Abstract
152Eu and 241Am recovery from HNO3 by conventional and micelle mediated extraction are studied. It is stated that radionuclides distribution ratios D (K D) in micelle mediated extraction are significantly higher than those of conventional extraction, with 241Am is slightly less extracted than 152Eu. Distribution ratios dependence on medium acidity is similar for both processes, with extraction maximum at C (HNO3) = 0.2–1 mol L−1. Microscopic research and dynamic light scattering prove micellar nature of calixarene solutions. Nano-scale of particles, which accumulate radionuclides, is confirmed by ultramicrofiltration. This method is also applied for studies of radionuclides re-extraction and electrochemical deposition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Micelle mediated (cloud point) extraction (MME) is a kind of homogeneous extraction, where pre-organized solutions of surfactants play role of extractants. It is known that the solubility of non-ionic and zwitterionic surfactants is depressed above so-called cloud point temperature. Above this point homogeneous micellar system separates into macrophases. This property can also manifest itself with pH or ionic forth changes. Thus, the MME method is based on the process of micellization, which proceeds in a certain for every surfactant temperature range, and its main characteristics are:
-
kraft point (lower temperature limit below which the solubility of the surfactant is no longer sufficient for the formation of micelles),
-
cloud point (upper temperature limit, followed by the separation of homogeneous micellar system into macrophases),
-
critical micelle concentration (surfactant concentration, at which micelles are stable in solution and exist in significant amounts in thermodynamic equilibrium with non-associated molecules) [1].
MME application for metals separation was first proposed by Watanabe in 1976. Earlier MME was widely used as a first step of proteins purification [2]. Brief survey of MME application for metals separation was published in 1982 [3], but comprehensive review of this issue appeared in publication [4] in 1985.
First data of lanthanides extraction by MME method with 8-hydroxyquinoline as chelating agent and Triton X-114 were published in 2001 [5]. Erbium(III) extraction by 2-(3,5-dichloro-2-pyridylazo)-5-dimethylaminophenol as chelating agent and PONTE-7.5 as surfactant was carried out by Silva et al. in 1997 [6]. Later similar procedure was used for Dy(III) preconcentration in urine [7].
Capability of various macrocyclic compounds bearing large structural cavities, in particular calixarenes, to form different colloidal aggregates (associates, micelles, clusters) in water due to hydrophobic interactions, is well known. Formation of aggregates (from 3 to 30 nm to 103 nm in diameter) was observed for some water soluble cationic calixarenes, and this process resembles micellization in colloidal surfactants and can be characterized by critical aggregate concentration, which is similar to critical micelle concentration for amphiphilic surfactants [8]. Association mechanisms for these compounds are different. At least two models of association are considered to be typical for amphiphilic molecules [9]. Amphiphilic calix[4]arenes, oxyethylated at the lower rim, were synthesized and examined in 2010 [10, 11]. Attraction of these compounds is in the possibility to observe multitypical association due to the simultaneous presence of hydrophobic fragments, aromatic rings and cavities in one molecule. It is stated that in systems “calixarene–Triton X-100” aggregation behavior consists in formation of small micelle-like aggregates, characteristic for non-ionic surfactants, and lamellar big aggregates. Use of similar mixtures, based on p-sulphonate thiacalixarene, tetra-sulphonatometylated calix[4]resorcinarene and calix[4]resorcinarene phosphonic acid for Ln(III), Gd(III) and Yb(III) micellar extraction showed moderate efficiency of such chelating agents [12].
Calix[4]arene phosphine oxides [13], regarded in this work as alternative ligands for radionuclides recovery from nitric acid aqueous solutions, possess enhanced solubility in water. The peculiarity of proposed scheme of MME consists in the possibility to avoid the introduction of additional surfactant (for example, Triton X-100) in the extraction system, because aqueous solutions of studied calixarenes are micellar themselves.
Experimental
Following materials and reagents were used:
-
phosphorylated calix[4]arenes with diethyl- and dimethyl-methylenphosphine oxide substituents at the upper rim (Fig. 1). Synthesis and purity tests by NMR (1H and 13C) were carried out in the Institute of Organic Chemistry (Kyev) [14–16];
-
m-nitrobenzotrifluoride (m-NBTF, “RHODIA”, France) was used as a diluent in liquid–liquid extraction;
-
152Eu and 241Am isotopes were produced by Khlopin Radium Institute. Salt of stable europium (10−5 mol L−1) was added in solutions as a carrier.
Liquid–liquid extraction
In accordance with preliminary kinetics investigation, experiments were performed in the following manner: similar volumes of organic and aqueous phases were placed in plastic tube and stirred at (23 ± 2) °C during 3 min. Then organic and aqueous phases were separated by centrifugation.
To determine radionuclides distribution ratios samples of separated phases (V = 0.4 mL) were measured on a DeskTop scintillation γ-ray spectrometer “DeskTop InSpector” (“Canberra”) with NaI detector (51 × 51 mm). For 241Am was used only one energy region—0.059 MeV, for 152Eu were used 2 energy regions: 1.41 and 0.344 MeV.
Distribution ratios were calculated by the equation \(D\, = \;\frac{{A_{\text{org}} }}{{A_{\text{aq}} }}\), where \(A_{\text{org}}\) and \(A_{\text{aq}}\) are isotope activities in organic and aqueous phases respectively. Time of sample measurement was chosen so that the measurement error did not exceed 15%.
Micelle mediated extraction (MME)
Recovery of metal cations in MME was performed by two methods.
Method 1 is suitable for solutions with C (HNO3) > 1 mol L−1. Calixarene (0.1 mL of aqueous solution, 0.1 mol L−1) is placed in a plastic tube, containing 0.9 mL of nitric acid solution, containing radionuclide. White amorphous phase is formed immediately. Mixture is stirred 10 min, and then phases are separated by centrifugation.
Method 2 is suitable for solutions with C (HNO3) < 1 mol L−1. Under these conditions addition of calixarene into the nitric acid solution, containing radionuclide, leads to colloid solution formation. Obtained mixture is heated up to 60 °C with stirring. Coagulation of colloidal particles takes place. Formed second phase is separated by centrifugation. Activities of aqueous phase before and after extraction were determined on a DeskTop scintillation γ-ray spectrometer “DeskTop InSpector” (“Canberra”) as described above.
Radionuclide distribution ratio is calculated by the equation:
where \(A^{0}\) and \(A^{{\prime }}\)—aqueous phase activities before and after extraction, respectively; m—mass of formed second phase, V—volume of aqueous phase.
Dynamic light scattering (DLS)
Sizes of formed micelles were studied by dynamic light scattering method with PhotoCor Complex (Milton Roy, United States) and Malvern Instrument ZetaSizer Nano (Malvern Instruments, England) instruments. The measured autocorrelation functions were analyzed by Malvern DTS Software, the DynaLS program and the second-order cumulant expansion methods. The effective hydrodynamic radius (R h) was calculated according to the Einstein–Stocks relation: D S = k B T/6πηR h, where D S is the diffusion coefficient, k B is the Boltzmann constant, T is the absolute temperature and η is the viscosity. The diffusion coefficient was measured at least three times for each sample. The average error in these experiments was approximately 4%. Studied solutions were filtered with Millipore filters, to remove dust particles from the scattering volume.
Atomic-force microscopy (AFM) and transmission electron microscopy (TEM) visualization
Sizes and shape of micelles were studied using atomic-force microscope Multimode V (Veeco, USA) and transmission electron microscope HT 7700 (Hitachi, Japan). AFM visualization of micelles was carried using discontinuous contact AFM technique and performed at low force set points. Phosphorus doped silicon probes RTESP (Veeco, USA) were utilized. TEM images were acquired at an accelerating voltage of 80 kV.
Aqueous solution of calixarene C67 at concentration 10−5 mol L−1 was ultrasonicated for 10 min and then 10–25 μL of the solution were dispersed on a suitable substrate and dried at 65 °C for 2 h. An atomically flat mica was used as a substrate for AFM and 200 mesh copper grids with continuous formvar films were utilized for TEM visualization.
Studies of radionuclides accumulating micelles: size of particles and ultrasound influence on their integrity
Colloidal and aqueous phases separation was prepared by centrifugal ultrafiltration in tubes with Amicon membranes (pore diameter 0.45–3 kDa). To study ultrasound influence on dispersion degree of micelles ultrasonic bath Elmasonic S40 (ultrasound frequency is 37 kHz, total energy consumption 320 W, effective ultrasound power is 120 W, maximum peak ultrasound power is 480 W, temperature range 0–80 °C, duration of sample treatment period—from 1 min to unlimited time) was used. In the audio range of frequencies acoustic system MS691 was used.
Re-extraction experiments: electrochemical deposition
For electrochemical deposition of 241Am on the steel target pseudo calixarene precipitate was dissolving in ethanol (50 mL) for 2 min. Then 0.1 mol L−1 phosphonium hexafluorophosphate [PH4]+PF6 − was added. Electrolysis duration was 120 min. Experiment was carried out as described in [17].
Results and discussion
Earlier we showed, that the most efficient extractants for rare earth (REE) and transplutonium elements (TPE) micelle mediated extraction are calix[4]arene dialkylmethylenphosphine oxides C45 and C67 [18]. Present paper is devoted to study influence of various factors on Am and Eu micelle mediated extraction by mentioned calixarenes from nitric acid solutions.
As for dependence of europium distribution ratios K D on the mode of calixarene addition and stirring intensity, neither mode of calixarene addition, nor stirring intensity do not affect europium distribution ratios. Formation of isolated phase occurs immediately, and further stirring has no influence on Eu distribution between phases.
To study the influence of added calixarene amount on Eu distribution between micellar and aqueous phases three experiments with various C45 weights were carried out. Experiments, where Method 1 was applied, demonstrated that with calixarene volume increasing (decrease of initial solutions acidity) Eu distribution ratios also increase. However, when the same volumes of calixarene solution with different concentrations are added, acidity of aqueous phase does not change, but with increasing of calixarene initial concentration (at constant acidity) Eu distribution ratios decrease. On the other hand, experiments, carried out by Method 2, showed that Eu distribution ratios stay practically constant with addition of different weights of calixarene at constant acid concentration in the aqueous phase. Thus, increase of calixarene concentration in the process of europium extraction at constant acidity by Methods 1 and 2 leads to different results.
Influence of medium acidity on MME process
Liquid–liquid extraction of europium from acid solutions by calixarenes C45 and C67 into m-NBTF was studied in paper [19]. Experiments on europium recovery by aqueous solutions of calixarenes C45 and C67 from nitric acid in the same intervals of HNO3 concentration were performed to compare processes of liquid–liquid and micelle mediated extraction. Obtained data are presented on Fig. 2.
As one can see, maximum of efficiency both in micelle-mediated and liquid–liquid extraction by C45 is observed at 1 mol L−1 of nitric acid. With increasing of nitric acid concentration efficiency of MME decreases, as well, as that of liquid–liquid extraction. It is related with conditions of isolated phase formation. At the acidity less than 0.3 mol L−1 after C45 addition colloidal solutions form, and only after solution heating up to T = 60 °C they coagulate and fall down as the isolated phase. At the acidity higher than 0.3 mol L−1 isolated phase forms immediately after addition of calixarene solution. C67 is insoluble already in 0.1 mol L−1 of nitric acid [19], and isolated phase falls down immediately after addition of calixarene solution. Thus, MME efficiency is due to the mechanism of isolated phase formation.
However, trends of distribution ratios dependence on medium acidity are similar in liquid–liquid and micelle mediated extraction: there is gradual increase of K D values with increase of aqueous phase acidity up to the maximum value (corresponding to such medium acidity, when extractant stops to wash out into the aqueous phase, and the mechanism of isolated phase formation changes) and gradual decrease of K D values, which is caused by competitive nitric acid extraction in liquid–liquid extraction process.
Influence of salting-out agents on europium MME process
The salting-out effect consists in the addition of nitric acid or its salts (e.g. alkali nitrates) in the solution. This increases the concentration of nitrate ions, which bind water. This results in a decrease of the hydration of the recovered cation.
MME tests were performed with C45, using different concentrations of salting-out agents, at 1 mol L−1 HNO3 and at 0.3 mol L−1 HNO3. Liquid–liquid extraction experiments with C45 solution in m-NBTF were performed in the same conditions. Lithium and sodium nitrates were used as salting-out agents. Trends in distribution ratios dependence on salting-out agent concentration were practically the same: in the case of 1 mol L−1 HNO3 extraction proceeded at room temperature, the isolated phase was falling down immediately after addition of the calixarene solution (Method 1). For 0.3 mol L−1 HNO3 extraction proceeded at 60 °C, because of colloidal solution formation, which required heating to coagulate (Method 2). Liquid–liquid extraction tests were performed in analogous conditions. Results are illustrated on Fig. 3.
Obtained data show that the salting-out agent concentration has greater influence on europium distribution ratios in MME, especially at 0.3 mol L−1 HNO3. In liquid–liquid extraction this influence is less. Thus the effect of salts influence in MME is due to conditions and the mechanism of isolated phase formation. At smaller HNO3 concentration Eu distribution ratios increase dramatically, because stability and micellization completeness increase with the salt concentration in the aqueous phase.
However, with the further increase of the salting-out agent concentration aggregate state of micelles stays constant and Eu distribution ratios decrease, as at liquid–liquid extraction. The last one occurs, evidently, because of increasing co-extraction of nitric acid.
Composition of europium: C45 solvates, obtained by MME process
To determine composition of extracted solvates one can use method of equilibrium shift or method of saturation. The method of equilibrium shift consists in changing of component concentration in extract by changing of component concentration in organic phase. Change of distributed component initial concentration up to complete extractant saturation is characteristic for this method.
In the case of europium liquid–liquid extraction by C2 (C67 analogue with butyl radicals at phosphorus atom) solutions disolvates formation was demonstrated by method of equilibrium shift and monosolvates formation was demonstrated by method of saturation [20]. Results of experiments on determination of europium solvate composition are presented in Table 1.
As it is shown in Table 1, saturation of extractant is observed at C (Eu) = 0.1 mol L−1, and [Eu]/[C45] becomes constant: about 2 mol of Eu on 1 mol of C45, that corresponds to half-solvate formation.
Study of micelle sizes and properties in calixarenes solutions
Preliminary tests demonstrated that C45 is readily soluble in water giving opalescent solution. It is regarded as indirect evidence of micellization. Strong binding of P=O groups of C45 with water molecules, not typical for aqueous solutions of other phosphine oxides, was observed in paper [13]. Moreover, it is stated that all four P=O groups of calixarene C45 molecule are bound with nitric acid protons, forming two (P=)O–H+–O(=P) fragments. Calixarene C45 in 3 mol L−1 HNO3 forms water insoluble compound [C45·2H+][O2NO–H–ONO2] −2 .
Experiments on the determination of sizes of C67 aggregates by dynamic light scattering were carried out to ensure micellar nature of studied calixarenes aqueous solutions. It was stated that in methylene chloride C67 exists as a homogeneous phase only for a short time, converting into the flaky precipitate, impossible to examine by DLS method and, probably, unable to capture cations in such state. This correlates with data, obtained earlier for Am and Eu liquid–liquid extraction by C67 into methylene chloride (%E Am = 6.9 ± 0.4, %E Eu = 13 ± 3) [21]. On contrary, in dichloroethane this calixarene forms aggregates of about 335 nm (Table 2).
In aqueous solutions were recorded particles of 4 nm size at 0.1 mol L−1 of calixarene and larger particles—up to 200 nm—at lower concentrations of calixarene (10−5 mol L−1).
Results for C67 aggregation in aqueous solution correlate with data of atomic-force and transmission electron microscopy (Figs. 4, 5). On the AFM images several types of particles were found. The first type are round particles which have height of 2.1–2.5 and 50–75 nm in lateral sizes. The second type are also round particles with characteristic diameter of 200–350 nm and height of 10–15 nm. The third type are crystallite like particles with lateral sizes of 1–2 μm and 10–15 nm in height. And the last one are particles with characteristic sizes of 0.7–2 μm.
AFM visualization of calix[4]arene C67 aqueous solutions applied on mica and dried: a AFM image with z scale—3 nm and profile of the particle; b, c AFM images both with z scale—20 nm and corresponding profiles of the particles; d AFM image with z scale 30 nm and corresponding roughness histogram (area of 10 × 10 μm)
TEM also showed the formation of the same type of particles and their aggregates. Most of particles have diameter of about 120 nm. And the most of larger particles are aggregates of the first ones. But also there are particles larger than 0.8 μm which inner structure cannot be seen. Crystallite like particles with characteristic sizes of 1.5–2 μm were also found.
Ultramicrofiltration experiments
Solubility of studied calixarenes decreases with increasing of aqueous phase acidity. On achieving the critical micelle concentration in solution colloidal particles form. They usually consist of an insoluble core of very small size, surrounded by a stabilizing shell of adsorbed ions and solvent molecules. The average size of micelles is of 1–100 nm. Metal ions react with calixarene forming hydrophobic complex. Obtained micelles capture metal complex in the hydrophobic core, than the phase, enriched by surfactant, can be separated from the bulk of the aqueous phase by centrifugation [11, 12]. However, taking into account micelles of nanoscale, their isolation requires special conditions.
C67 was chosen to solve the task of 241Am recovery in the MME, as it has already proved to be an efficient reagent to recover europium [19].
It appeared that with initial C67 concentration increasing (at constant aqueous phase acidity) Am and Eu recovery extent decreases (Fig. 6). Moreover, americium recovery extent is higher, than that of europium, although its concentration is three orders lower. This indicates that the metal in such case can’t be regarded as micellizing complexing agent, and micelles consist of calixarene molecules associates (lyophilic sol). Thus, one can conclude that the efficiency of micelle mediated extraction by C67 is affected by the mechanism of formation and dispersion state of pseudo precipitate.
Americium distribution in colloidal particles in 0.1 mol L−1 nitric acid solution with addition of C67 solution of various concentrations was examined to study micelles formation mechanism (Fig. 7).
It was stated that experimentally determined Am recovery extent depends on types of membranes, used for micellar phase separation. With decrease of calixarene concentration from 0.005 to 0.0025 mol L−1 extent of Am recovery by ultrafiltration membranes increases. At the same time, at C67 concentration 0.02 mol L−1 Am recovery extent does not depend on the size of forming colloidal particles, remaining constant in the whole range of applied filters. As diagrams of Fig. 7 show, at C67 concentration 0.02 mol L−1 americium is captured only by large particles (>0.45 μm). Recovery extent is low (20%). When reducing the calixarene concentration to 0.005 mol L−1, recovery extent is 100%. The main accumulating particles range in size from 10 to 100 kDa. By reducing the concentration of calixarene to 0.0025 mol L−1 recovery extent decreases slightly by 1%, but 50% of americium contains generally in monodisperse particles of 30–100 kDa.
Thus, micelles, which efficiently capture radionuclides, are of very small size, which makes difficult their isolation from the solution. At the same time, aggregated particles larger than 0.45 μm bind americium weaker, possibly due to the decrease of the specific surface. Therefore, it is better to employ methods that promote micelles coagulation only after capture of americium.
Attempts to stimulate the coagulation of colloidal particles in the system under study by treatment of sound waves (similar to the flotation process) were not successful. With the electrolyte introduction in the aqueous phase K D (Am) values increase dramatically with electrolyte concentration but then gradually decrease (Fig. 8). The growth of americium recovery extent with the electrolyte introduction can be explained by the increase of micelles stability, subsequent reduction of recovery extent—by reduction of water activity and of metal activity coefficients. For example, in the liquid–liquid extraction by calixarenes europium recovery extent also decreases slowly with sodium nitrate or lithium nitrate concentrations decrease [19].
One can explain this regularity, considering the diagram of americium distribution in colloidal particles of various sizes (Fig. 9).
As can be seen from obtained data, colloidal particles coarsen with lithium nitrate addition into the aqueous phase. Americium is practically absent in particles smaller than 30 kDa. Concentration of americium, accumulated by particles larger than 0.1 μm, increases slightly, americium content increases significantly only in the particles fraction of 0.1 kDa—30 μm. In the whole range of electrolyte concentration americium recovery extent is close to 100%, and a 30 kDa membrane with sufficient intensity of filtration rate can be applied for the isolation of particles.
Similar experiments were conducted with europium. In this case, the calixarene concentration was 0.001 mol L−1, and nitric acid concentration was 3 mol L−1 in order to obtain Eu distribution ratio values close to 1. Thus, the bulk of Eu accumulating colloidal particles belongs to nanoparticles of 3–30 nm. Such particles are difficult to be separated from the solution. It is therefore advisable to employ methods that improve particles aggregation, including introduction of the optimal salt amount into the aqueous solution.
Ultrasonic waves influence on micellar state of radionuclides complexes with calixarenes and re-extraction of target elements
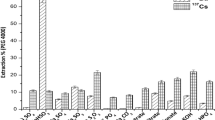
Although in the framework of this paper micelle mediated extraction is regarded only as alternative method for actinides analysis, and there is no need of extractant recycling, some experiments with re-extraction were carried out. To extract the desired component from precipitates obtained by MME one can use their chemical decomposition by strong acids or sodium carbonate. However, experiments have shown that pseudo-precipitates of calixarene compounds with REE and TPE dissolve very slowly in 4–8 mol L−1 of HNO3, and re-extraction of radionuclides proceeds only on 80–90%. Furthermore, the use of concentrated solutions of strong mineral acids and the formation of destruction products of cellulose organic residues of ultrafiltration membrane prevent re-extraction. Therefore, following techniques were proposed to recover radionuclides (241Am in particular) from the colloidal fraction: destruction of micelles by ultrasound or dissolution of pseudo-precipitate, followed by electrochemical deposition of target element.
Influence of ultrasonic treatment on colloidal particles state was examined in the solution (Fig. 10) and on the filter (Fig. 11). Calixarene solution of 0.005 mol/L) and 0.1 mol L−1 HNO3 were used.
As demonstrates Fig. 10, ultrasound rapidly destroys large particles in the solution: even 1 min is enough to destroy micron particles. However, nanoscale particles are destroyed in less extent. Therefore, to extract americium from the filter hydrochloric acid solution (1 mol L−1) and sonication were applied. Data are presented on Fig. 11.
The composition of the aqueous phase was chosen in accordance with technical conditions of applied membranes, in order to reduce entering of cellulose into solution. The reason is that filter membranes are resistant to 1 N HCl, and this acid was used as the only mineral acid, where membranes are stable.
As it is seen on Fig. 11, proposed method appeared very efficient: after a 10 min treatment americium recovery extent on the filter was 99%.
Experimental results for electrochemical deposition of 241Am on the steel target demonstrated that over 60% of the americium are already deposited on the target after 30 min of electrolysis. About 120 min is enough to achieve over 99% of element recovery.
Both methods—ultrasonic micelles destruction and electrochemical deposition of the target element are efficient enough to transfer radionuclides in solution and to separate them on a target for further analytical purposes.
Conclusions
Experiment showed the possibility of using water-soluble calix[4]arenes with phosphine oxide substituents at the upper rim as micellizing extractants to recover from nitric acid solutions such radionuclide as 152Eu and 241Am. Compared with the known method of micelle mediated extraction, phosphorylated calixarenes C45 and C67 are at the same time chelating agents and surfactants.
The DLS method showed formation of calixarene aggregates of various sizes in dichloroethane (335 nm) and in water (~4 nm for 0.1 mol L−1 of C67 and ~200 nm for 10−5 mol L−1 of C67). This correlates with data of atomic-force and transmission electron microscopy, which prove existence of at least 4 types of calixarene aggregates (round particles of different sizes, crystallite like particles, small particles of 0.7–2 μm).
The efficiency of 152Eu and 241Am MME process of micellar extraction provide mainly micelles of minimum size.
New methods of re-extraction of radionuclides from the micellar phase are proposed: the ultrasonic treatment and the electrochemical deposition, which allows to quantitatively recover americium on a target.
Study of the influence of acoustic waves not only for the destruction of the micellar state extracts, but also on the possible coagulation of the micelles in the extract for their easier microfiltration separation can be considered as promising directions for further research.
References
Mittal K (ed) (1977) Micellization, solubilization and microemulsions. Plenum, New York
Paleologos E, Giokas D, Karayannis M (2005) Micelle-mediated separation and cloud-point extraction. Trends Anal Chem 24(5):426–436
Watanabe H (1982) In: Mittal K, Fendler E (eds) Solution behavior of surfactants. Plenum Press, New York
Pelizzetti E, Pramauro E (1985) Analytical applications of organized molecular assemblies. Anal Chim Acta 169:1–29
Paleologos E, Stalikas C, Tzouwara-Karayanni S, Karayannis M (2001) Selective speciation of trace chromium through micelle-mediated preconcentration, coupled with micellar flow injection analysis-spectrofluorimetry. Anal Chim Acta 436:49–57
Silva M, Fernandes L, Olsina R, Stacchiola D (1997) Cloud point extraction, preconcentration and spectrophotometric determination of erbium(III)-2(3,5-dichloro-2-pyridylazo)-5-dimethylaminophenol. Anal Chim Acta 342:229–238
Ortega C, Cerutti S, Olsina R, Silva M, Martinez L (2003) On-line complexation/cloud point preconcentration for the sensitive determination of dysprosium in urine by flow injection inductively coupled plasma-optical emission spectrometry. Anal Bioanal Chem 375:270–274
Vodolazkaya N, Mhchedlov-Petrossyan N, Bogdanova L et al (2012) In: Ryabchenko V (ed) From molecules to functional architecture. Supramolecular interactions. East Publisher House, Donetsk
Zakharova L, Kudryashova Yu, Selivanova N, Voronin M, Ibragimova A et al (2010) Novel membrane mimetic systems based on amphiphilic oxyethylated calix[4]arene: aggregative and liquid crystalline behavior. J Membr Sci 364:90–101
Kudryashova Yu, Valeeva F, Zakharova L, Solovieva S et al (2012) New organized systems based on amphiphilic oxyethylated Calix[4]arene. Colloidal J 74:67–77
Mustafina A, Zakharova L, Elistratova J, Kudryashova Yu, Solovieva S et al (2010) Solution behavior of mixed systems based on novel amphiphilic cyclophanes and Triton X100: aggregation, cloud point phenomenon and cloud point extraction of lanthanide ions. J Colloid Interface Sci 346:405–413
Mustafina A, Elistratova J, Burilov A, Knyazeva I et al (2006) Cloud point extraction of lanthanide (III) ions via use of Triton X-100 without and with water-soluble calixarenes as added chelating agents. Talanta 68:863–868
Smirnov I, Karavan M, Babain V, Kvasnitskiy I et al (2007) Effect of alkyl substituents on extraction properties and solubility of calix[4]arene dialkylphosphine oxides. Radiochim Acta 95:97–102
Atamas L, Klimchuk O, Rudzevich V, Pirozhenko V et al (2003) New organophosphorus calix[4]arene ionophores for trivalent lanthanide and actinide cations. J Supramol Chem 2(4–5):421–427
Klimchuk O, Atamas L, Miroshnichenko S, Kalchenko V et al (2004) New wide rim phosphomethylated calix[4]arenes in extraction of americium and europium. J Incl Phenom Macrocycl Chem 49:47–56
Klimchuk O (2004) Design, synthesis and properties of phosphorylated ionophoric calix[4] arenes, Abstract of PhD Thesis, Kyev
Novikov A, Ryleeva V, Abramova A, Pribylova G, Smirnov I (2014) Electrodeposition of americium on the stainless steel support for the purpose of radiochemical assay. J Radioanal Nucl Chem 302:543–544
Karavan M, Smirnov I, Miroshnichenko S, Kal’chenko V (2008) Water-soluble Calix[4]arene Dialkylphosphine Oxides in the process of cloud point extraction. In: Proceedings of XV International conference on phosphorous compounds ICCPC-XV, Saint-Petersburg
Karavan M (2009) Phosphorylated calix[n]arenes as perspective extractants for actinides and some fission products from nitric acid medium, Abstract of PhD Thesis, Saint-Petersburg
Smirnov I (2009) Extraction of actinides and fission products by multifunctional and macrocyclic compounds: general patterns in the processing of HLW. Thesis for the degree of Doctor of Science, Saint-Petersburg
Karavan M (2009) Phosphorylated calixarenes for the recognition of f-elements, PhD Thesis, University of Strasbourg
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karavan, M.D., Smirnov, I.V., Kleshnina, S.R. et al. Micelle mediated extraction of americium and europium by calix[4]arene phosphine oxides from nitric acid media. J Radioanal Nucl Chem 311, 599–609 (2017). https://doi.org/10.1007/s10967-016-5096-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5096-7