Abstract
In the present investigation, the activity concentrations of radionuclides in the soils of Cauvery river environs were measured using HPGe gamma ray spectrometer. FTIR spectroscopy was used to find minerals present in soil samples. The mean values of 40K, 226Ra and 232Th in the soil samples was found to be 132.9, 22.95 and 26.88 Bq kg−1 respectively. The estimated absorbed dose rate and hazard indices were found to be within the safety limits. The extinction coefficients for quartz, sepiolite and kaolinite in soil varied from 0.64 to 37.24, 0.39 to 34.47 and 9.66 to 35.81 respectively. The correlation matrix showed that the clay mineral like kaolinite influences the increase in activity concentration of radionuclides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The assessment of gamma radiation dose delivered to the human population through natural background radiation is important because the natural radiations are the largest contributors to the population dose around the world [1]. The total radiation dose received by human population is about 2.8 mSv per year, out of this 85% (2.4 mSv) comes from the natural background radiation exposure [2]. The main sources of natural background radiation are cosmic rays and terrestrial radioactive decay series 238U and 232Th and singly occurring radionuclides like 40K present in soil [3]. The activity concentration of radionuclides in soil depends on the geochemical, geological and geographical circumstances of the location. Some investigations have reported that the mobility and activity of radionuclides depends on the vital physico-chemical parameters [4]. In the soil, clay minerals are one of the major constituents. Organic matter and clay are known to have high cation exchange capacity, that helps to absorb and exchange the cations to fix the radionuclides [5]. Clay mainly contains alumina, silica, and water, which also contains small quantities of iron, alkali metals, and alkaline earths. Clay materials are classified into different layered structure based on the difference in their layered structure. There are few classes of clay, such as mica (illite), serpentine, kaolinite, smectites (montomorillonite, saponite), vermiculite, pylophyllite (talc), and sepiolite [6]. Therefore, the measurement of natural radionuclides concentrations and associated dose rate is important. The characterization of soil and its minerals is vital to understand the influence to the increase of activity. In view of this, in the present investigation the role of minerals in the natural radioactivity level of Cauvery river basin soil has been studied.
Materials and methods
Study area
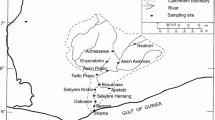
The Cauvery river is one of the major rivers in South India, which originates at Brahmagiri hills in the Western Ghats and it flows through Karnataka, Kerala and Tamil Nadu. The length of the river within Karnataka is 320 km (total 800 km) from the origin of the river and it covers an area of 8115 km2 (2.47% of the total geographical area of India). The river basin is bound between the longitude of 75°30′ E to 79°45′ E and latitude of 10° 5′ to 13°30′ N.
The river basin experiences tropical monsoon climate with bimodal rainfall pattern and the temperature ranges from 13.5 to 41 °C depending on the climatic changes [7]. The river basin covers a part of peninsular India and drastic changes in the climatic condition are also observed along the river drainage and their tributaries. The river basin contains different rock types such as supracrustal rocks consists of predominantly athletic and minor komatiitic volcanic rocks, felsic volcanic rocks, caustic and chemical sedimentary rocks [8].
Sample collection and preparation
The sampling locations along the river Cauvery was identified based on geological study of the area and the locations were nearly 20–25 km apart from each other. The sampling locations, K1–K14, of the river basin are shown in Fig. 1. The positions of sampling locations were recorded using a global positioning system (GPS) (Model: GARMIN GPSMAP 64) unit. The soil samples were collected from the riverbank following the standard protocol (EML procedure manual) during the period of January 2014 [9]. The sampling was done on undisturbed surface area away from the residential area. The soil was collected within the top 10 cm of soil after removing the extraneous materials like roots, pebbles, plant materials. About 4 kg of soil sample was stored in polyethylene bags at each location and brought to the laboratory for further processing. The samples were dried in a hot air circulated oven until dry weight was obtained and sieved through a 250 µ mesh and stored in an air tight PVC container for 30 days to obtain a secular equilibrium between 226Ra and its progeny [10].
Activity measurement
The activity concentration of natural radionuclides 226Ra, 232Th, and 40K in the soil samples were determined by gamma spectrometry technique using a high-resolution n-type HPGe detector (NGC 3019, DSG). The efficiency and resolution of the detector was 30% and 1.9 keV at 1.33 MeV energy [7]. The detector was shielded using lead of 10–15 cm. The gamma ray spectrometry system contains the 16 K multi- channel analyzer (MCA-3 series) which gives the analyzed output in the form of spectrum. The gamma spectrum was recorded for 30,000 s and the activity concentration of radionuclides was determined. The corresponding energy of the radionuclides is 1.46 MeV for 40K, 0.911 MeV for 223Ac (232Th) and 0.609 MeV for 214Bi (226Ra) and its counts give the activity of the corresponding radionuclides [11].
FTIR analysis
In the present study, 30–50 mg of 53 mm grain size soil was taken in an agate mortar for grinding and with 20–25 drops of ethanol. The grinded sample was kept in a hot air oven to remove the moisture and making the pellet mixing with KBr in the ratio of 1:30 [12]. The mixture was taken in a dye to prepare disc for the further analysis. Using the Perkin Elmer RX1 FTIR spectrometer to record the infrared spectra of soil samples in the region 4000–400 cm−1. The instrument has been calibrated each time using standard polystyrene films and it shows the resolution of 0.001 cm−1 and the accuracy was 4 cm−1 [13].
Results and discussion
Activity concentration of 226Ra, 232Th and 40K
The activity concentration of 226Ra, 232Th and 40K was determined using HPGe gamma ray spectrometer and the results are shown in Table 1 and Fig. 2. The measured activity concentration of 226Ra varied from 2.7 to 53.9 Bq kg−1 with an average value of 22.9 Bq kg−1 and activity of 232Th varied from 4.7 to 52.2 Bq kg−1 with an average value of 26.9 Bq kg−1 respectively. The activity concentration of 40K varied from 19.1 to 320.0 Bq kg−1 with a mean value of 132.9 Bq kg−1. The activity concentration of radionuclides at various locations varied [14]. The variation in activity may be due to the changes in geology of individual location, altitude, climate and physico-chemical properties of the soil [7]. Along with these natural processes, some artificial activities also contribute to radioactivity. The activity concentration of 40K is higher than the concentration of 226Ra and 232Th activity normally happens in the soils. This could be due to abundance of potassium bearing minerals presence in the soils. The obtained result shows that the activity concentration of 226Ra and 232Th was high in upper reaches of the river as compared to the lower reaches. The upper reaches of the river comes under Coorg district, which contains red loamy type soil [8] and different types of rocks with high activity of uranium and thorium minerals. Mainly the rock types are granites, gneisses, charnockites and amphibolites rocks. The activity of radionuclides is very high in granites and gneisses, which contribute to the high activity in upper reaches of the river.
The activity concentration of Cauvery river environment soil samples were compared with other literature values and World average as presented in Table 2. The activity concentration found in soil is within the Indian and world average values (Indian average 29, 64 and 400 Bq kg−1 for 226Ra, 232Th and 40K respectively and world average of 226Ra, 232Th and 40K is 35, 30 and 400 in Bq kg−1, respectively UNSCEAR, 2000) [1]. The average activity of 232Th in study area is higher than the corresponding values of Kali and Sharvathi river but it is lower than the 232Th activity values of all other regions [10]. The higher activity of thorium is due to the presence thorium bearing rocks presence in the river basin. The 226Ra activity concentration was found to be high in Nethravathi, Kali and Sharvathi river basin compared to the Cauvery river soils. The Kali river basin consists of gneisses, schists and quartzite and Sharavathi river basin consist of gneisses, local ferruginous laterities, Meta volcanic and dolomites types of rocks. The activity of 226Ra, 232Th in Kallada river is higher than the corresponding values of Cauvery river soil samples, the higher activity in Kallada river due to the presence of thorium bearing monazite sand in the soil samples [11]. But the values are comparable to the other environments such as Pra river western regions of Ghan, Dez river Iran and Wei river China [15, 20]. The activity concentration of 226Ra and 40K were found to be lower than the literature values. Table 3 shows the statistical analysis values of 226Ra, 232Th and 40K in the soil. The frequency distribution graphs for the soil are shown in Fig. 3. The frequency distribution for natural radionuclides activity in the soil is symmetric with right and left. The value of skewness for 226Ra, 232Th and 40K is positive. The distribution was observed to be flat due to the negative values of kurtosis. The values corresponding to the present study (skewness and kurtosis) show the normal distribution, these values are in good agreement with the theoretical frequency.
The importance of measuring the gamma emitting natural radionuclides is to estimate the gamma dose likely to be received by the population. To estimate the risk due to the gamma dose in the river basin soil, different radiological risk indicating parameters were calculated using the standard equations and the obtained results are shown in Table 4.
Absorbed dose rate (D)
The absorbed dose rate D (nGy h−1) in air due to the produced from the radionuclides presence of soil was calculated using the equation with the conversion factors [21]
where ARa, ATh and AK are the mean activity concentration of 226Ra, 232Th and 40K respectively. The absorbed dose rate in air found to be 8.5–57.6 nGy h−1 with a mean value of 32.4 nGy h−1, which is lower than the recommended limit by UNSCEAR (2000) [1].
Hazard index
The calculation of external and internal hazard index is the one of the ways to find the radiological suitability of the materials and hazardous to the respiratory due to the inhalation of radon. Therefore in the present investigation both the external and internal–external hazard index was calculated using the below equations [22].
where ARa, ATh and AK are the mean activity concentration of 226Ra, 232Th and 40K respectively. The mean value of hazard indices is less than unity (Hex = 0.19 and Hin = 0.25). Therefore there is no associated health risk to the population.
Annual effective equivalent dose
The annual effective dose from indoor terrestrial gamma radiation was estimated by taking into account of dose rate, indoor and outdoor occupancy factor. The AEED was calculated using the following equations, which contains the conversion factors such as outdoor occupancy factor (0.2) and indoor occupancy factor (0.8), absorbed dose rate in air (D) and effective dose received by adults (0.7 Sv Gy−1) [23, 24].
The estimated mean value of AEED for indoor and outdoor is 39.7 and 158.9 µSv year−1, which is marginally lower than the ICRP (International Commission on Radiological Protection) recommended annual effective dose limit of 20 mSv year−1 for the general public [25].
Radium equivalent activity (Ra eq)
The radium equivalent activity is a single index to describe the gamma output coming from combining of 226Ra, 232Th and 40K in the samples from an individual location. The radium equivalent activity was estimated using the following equation [26, 27]
where ARa, ATh and AK are the mean activity concentrations of 226Ra, 232Th and 40K in Bq kg−1, respectively. The Radium equivalent activity (Raeq) ranged from 24.5 to 130.6 Bq kg−1 with a mean value of 71.6 Bq kg−1, which is lower than the safety limit (recommended level < 370 Bq kg−1).
FTIR studies
The results obtained from the FTIR studies on the soil samples along with corresponding mineral are presented in Table 5. A representative FTIR spectrum (soil K3) is shown in Fig. 4. The identification of abundant minerals in the soils using FTIR spectrum is helpful. In the present study the obtained spectrum of soils are compared with the literature values for the identification of concerned mineral [13]. The hydrous Mg silicate clay mineral Sepiolite is present in all the samples with a peak of 428–432 cm−1. The silicate mineral like Quartz which is present normally in all samples in the range of 460–462 cm−1 and other two, 693–695 cm−1 due to the Si–O symmetrical bending vibrations, 794–796 cm−1 due to the Si–O symmetrical stretching vibrations found in K1–K5, K7, K8, K10–K14 samples. 914 cm−1 indicate the abundance of clay mineral constituents such as kaolinite, which is found in all samples. The peak presence in the region of 1369–1372 cm−1 indicates the presence of magnesium oxalate in the top samples. Peaks presence in the region of 2852–2854 and 2923–2927 cm−1 show the presence of organic carbon in all the samples. Hematite is one of the most abundant iron mineral, which is identified in all the sample in the region 538–539 cm−1. Similar findings have been reported by Fysh and Fredericks (1983) [28]. Palygorskite peak in the range of 1630–1650 cm−1 was observed in all the locations. The clay mineral illite, Carbonate mineral aragonite and gibbsite found in few samples as shown in table with corresponding wave number (cm−1) [29].
Relative distribution of minerals in the soil samples
In the present study, the minerals quartz, sepiolite, and kaolinite are considered as the important minerals and others are associated minerals (Table 6). Therefore, it is interesting to find the relative distribution of major minerals in the Cauvery river soil samples. The relative distribution of the minerals was estimated through the calculation of extinction co-efficient of individual mineral. Using the following equations the extinction co-efficient of quartz, sepiolite, and kaolinite was calculated for all soil samples [13].
where K is the extinction co-efficient, A is the area of the pellet, and m is the mass of the pellet. D is the optical density and it can be calculated using the relation.
where I0 is the intensity of the incident radiation and I is the intensity of the transmitted radiation [14].
The calculated values of extinction co-efficient are presented in Table 5. The extinction co-efficient value of quartz, seopilite, and kaolinite in the soil varies from 0.64 to 37.24, 0.39 to 34.47, and 9.66 to 35.81 respectively. The correlation analysis between activity concentration of natural radionuclides and extinction co-efficient value of quartz, seopilite, and kaolinite to understand the dependence of radionuclides with clay minerals was done using SPSS statistical software. The values are presented in Table 7. The bold letter indicated in the table show good correlation coefficients and correlation is significant at the level of 0.01 with confidence level is two tail (95%) in SPSS 22. A positive correlation of 0.84 was found between activity of 226Ra and 232Th which shows that uranium and thorium decay series occur together in the examined soil samples nature. The extinction coefficient of kaolinite and activity of 226Ra are in good correlation with correlation coefficient of 0.60. The 232Th activity show slightly positive correlation with kaolinite with correlation coefficient 0.46.
Conclusion
The activity concentration of natural radionuclides in Cauvery river soil samples were measured by HPGe gamma ray spectroscopy method. The measured activity concentrations of 226Ra, 232Th and 40K in this study were found to be within the world average value. Significant correlation was observed between 226Ra and 232Th activity concentration. The calculated absorbed dose rate in air was lower than the world average value (57 nGy h−1). The internal and external hazard indices were found to be less than unity, which shows the radiation risk is below the safety limit. The estimated value of radium equivalent in all soil samples were less than the recommended limit 370 Bq kg−1. The correlation matrix showed that the clay mineral like kaolinite influences the increase in activity concentration of radionuclides.
References
UNSCEAR (2000) United Nations Scientific Committee on the effect of atomic radiation. Sources and effects of ionizing radiation. Report to General Assembly, with Scientific Annexes, United Nations, New York
Rangaswamy DR, Sannappa J (2016) Distribution of natural radionuclides and radiation level measurements in Karnataka State, India: an overview. J Radioanal Nucl Chem 310(1):1–12
Narayana Y, Rajashekara KM, Siddappa K (2007) Natural radiovactivity in some major rivers of costal Karnataka on the south west coast of India. J Environ Radioact 95:98–106
Aytas S, Yusan S, Aslani MA, Karali T, Turkozu DA, Gok C, Erenturk S, Gokse M, Oguz FK (2012) Natural radioactivity of riverbank sediments of the Maritza and Tundja Rivers in Turkey. J Environ Sci Health Part A 47:2163–2172
Ramasamy V, Paramasivama K, Suresh G, Jose MT (2014) Role of sediment characteristics on natural radiation level of the Vaigai river sediment, Tamilnadu, India. J Environ Radioact 127:64–74
Shichi T, Takagi K (2000) Clay minerals as photochemical reaction fields. J Photochem Photobiol C 1:113–130
Narayana Y, Kaliprasad CS, Sanjeev Ganesh (2016) Natural radionuclide levels in sediments of Cauvery riverine environment. Radiat Prot Dosim 171(2):229–233
Kaliprasad CS, Narayana Y (2016) Speciation and behaviour of 210Po and 210Pb in the riverine ecosystem of Cauvery, a major river of south India. Radiochemistry 58(4):431–437
EML Procedure Manual (1983) In: Herbert L. volchok, Gail de Planque (ed), 26th edn, Environment Measurement Laboratory, U.S. Department of energy
Narayana Y, Rajashekara KM, Siddappa K (2007) Natural radiovactivity in some major rivers of costal Karnataka on the south west coast of India. J Environ Radioact 95:98–106
Venunathan N, Kaliprasad CS, Narayana Y (2016) Natural radioactivity in sediment and river bank soil of Kallada river of Kerala, South India and its associated radiation risk. Radiat Prot Dosim 171(2):271–276
Mullainathan S, Nithiyanantham S (2016) FTIR spectroscopic studies of rock sediments in Namakkal, Tamil Nadu, South India, for vegetations. Environ Earth Sci 75:692
Rajesh P, Joseph Vedhagiri S, Ramasamy V (2013) FTIR characterisation of minerals in charnockite rocks of Kalrayan Hills, India. Arch Phys Res 4(4):5–13
Krishnamoorthy N, Mullainathan S, Mehra R (2015) Variation of naturally occurring radionuclides, dose rate and mineral characteristics with particle size and altitude in bottom sediments of a river originating from Anamalai hills in the Western Ghats of India. Environ Earth Sci 74(4):3467–3483
Santawamaitre T (2012) An evaluation of the level of naturally occurring radioactive materials in soil samples along the Chao Phraya river Basin. PhD thesis. University of Surrey
Adukpo OK, Faanu A, Lawluvi H, Tettey Larbi L, Emi Reynolds G, Darko EO, Kansaana C, Kpeglo DO, Awudu AR, Glover ET, Amoah PA, Efa AO, Agyemang LA, Agyeman BK, Kpordzro R, Doe AI (2015) Distribution and assessment of radionuclides in sediments, soil and water from the lower basin of river Pra in the Central and Western Regions of Ghana. J Radioanal Nucl Chem 303:1679–1685
Lu X, Zhang X, Wang F (2008) Natural radioactivity in sediment of Wei river, China. Environ Geol 53(7):1475–1481
Akozcan S (2014) Annual effective dose of naturally occurring radionuclides in soil and sediment. Toxicol Environ Chem 96(3):379–386
Nasrabadi MN, Mostajaboddavati M, Hajialiani G (2014) Natural radioactivity distribution in riverbank soils along the Dez river basin of Iran. World J Environ Res 4(1):07–22
Kurnaz A, Kucukomeroglu B, Keser R, Okumusoglu NT, Kprkmaz F, Karahan G, Cevik U (2007) Determination of radioactivity levels and hazards of soil and sediment samples in Firtina Valley (Rize, turkey). Appl Radiat Isot 65:1281–1289
Yadav Manjulata, Rawat Mukesh, Dangwal Anoop, Prasad Mukesh, Gusain GS, Ramola RC (2015) Analysis of natural radionuclides in soil samples of purola area of Garhwal himalaya, India. Radiat Prot Dosim 167:215–218
Beretka J, Mathew PJ (1985) Natural radioactivity of Australian building materials, waste and by-products. Health Phys 48:87–95
Sac MM, Ortabuk F, Kumru MN, Ichedef M, Sert S (2012) Determination of radioactivity and heavy metals of Bakirçay river in Western Turkey. Appl Radiat Isot 70:2494–2499
Kritsananuwat R, Sahoo SK, Fukushi M, Pangza K, Chanyotha S (2015) Radiological risk assessment of 238U, 232Th and 40K in Thailand coastal sediments at selected areas proposed for nuclear power plant sites. J Radioanal Nucl Chem 303(1):325–334
International Commission on Radiological Protection (2007) Recommendations of the ICRP, publication 103. Pergamon Press, Oxford
El-Arabi AM (2007) 226Ra, 232Th and 40K concentrations in igneous rocks from eastern desert, Egypt and its radiological implications. Radiat Meas 42:94–100
Zaim Nimet, Atlas Hakan (2016) Assessment of radioactivity levels and radiation hazards using gamma spectrometry in soil samples of Edirne, Turkey. J Radioanal Nucl Chem 310(3):959–967
Fysh SA, Fredericks PM (1983) Fourier transform infrared studies of aluminous goethites and haematites. Clays Clay Miner 31(5):377–381
Ramasamy V, Rajkumar P, Ponnusamy V (2009) Depth wise analysis of recently excavated Vellar river sediments through FTIR and XRD studies. Indian J Phys 83(9):1295–1308
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaliprasad, C.S., Vinutha, P.R. & Narayana, Y. Studies on distribution of radionuclides and behavior of clay minerals in the soils of river environs. J Radioanal Nucl Chem 316, 609–617 (2018). https://doi.org/10.1007/s10967-018-5825-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5825-1