahydrate, N,N-Dimethylethylamine, trimethylammonium and bistrifluoromethanesulfonimide lithium salt were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Arsenazo III purchased from the Aladdin Chemistry Co., Ltd. (Shanghai, China) was used to quantitatively analyze the concentration of the europium ion.
Instruments
FT-IR spectrums were measured in ATR mode using a Ge crystal plate on a Vector Model (Bruker, Switzerland) spectrometer. 13C NMR and 1H NMR spectrums were recorded in an Avance DMX500 (Bruker, Switzerland) spectrometer. The metal ion concentration was determined by using a 721E model UV–Vis spectrophotometer (Shanghai Spectrum, China).
Preparation of [DHbet][Tf2N] and [THbet][Tf2N]
[DHbet][Tf2N] was synthesized according to the following the two-step method. A 500 mL three-necked flask with a reflux condenser was filled with chloroacetic acid (0.317 mol, 30 g) and DMF (100 mL). The system was stirred to form a homogeneous phase, to which N,N-dimethylethylamine (0.547 mol, 40 g) was batch-wise added. The mixture was heated to a constant temperature of 60 °C for 6 h followed by suction filtration, vacuum drying. After that, a white powder solid as the crude product was obtained. The further purification was operated by the recrystallization method. The pure product, N-(carboxymethyl)-N,N-dimethylethanaminium hydrochloride, was separated out from 150 mL ethyl alcohol.
The ionic liquid, [DHbet][Tf2N], was obtained by the replacement reaction. The method in detail was described as the following. N-(carboxymethyl)-N,N-dimethylethanaminium (0.0871 mol, 14.45 g) and bistrifluoromethanesulfonimide lithium (0.0871 mol, 25 g) were added into distilled water (20 mL), followed by stirring for 1 h at room temperature. Then the mixture was centrifuged to separate the sublayer ionic liquid, which was washed five times to remove the reaction byproduct, LiCl. Afterward, the pure ionic liquid, [DHbet][Tf2N], was obtained. FT-IR (cm−1): 1740, 1468, 1415, 1340, 1340, 1129, 1045, 1179, 735, 653, 607; 1H-NMR (300 MHz, DMSO, δ/ppm): 4.22 (s, 2H, CH2), 3.51 (s, 2H, CH2), 3.22 (s, 6H, 2 × CH3), 1.34 (s, 3H, CH3); 13C-NMR (75 MHz, DMSO, δ/ppm): 167.28 (COO), 120.93, 118.10 (CF3), 60.38 (CH2), 50.93 (CH3), 40.53 (CH2).
According to the above path [THbet][Tf2N] was synthesized from the initial raw material of trimethylammonium. FT-IR (cm−1): 1731, 1480, 1335, 1130, 1046, 1177, 739, 785, 613. 1H-NMR (300 MHz, DMSO, δ/ppm): 3.56 (s, 2H, CH2), 2.77 (s, 2H, CH2), 3.06 (s, 9H, 3 × CH3). 13C-NMR (75.47 MHz, DMSO, δ/ppm): 172.55 (COO), 121.29, 118.91 (2 × CF3), 61.58 (CH2), 44.16 (CH2–CH2), 54.02 (CH3).
Homogeneous solvent extraction study
Prior to a homogeneous extraction operation, the ionic liquid ([DHbet][Tf2N] or [THbet][Tf2N]) was preequilibrated to a certain pH by once contacting with an identical volume of metal-free solution of specific pH. The pH of aqueous phase was adjusted with 0.5 mol/L nitric acid and NaOH solution. Therefore, equal volume of IL and the aqueous phase containing europium of a certain concentration were set into a conical flask and heated to the critical thermomorphic temperature to form a homogeneous system. After a certain period, the solution was cooled down to form two phases. The residual europium concentration in the aqueous solution was detected with the arsenazo III method with the UV–Vis spectrophotometer. The distribution ratio (D) was calculated by taking the ratio of equilibrium concentration of metal ion in the organic phase and in the aqueous phase (Eq. 1).
where [C]org and [C]aq represent europium content in the ionic liquid phase and the aqueous phase (mol/L), respectively; Ci and Cf are the initial and residual concentration of europium (mol/L), respectively; Vaq and Vorg are volumes of the aqueous phase and ionic liquid (mL). All extraction experiments were duplicated at least to make reliable and repeatable results.
Results and discussion
Effect of pH on the extraction
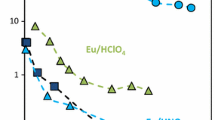
The solution pH plays essential role in the metal species distribution and the extraction extent [31, 32]. Considering europium hydrolysis beyond pH 7.0 [33, 34], the effect of pH in the range of 1.0–7.0 on the extraction was investigated. The result is shown in Fig. 2a. One could see that as pH increased from 1.0 to 3.0, the distribution ratio (D) increased from 1.39 to 1.91 for [DHbet][Tf2N] and from 1.11 to 1.73 for [THbet][Tf2N], further slightly decreased to 1.85 for [DHbet][Tf2N] and to 1.67 for [THbet][Tf2N] as pH increased to 7.0. The explanation for the plot was the following. The contributing coordination group when extracting europium was the carboxyl in the chemical structure of two ILs [35]. As pH increased from 1.0 to 3.0, the less protonated carboxyl group coordinated europium effectively. Once pH beyond 3.0 the slight protonation exerted weak influence on the extraction and the value of D kept constant. Further experiments were conducted at pH = 3.0.
As shown in Fig. 2b, the extraction using [DHbet][Tf2N] and [THbet][Tf2N] at pH = 3.0 weakly dependent on ionic strength was accordance with the chemical process in nature [36,37,38].
Contact time
The extraction at different contact times was researched and the result was shown in Fig. 3. It could be seen that in the first 5 min D reached 1.62 for [THbet][Tf2N] and 1.70 for [DHbet][Tf2N]. After 10 min the values were 1.69 for [THbet][Tf2N] and 1.90 for [DHbet][Tf2N] and kept constant further, illustrating the accomplishment of equilibrium. The results proved that the extractions were fast processes. The traditionally immiscible two-phase extraction is based on two immiscible solvents, in which the limited contact interface depresses the substance transmission, leading a relatively long equilibrium time. For example, Tan et al. [39] found that 2,6-bis(5,6-diethyl-1,2,4-triazin-3-yl)pyridine dissolved in room temperature ionic liquids exhibited slow kinetics over 24 h in a two-phase extraction toward europium. The extraction herein utilized the ability of the specific ionic liquid to form a homogeneous phase with aqueous solution at a certain temperature. The homogeneous system made the contact interface infinite, resulting sufficiently reaction between the ILs and target in a short time.
Effect of initial concentration of europium on the extraction
The experiments varying the initial europium concentration at 0.5, 1.0, 3.0, 5.0, 10.0, 15.0, 40.0 mmol/L were conducted to investigate the maximum extraction capacity of [DHbet][Tf2N] and [THbet][Tf2N]. As shown in Fig. 4, the europium concentration in ionic liquid phase increased rapidly from 0.3 to 3.29 mM for [DHbet][Tf2N] and from 0.3 to 3.16 mM for [THbet][Tf2N] as the initial europium concentration varied from 0.5 to 5.0 mM. This was attributed to the greater gradient caused by the higher europium concentration. On the other hand, the concentration of europium extracted kept at constant once the initial europium concentration beyond 5.0 mM. This was because the ionic liquid phases reached saturation. The maximum europium extraction capacity were determined as 3.29 mmol/L for [DHbet][Tf2N] and 3.16 mmol/L for [THbet][Tf2N].
Stripping performance
The stripping research was carried out using hydrochloric acid as stripping agent. Solutions of ionic liquids saturated with europium were prepared in advanced. The stripping experiment was conducted by mixing the stripping hydrochloric acid solution of 0.2, 0.4, 0.6, 0.8, or 1.0 mol/L with the equal volume of the europium saturated ionic liquids. The europium recovery efficiency corresponding to the hydrochloric acid concentration is shown in Fig. 5. It could be seen that as the acid concentration increased, the recovery percentage increased. The hydrochloric acid of 1.0 mol/L could recovery 94% europium from Eu-[DHbet][Tf2N] and 93% europium from Eu-[THbet][Tf2N], respectively.
Complex formation and dissociation constants of Eu-[DHbet][Tf2N] and Eu-[THbet][Tf2N]
In the mole-ratio method, a series of aqueous samples were made in which the concentration of ionic liquid ([DHbet][Tf2N] or [THbet][Tf2N]) kept constant while that of europium varies. The maximum absorbance–wavelength of the complex were qualitatively measured as 281 nm for [DHbet][Tf2N] and as 289 nm for [THbet][Tf2N] firstly which were located in the ultraviolet spectral region due to the weak conjugation effect. Then the absorbance of each aqueous sample was surveyed and plotted versus the mole ratio between europium and the ionic liquid. It is a reasonable prediction that the absorbance would increase as the mole ratio increased and reach a constant after the complex concentration come up to the maximum. In the curve, a break appears at the mole ratio of the complex composition. As shown in Fig. 6, two tangents intersecting at (1.0, 0.79) for [DHbet][Tf2N] and (1.0, 0.74) for [THbet][Tf2N] indicated that Eu and the ionic liquids reacted in 1:1 ratio that were mononuclear complex.
For a typical complex dissociation reaction:
The dissociation constant of the complex, K, can be expressed as the following equation (Eq. 2).
where α is the dissociation degree, equal to the difference value of 1.0 and the ratio for a defined coordination value (Ad) to the theoretical maximum absorbance number for coordination (A0), namely 1.0-Ad/A0, and C is the complex concentration at the break point.
Since both m and n values had been determined as 1.0 and α value of Eu-[DHbet][Tf2N] was calculated as 0.113, the value for Eu-[THbet][Tf2N] was 0.108, C = 1.0 mmol/L, the dissociation constant for Eu-[DHbet][Tf2N] was 0.0143 and for Eu-[THbet][Tf2N] was 0.0131.
Multistage countercurrent extraction process
Multistage countercurrent extraction process is an important separation technique and is often simulated using the cascade extraction in lab [40]. This study used a four-stage cascade extraction (shown in Fig. 7), in which the europium concentration of the feed solution = 5.0 mmol/L, Vaq/Vorg = 1, contact time = 20 min, and T = 358.15 K. The europium concentrations in every round raffinate for [DHbet][Tf2N] were C(R1) = 15.31 mg/L, C(R2) = 14.03 mg/L, C(R3) = 16.98 mg/L, C(R4) = 21.29 mg/L and for [THbet][Tf2N] were C(R1) = 15.31 mg/L, C(R2) = 14.03 mg/L, C(R3) = 16.98 mg/L, C(R4) = 21.29 mg/L, respectively. Theoretically C(Rx) increased as the cascade number increased. Generally C(R1) should be less than C(R2) due to the more fresh ionic liquid was used in every vessel. However the value of C(R1) was greater than C(R2) in this study. This was attributed to unsteady state in the first two rounds. Thus one could see that C(R3) and C(R4) values were greater than C(R1) and C(R2) values, and C(R3) < C(R4). The total extraction efficiencies were calculated as 91.12% europium for [DHbet][Tf2N] and as 90.55% europium for [THbet][Tf2N] by using Eq. 3. It was convincible that the extraction efficiency could be further improved by more than 4-stage extraction.
Conclusion
In consideration of drawbacks including limited contact interface and relatively long equilibrium time of the traditionally immiscible two-phase extraction, a homogeneous solvent extraction technique toward europium was initially developed herein based on ionic liquid [DHbet][Tf2N] and [THbet][Tf2N]. Effects of pH, contact time and ionic strength on the europium extraction were studied. It was demonstrated that the europium extraction was dependent on pH. The optimal value was 3.0. Almost infinite contact interface created by the homogeneous phase state possessed fast mass transfer kinetics, made the extraction reach equilibrium within 10 min. The maximum europium extraction capacity were determined as 3.29 mmol/L for [DHbet][Tf2N] and 3.16 mmol/L for [THbet][Tf2N]. The extracted europium was effectively stripped by hydrochloric acid. Once contact with hydrochloric acid (1.0 mol/L) could strip ca. 94% of the extracted europium. The mole-ratio method demonstrated europium and ILs reacted in 1:1 ratio that is mononuclear complex. Simulating four-stage counter-current operation extracted more than 91% europium for [DHbet][Tf2N] and more than 90% europium for [THbet][Tf2N]. The study shows that the homogeneous solvent extraction process based on [DHbet][Tf2N] and [THbet][Tf2N] are effective for europium separation.
References
Wang Y, Liu Z, Li Y, Bai Z, Liu W, Wang Y, Xu X, Xiao C, Sheng D, Diwu J, Su J, Chai Z, Albrecht-Schmitt TE, Wang S (2015) Umbellate distortions of the uranyl coordination environment result in a stable and porous polycatenated framework that can effectively remove cesium from aqueous solutions. J Am Chem Soc 137:6144–6147
Xie J, Wang Y, Liu W, Yin X, Chen L, Zou J, Diwu J, Chai Z, Albrecht-Schmitt TE, Liu G, Wang S (2017) Highly sensitive detection of ionizing radiations by a photoluminescent uranyl organic framework. Angew Chem Int Ed 56(26):7500–7504
Zheng T, Yang Z, Gui D, Liu Z, Wang X, Dai X, Liu S, Zhang L, Gao Y, Chen L, Sheng D, Wang Y, Diwu J, Wang J, Zhou R, Chai Z, Albrecht-Schmitt TE, Liu G, Wang S (2017) Overcoming the crystallization and designability issues in the ultrastable zirconium phosphonate framework system. Nat Commun 8:1–11
Wu Y, Jiang J, Wang M, Jin M (2011) A fusion-driven subcritical system concept based on viable technologies. Nucl Fusion 51:103036–103042
Xian L, Tian G, Beavers CM, Teat SJ, Shuh DK (2016) Glutarimidedioxime: a complexing and reducing reagent for plutonium recovery from spent nuclear fuel reprocessing. Angew Chem Int Ed 55(15):4671–4673
Rim JH, Armenta CE, Gonzales ER, Ünlü K, Peterson DS (2016) Evaluating bis(2-ethylhexyl) methanediphosphonic acid (H2DEH[MDP]) based polymer ligand film (PLF) for plutonium and uranium extraction. J Radioanal Nucl Chem 307(3):2327–2332
Williams NJ, Dehaudt J, Bryantsev VS, Luo H, Abney CW, Dai S (2017) Selective separation of americium from europium using 2,9-bis(triazine)-1,10-phenanthrolines in ionic liquids: a new twist on an old story. Chem Commun 53(18):2744–2747
Nilsson J, Bauden MP, Nilsson JM, Strand S-E, Elgqvist J (2015) Cancer cell radiobiological studies using in-house-developed α-particle irradiator. Cancer Biother Radiopharm 30(9):386–394
Shu Q, Khayambashi A, Zou Q, Wang X, Wei Y, He L, Tang F (2017) Studies on adsorption and separation characteristics of americium and lanthanides using a silica-based macroporous bi(2-ethylhexyl) phosphoric acid (HDEHP) adsorbent. J Radioanal Nucl Chem 313(1):29–37
Muthukumar K, Lakshmi DS, Gujar RB, Boricha AB, Mohapatra PK, Bajaj HC (2016) Synthesis and characterization of magnetic copper-iron-titanate and uptake studies of americium from nuclear waste solutions. RSC Adv 6(113):111822–111830
Ekberg C, Löfström-Engdahl E, Aneheim E, Foreman MR, Geist A, Lundberg D, Denecked M, Ingmar P (2015) The structures of CyMe4-BTBP complexes of americium(III) and europium(III) in solvents used in solvent extraction, explaining their separation properties. Dalton Trans 42:18395–18402
Chapron S, Marie C, Arrachart G, Miguirditchian M, Pellet-Rostaing S (2015) New insight into the americium/curium separation by solvent extraction using diglycolamides. Solvent Extr Ion Exc 33(3):236–248
Jensen MP, Chiarizia R, Ulicki JS, Spindlerb BD, Murphyb DJ, Mahmun Hossainb M, RocaSabioc A, Andrés B, Rodríguez-Blas T (2015) Solvent extraction separation of trivalent americium from curium and the lanthanides. Solvent Extr Ion Exc 33(4):329–345
Noronha DM, Pius IC, Chaudhury S (2017) Co-precipitation of plutonium(IV) and americium(III) from nitric acid–oxalic acid solutions with bismuth oxalate. J Radioanal Nucl Chem 313(3):523–529
Luo L, Qin X, Wu J, Liang G, Li Q, Liu M, Kang F, Chen G, Li B (2018) Interwoven MoO3@CNT scaffold interlayer for high-performance lithium-sulfur batteries. J Mater Chem A 6(18):8612–8619
Liu S, Liu H, Huang Y, Yang W (2015) Solvent extraction of rubidium and cesium from salt lake brine with t-BAMBP-kerosene solution. Trans Nonferrous Met Soc China 25(1):329–334
Silva M, Fernandes L, Olsina R, Stracchiola D (1997) Cloud point extraction, preconcentration and spectrophotometric determination of erbium(III)-2-(3,5-dichloro-2-pyridylazo)-5-dimethylaminophenol. Anal Chim Acta 342:229–238
Paleologos E, Giokas D, Karayannis M (2005) Micelle-mediated separation and cloud-point extraction. Trends Anal Chem 24(5):426–436
Karavan M, Smirnov I, Kleshnina S, Solovieva S, Kadirov M, Antipin I, Safiullin R, Gorbacheva S, Novikov A (2017) Micelle mediated extraction of americium and europium by calix [4] arene phosphine oxides from nitric acid media. J Radioanal Nucl Chem 311(1):599–609
Yuan LY, Liao XH, Liu ZR, Chai ZF, Shi WQ (2017) U(VI) extraction by 8-hydroxyquinoline: a comparison study in ionic liquid and in dichloromethane. Radiochim Acta 105(6):441–448
Yuan LY, Sun M, Mei L, Wang L, Zheng LR, Gao ZQ, Zhang J, Zhao YL, Chai ZF, Shi WQ (2015) New insight of coordination and extraction of uranium(VI) with N-donating ligands in room temperature ionic liquids: N,N′-Diethyl-N,N′-ditolyldipicolinamide as a case study. Inorg Chem 54:1992–1999
Chemat F, Fabiano-Tixier AS, Vian MA, Allaf T, Vorobiev E (2015) Solvent-free extraction of food and natural products. TrAC-Trends Anal Chem 71:157–168
Asrami MR, Saien J (2018) Salting-out effect on extraction of phenol from aqueous solutions by [Hmim][NTf2] ionic liquid: experimental investigations and modeling. Sep Purif Technol 204:175–184
Wilson M, Kore R, Ritchie AW, Fraser RC, Beaumont SK, Srivastava R, Badyal JPS (2018) Palladium–poly(ionic liquid) membranes for permselective sonochemical flow catalysis. Colloid Surf A 545:78–85
Zhang Y, Liu Y, Ma X, Ma X, Wang B, Li H, Huang Y, Liu C (2018) An environmentally friendly approach to the green synthesis of azo dyes with aryltriazenes via ionic liquid promoted C–N bonds formation. Dyes Pigm 158:438–444
Li K, Qian L, Song W, Zhu M, Zhao Y, Miao Z (2018) Preparation of an ionic liquid-based hydrogel with hyperbranched topology for efficient removal of Cr(VI). J Mater Sci 53(20):14821–14833
Han M, Li Y, Gu Z, Shi H, Chen C, Wang Q, Wan H, Guan G (2018) Immobilization of thiol-functionalized ionic liquids onto the surface of MIL-101(Cr) frameworks by S–Cr coordination bond for biodiesel production. Colloid Surf A 553:593–600
Vaezzadeh M, Shemirani F, Majidi B (2012) Determination of silver in real samples using homogeneous liquid-liquid microextraction based on ionic liquid. J Anal Chem 67(1):28–34
Onghena B, Binnemans K (2015) Recovery of scandium(III) from aqueous solutions by solvent extraction with the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl) imide. Ind Eng Chem Res 54(6):1887–1898
Kelley C, Mielke RE, Dimaquibo D, Curtis AJ, Dewitt JG (1999) Adsorption of Eu(III) onto roots of water hyacinth. Environ Sci Technol 33(9):1439–1443
Peng J, Song Y, Yuan P, Cui X, Qiu G (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161(2–3):633–640
Wang C, Lan J, Feng Y, Wei Y, Zhao Y, Chai Z, Shi W (2014) Extraction complexes of Pu(IV) with carbamoylmethylphosphine oxide ligands: a relativistic density functional study. Radiochim Acta 102(1–2):77–86
Sheng G, Yang S, Zhao D, Sheng J, Wang X (2012) Adsorption of Eu(III) on titanate nanotubes studied by a combination of batch and EXAFS technique. Sci China Chem 55(1):182–194
Tan X, Fang M, Li J, Lu Y, Wang X (2009) Adsorption of Eu(III) onto TiO2: effect of pH, concentration, ionic strength and soil fulvic acid. J Hazard Mater 168(1):458–465
Mori T, Takao K, Sasaki K, Suzuki T, Arai T, Ikeda Y (2014) Homogeneous liquid–liquid extraction of U(VI) from HNO3 aqueous solution to betainium bis(trifluoromethylsulfonyl)imide ionic liquid and recovery of extracted U(VI). Sep Purif Technol 155:133–138
Wang X, Chen C, Du J, Tan X, Xu D, Yu S (2005) Effect of pH and aging time on the kinetic dissociation of 243Am(III) from humic acid-coated γ-Al2O3: a chelating resin exchange study. Environ Sci Technol 39(18):7084–7088
Yang X, Yang S, Yang S, Hu J, Tan X, Wang X (2011) Effect of pH, ionic strength and temperature on sorption of Pb(II) on NKF-6 zeolite studied by batch technique. Chem Eng J 168(1):86–93
Fan Q, Shao D, Lu Y, Wu W, Wang X (2009) Effect of pH, ionic strength, temperature and humic substances on the sorption of Ni(II) to Na-attapulgite. Chem Eng J 150(1):188–195
Tan C, Zhang X, Cao S, Li S, Guo H, Yuan Tian, Chen D, Tian W, Wang L, Zhi Q (2018) Solvent extraction of americium(III) and europium(III) with 2,6-bis(5,6-diethyl-1,2,4-triazin-3-yl) pyridine in ionic liquids: experimental study and molecular dynamics simulation. Sep Purif Technol 192:302–308
Racheva R, Rahlf AF, Wenzel D, Müllera C, Kernerc M, Luinstrab GA, Smirnovaa I (2018) Aqueous food-grade and cosmetic-grade surfactant systems for the continuous countercurrent cloud point extraction. Sep Purif Technol 202:76–85
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (11605027, 21866003, 41461070, 11475044, 21561002, 21501025, 21761002), the China Postdoctoral Science Foundation (2016M600981) and Natural Science Foundation of Jiangxi Province (No. 20171BAB213020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, Y., Cao, B., Zhong, S. et al. Homogeneous liquid–liquid extraction of europium from aqueous solution with ionic liquids. J Radioanal Nucl Chem 319, 1219–1225 (2019). https://doi.org/10.1007/s10967-019-06419-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06419-7