Abstract
Minerals of 15 rock sediments of Kollimalai Hills, Namakkal district, Tamil Nadu, South India, were determined using FTIR spectroscopy Crystallinity, extinction coefficient and relative distribution for different types of vegetation were analyzed owing to its importance and the suitability and those parameters were compared with those of many reported relevant data. The possible vegetation of each city is interpreted in light of qualitatively and quantitatively observed measurements of relevant parameters such as crystallinity index, relative distribution and extinction coefficients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tamil Nadu is bounded by Karnataka and Andhra Pradesh in the north and Kerala on the west. Tamil Nadu is the southernmost state of India, which is estimated to have an area of 3305 km2, equivalent to that of France. It is a state nestled in the southern Indian peninsula, on the shores of the Bay of Bengal and the deep blue Indian Ocean. The eastern and western tips of the state are defined by the Point Calimere and Mudumalai wildlife sanctuaries, while the southernmost tip is defined by Cape Comorin or Kanyakumari, the land’s end of India.
Although Tamil Nadu is one of the most urbanized states of India, it is still rural land; agriculture is the mainstay of life for about three quarters of the rural population. The principal food crops are rice, maize, jowar, bajra, ragi and pulses. The cash crops include cotton, sugarcane, oilseeds, coffee, tea, rubber and chilies. Tamil Nadu is considered the main textiles producer in India and an important exporter of leather products, cotton goods, tea, coffee, spices, tobacco, etc. Chennai is the capital city of the state.
Geological locations of Namakkal district: Namakkal is a historic town dating back to at least the 14th century. The name Namakkal derives from Namagiri, which is the name of the single rock formation at the center of the town. The rock is 65 m high and more than a kilometer in circumference. Namakkal is located at 11.23°N 78.17°E, has an average elevation of 218 m (715 feet), and is close to Kolli Hills, which is part of the ‘Eastern Ghats’, and the closest river is Kaveri. The city is approximately 380 km southwest of Chennai, 250 km south of Bangalore, 50 km south of Salem and 85 km northwest of Tiruchirapalli (Trichy).
Kolli Hills is a small mountain range located in central Tamil Nadu, India. The mountain ranges are about 1000–1300 m in height and cover an area of approximately 280 km2. Kolli Hills is part of the Eastern Ghats, which is a mountain range that runs mostly parallel to the east coast of South India. The mountains are relatively untouched by tourism and still retain their natural beauty.
Farming and vegetation Apart from its historical significance, the mountains are covered with ‘evergreen forests, but there the soil surface area has been cleared for farming use’. Important farm products of the mountain ranges include coffee, tea, jackfruit, pineapple, black pepper and other spices. Rice and other minor millets form are also grown for livestock feeding and self-consumption of tribal people who inhabit these mountains. The jackfruit grown on these mountains is well known for its delicious taste and fragrance, and it is best savored by soaking in wild honey, which is also harvested from these mountains. The mountains are covered by lush green vegetation in the spring and monsoon season, and several streams pass through them.
Agaya Gangai waterfalls A popular attraction on these mountains is the Agaya Gangai waterfalls. They can, however, tack a dip in the river near the beginning of the trail. The end of the trail is separated from the base of the waterfalls by a body of flowing water and is just an easy swim away (Kumar and Reddy 2004).
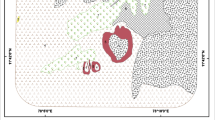
Sample collection: Fifteen locations were selected at various altitudes from the top of the mountain and the rock samples are collected at each location and stored in thick polythene bags. The samples are numbered as 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and 15. Each sampling site was separated 100 m approximately. The location of Kolli Hills in Namakkal district is visualized in Fig. 1 (Mullainathan and Nithiyanantham 2013).
Sample preparation and pretreatment
Wet grinding was carried out by placing 30–50 mg of the sample in an agate mortar along with 20–25 drops of ethanol. The ground samples were dried in a hot air oven at 110 °C to remove the moisture content. Using KBr pellet technique, the samples were mixed with KBr at various ratios, viz. 1:10, 1:20, 1:30, 1:40 and 1:50. The mixture was then pressed into a transparent disk in an evacuable die at sufficiently high pressure. The samples in the ratio 1:20 (sample: KBr) were taken for further analysis, since it gave rise to maximum transmittance and observable peaks. This ratio was checked for 2 to 3 times for its accuracy. The abovementioned samples were subjected to various pre-treatments in order to remove organic matter and other materials for improving the quality and for the spectra resolution. The KBr pellet technique was used in the present investigation and the samples are prepared in the usual way. All the chemicals used were of spectroscopic grade.
Experimental methods
As a part of outgoing research, the part of X-ray diffraction analysis is already reported in Mullainathan and Nithiyanantham (2013). In addition to XRD, the infrared spectroscopy gives an appropriate information about the structure of a compound. Unlike ultraviolet spectrum comprising relatively few peaks, this technique provides a spectrum containing a large number of absorption bands from which a wealth of information can be derived about the structure of a compound. The absorption of infrared radiation (quantized) causes a change in the amplitude of the molecular vibrations, leading to the stretching and bending vibrations where a change in the inter-atomic distance or in the angle between two bonds occurs, respectively. The higher-energy near IR, approximately 14,000–4000 cm−1, can excite overtone or harmonic vibrations (Ramasamy et al. 2003a, c).
Fourier transform infrared (FTIR) spectroscopic technique
Infrared spectroscopy has been extensively used in the detailed characterization of molecular structures and used as a diagnostic tool since every species for which the molecular motion causes a change in dipole moment has a spectrum characteristic of its own structure. Although infrared techniques are most commonly developed for qualitative studies, a careful examination of band intensities can lead to provide a most useful tool for quantitative analysis as well (Clarence Karr 1975). One of the most important and value-added applications of infrared spectroscopic studies is the identification of minerals in rock samples.
The total energy of a molecule consists of the translational, vibrational and electronic energies. Transitions among these different types of energy levels occur in different regions of the electromagnetic spectrum. Absorption of infrared radiation is associated with the transition between rotational and vibrational energy states. Infrared spectra act as ‘finger print’ technique and yield information about the atomic groupings present in the sample. Using the infrared spectra, unique information about the group of minerals to which the specimen belongs, the degree of crystalline and non-crystalline impurities and reactions of minerals with chemicals in their environment can also be inferred. For mineral analysis, infrared spectrum in the range of 4000–400 cm−1 is of importance (Wilson 1987; White and Roth 1996; Clarence Karr 1975).
In FTIR spectrophotometers, the detector continuously monitors the full wave number range of radiation emitted by an infrared source, providing an inherently more sensitive system compared to a dispersive instrument. FT instruments are interferometers and require a dedicated computer to transform their output into an absorption spectrum. FTIR spectrophotometers may be either single beam, in which the sample spectrum must be rationed against a background, or double beam, in which rationing against background is carried out continuously. Martin has provided excellent texts on the details of FTIR spectrophotometers and also shown that FTIR is superior to sequential dispersive spectroscopy in applications requiring high resolution over a wide spectral range, in rapid scans applications and where the signal is so weak that an unacceptably long time is needed to measure the spectrum conveniently.
Qualitative and quantitative analysis
Infrared spectroscopic analysis is a potential tool in identifying the minerals present in geological samples. Qualitative analysis is used to determine the major and minor mineral constituents present in the samples. The minerals are identified from the band position or locations of the peaks by comparing literature values of pure and synthetic minerals. In addition to band positions, the sharpness or diffuseness of bands may be helpful in the identification of mineral components.
The quantitative analysis gives clear information on the exact amount of particular species in a given sample. Minerals do not bend themselves readily to quantitative analysis by infrared techniques as they are particulate in nature and light scattering phenomena impose relevant limitations on the relationships between the concentration and the infrared radiation. There are some problems associated with particle size reduction in mineral mixtures, as well as with the lack of standard reference minerals having the same structure, particle size distribution composition and spectral features since the component in the sample limits the success of quantitative determination.
The quantitative determination through infrared analysis may be achieved using Lambert–Beer’s law.
where A denotes absorbance, a denotes absorptivity (L mol−1 cm−1), b denotes path length (cm), c denotes concentration (mol L−1), and I o and I are the intensity of incident and transmitted radiation.
This equation states that a straight-line relationship should be obtained from a plot of absorbance against concentration. From this curve, to measure the absorbance of a particular mineral, its concentration in the sample can be determined. Since gases and liquids are homogeneous, they are suitable for the quantitative analysis of a high degree of accuracy.
The basis of quantitative infrared analysis is the direct or indirect by comparison of the absorbance of the unknown at a given wavelength of a strong absorption band with the absorbance of the same material in a standard of known concentration. Peak absorbance is easily measured and is directly related to concentration. For compounds and minerals whose composition is relatively invariant, e.g., kaolinite and gibbsite, it is easy to find reference for the unknown mineral similar to those in the sample being analyzed.
A Perkin-Elmer FTIR spectrophotometer was used in the present work for recording the FTIR spectra of samples at room temperature. It scans the spectra 16 times in 1 min. The resolution of this instrument is ±4 cm−1 and an accuracy is ±0.01 cm−1. In all the cases, this instrument was calibrated for its accuracy with the spectrum of a standard polystyrene film.
Results and discussion
Determination mineral species
All the collected samples are subjected to FTIR analysis and given in Table 1. The minerals are assigned for the peaks appeared at the particular wave numbers using available literatures (Stubican and Roy 1961a, b; Farmer and Russell 1964; Russell et al. 1970; White 1971; Hlavay et al. 1978; Russell 1987; Madejova 2003; Ramasamy et al. 2003a, b, c, 2004a; Ibrahim et al. 2008a, b). These assigned wave numbers from all the spectra are given in Table 2. The minerals such as quartz, orthoclase feldspar, microcline feldspar, albite, gibbsite, calcite, kaolinite, chlorite and nacrite are identified.Ì
Quartz, orthoclase feldspar, microcline feldspar and albite were found to be the major minerals present in the Kolli Hills rock samples. From now on, the present study will be focused on quartz feldspar, since both were the major minerals of the samples studied. Their crystallinity nature and the relative distribution of minerals phases are described in the following sections.
Crystallinity index of quartz
Crystallinity can be defined as the fraction of crystalline materials in a mixture of crystalline and non-crystalline materials. It is otherwise called degree of disorder. The crystallinity of quartz gives a clear indication of the crystalline form of other minerals, because quartz is the mineral that crystallizes last. If this crystallinity is maximum, then the other minerals are also expected to be equally well crystallized. Crystallinity cannot be found out directly. So it is determined from the crystallinity index, which is indirectly proportional to crystallinity.
The crystallinity index is computed according to Hlavay et al. (1978), and a peak in the range 799–804 cm−1 observed only in few sites shows a shoulder to the predominant peak in the range 776–780 cm−1. For the purpose of calculation of intensity ratios, leaving 779–804 cm−1 unused, the peak at around 780 cm−1 is considered for crystallinity index. The absorption intensity ratios between the bands at around 780 and 690–695 cm−1 that have been calculated for all the samples by constructing the tangent base lines on the basis of Parker’s (1969) and Ibrahim et al.’s (2009) study are reported in Table 3. The values are graphically represented with respect to the site numbers and are shown in Fig. 2. When the crystallinity index is maximum, the minerals are said to be in disordered state, and when it is minimum, the minerals are considered to be in ordered state (Ibrahim et al. 2008a).
According to Ramasamy et al. (2003a, b, c), the observation of a peak at around 695 cm−1 for quartz indicates the orderedness or crystallinity in structure. If the intensity of this peak increases, the crystallinity increases. In the view of Hlavay et al. (1978), the intensity of the absorption band at 695 cm−1 is due to the vibrations in octahedral site symmetry and that at 780 cm−1 is due to the vibrations of tetrahedral site symmetry. The tetrahedral symmetry is stronger than the octahedral symmetry. If any structural changes take place, the damage occurs first in octahedral and then in tetrahedral symmetry. Therefore, the intensity of the bands due to vibrations in these two symmetries will supply direct information about the crystallinity.
From Table 3, it is observed that the 695 cm−1 peak intensity decreases considerably and the ratio between I 777/I 695 cm−1 increases (crystallinity index increases). Parker (1969) showed that the crystallinity index of the samples can be classified into three groups: (a) well ordered, (b) intermediate and (c) disordered. If the values are below 0.75, then it is said to be ordered crystalline quartz. The intermediate crystalline quartz can be explained by values between 0.75 and 1.00. Any significant change in the crystallinity index can be attributed to the geological conditions of formation like transport or deposition or by mechanical treatment, such as grinding (Ramasamy et al. 2003a, b, c). As shown in Table 3, the crystallinity index varies from 0.823 to 0.939. This variation may be due to the minerals that are formed at different geological conditions. Thus, it is observed that the samples of sites having the crystallinity index values above 0.75 showed that the prevalence of low- or high-pressure minerals is due to the leaching or weathering process in each particular site. Since quartz is more harder than the other minerals present in these samples, there is no need of measuring the crystallinity for other minerals, i.e., quartz crystallizes last.
Relative distribution of quartz and orthoclase feldspar
From the above analysis, it is observed that minerals such as quartz, orthoclase feldspar and kaolinite are invariably present in all the experimental sites. Therefore, there is a need to study the distribution of these minerals in various sites of Palaru River and the relative distribution of quartz, orthoclase feldspar and kaolinite for all the 15 site samples can be calculated/inferred from the extinction coefficient of characteristic bands at 778, 640 and 369 cm−1, respectively. These bands are well separated from those of most other mineral bands. The selection of orthoclase feldspar gives an importance for this estimation, since microcline and albite feldspar show weak shoulder peaks and at the same time are not distinct for all the samples. The extinction coefficient (K) is given by the relation
where K—extinction coefficient, D—optical density, A—area of the pellet, m—mass of the sample.
The values are shown in Tables 1 and 4. The plots of site number versus extinction coefficient for quartz and orthoclase feldspar are drawn and are shown in Fig. 3 (Madejova 2003).
Quartz
From Table 1, it is shown that site no. 8 has a maximum extinction coefficient of 850.7 and site no. 12 has a minimum extinction coefficient of 227.6 (Fig. 4).
Orthoclase feldspar
The site no. 7 has a maximum extinction coefficient of 519.5. The site no. 15 has a minimum extinction coefficient of 83.3. The maximum value of extinction coefficient is taken as reference to have maximum quartz, orthoclase feldspar and kaolinite. With the maximum and minimum limits of these latter minerals, the other sites may be arranged for the containment of the same minerals quantitatively in an order.
Conclusions
Thus, from the above mineral determination, crystallinity index, relative distributions and the extinction coefficients analysis of the minerals such as quartz, orthoclase feldspar, microcline feldspar, albite, gibbsite, calcite, kaolinite, chlorite and nacrite are identified in the rock samples of Kolli Hills, Namakkal district, Tamil Nadu, India. The minerals quartz and feldspar are identified as major minerals. Hence, further analyses are made on these minerals by measuring the crystalline natures and relative distribution, and the well-separated bands are identified. The crystallinity index of quartz indicates that prevalence of low- or high-pressure minerals is due to the leaching or weathering in all the sites. With reference to the extinction coefficient values, the quartz is relatively higher than the other minerals present in the samples.
References
Beaufort D, Cassagnabere A, Petit S, Lanson B, Berger G, Lacharpagne JC, Johansen H (1998) Kaolinite-to-dickite reaction in sandstone reservoirs. Clay Miner 33:297–316
Brindley G, Chunkao W, Harriso JL, Li-sicas M, Raythatha R (1986) Relation between structural disorder and other characteristics of kaolinites and dickites. Clays Clay Miner 34:239–249
Chang CW, You CF, Huang CY, Lee TQ (2005) Rapid determination of chemical and physical properties in marine sediments using a near-infrared reflectance spectroscopic technique. Appl Geochem 20:1637–1647
Chester R, Elderfield H (1968) The infrared determination of opal in a series of siliceous deep sea sediments. Geochim Cosmochim Acta 32:1128–1140
Chester R, Green RN (1968) The Infrared determination of quartz in sediments and sedimentary rocks. Chem Geol 3:199–212
Clarence Karr (1975) Infrared and Raman Spectroscopy of Lunar and Terrestrial Minerals, Elsevier. ISBN - 978-0-12- 399950-4
Couty R, Velde B (1986) Pressure induced band splitting in infrared spectra of sanidine and albite. Amer Mineral 71:99–104
Fagel N, Alleman LY, Granina L, Hatert F, Thamo-Bozso E, Cloots R, Andre L (2005) Vivianite formation and distribution in Lake Baikal sediments. Global Planet Change 46:315–336
Farmer VC, Russell JD (1964) The infrared spectra of layer silicates. Spectro Chimica Acta 20:1149–1173
Hlavay J, Jonas K, Elek S, Inczedy J (1978) Characterisation of the particle size and the crystallinity of certain minerals by IR spectrometry and other instrumental methods: II. Investigations on quartz and feldspar. Clays Clay Miner 26(2):139
Ibrahim M, El-Aal MA (2008) Spectroscopic study of the interaction of Heavy metals with organic acids. Int J Env Pollut 35(1):99–110
Ibrahim M, Hameed AJ, Jalbout J (2008a) Molecular spectroscopic study of river Nile sediment in the greater cairo region. Appl Spectrosc 62(3):306–311
Ibrahim HS, Ibrahim MA, Samhan FA (2008b) Distribution and bacterial bioavailability of selected metals in sediments of Ismailia Canal Egypt. J Hazard Mater 168(2–3):1012–1016
Ibrahim M, Kühn O, Scheytt T (2009) Molecular spectroscopic study of water hyacinth dry matter. Open Chem Phys J 2:1–6
Keller WD, Pickett EE (1950) The absorption of infrared radiation by clay minerals. Am J Sci 248:264–273
Kim JG, Dizon JB, Chusuei CC, Deng Y (2002) Oxidation of chromium (111) to (VI) by manganese oxides. Soil Sci Soc Am J 66:306–315
Kumar PR, Reddy GK (2004) Radiaelements and heat production of an exposed Archaean crustal cross-section, Dharwar craton, South India. Earth Planet Sci Lett 224(3):309–324
Libowitzky E, Rossman GR (1997) An IR absorption calibration for water in minerals. Am Miner 82:1111–1115
Madejova J (2003) FTIR techniques in clay mineral studies. Vib Spectrosc 31:1
Mukherjee BK (1984) Infrared investigations of laterite profiles in shevaroys and Kolli Hills, Salem District. Tamilnadu. J Geol Soc India 25(9B):611–613
Mullainathan S, Nithiyanantham S (2013) Elemental Analysis of Some Rock Samples from Namakkal, Tamil Nadu, India. J Adv Phys 4:1–6
Parker TW (1969) A Classification of Kaolinites by Infrared Spectroscopy. Clay Miner 8:135
Perraki TH, Orfanoudaki A (2004) Minealogical study of Zeolites from Pentalofos. Area, Thrace,Greece. Appl Clay Sci 25:9–16
Ramasamy V, Dheenathayalu M, Ponnusamy V, Murugesan S, Mullainathan S (2003a) Charactersation of quartz and feldspars in white granites. J Curr Sci 3(1):181–190
Ramasamy V, Anandalakshmi K, Ponnusamy V (2003b) Rapid determination of quartz and structural characterization of feldspars in rocks using FTIR. Indian J Phys 77A(4):347–352
Ramasamy V, Dheenathayalu M, Ponnusamy V, Hemalatha J, Presannalakshmi P (2003c) FTIR-characterisation and thermal analysis of natural calcite and aragonite. Indian J Phys 77B(4):443–450
Ramasamy V, Ponnusamy V, Dheenathayalu M, Palani G (2004a) Quantitative determination of haematite and chalcopyrite in metamorphic rocks using infrared spectroscopy. Indian J Phys 78(7):563–568
Ramasamy V, Mullainathan S, Murugesan S (2004b) Fourier Transform Infrared analysis of some sediments from Palaru river, Tamil Nadu,India. J Curr Sci 5(2):599–606
Russell JD (1987) Infrared methods. In: Wilson MJ (ed) A hand book of determinative methods in clay mineralogy. Blackie, Glasgow, p 133–137
Russell JD, Farmer VC, Velde B (1970) Replacement of OH by OD in layer silicates and identification of the vibrations of these group in infrared spectra. Miner Mag 37:869–879
Schmidt M, Botz R, Rickert D, Bohrmann G, Hall SR, Mann S (2001) Oxygen isotopes of marine diatoms and relations to opal-maturation. Geochim Cosmochim Acta 65:201–211
Stubican V, Roy R (1961a) A new approach to assignment of infra-red absorption bands in layer-silicates. Z Kristallogr Kristallogeom 115:200–214
Stubican V, Roy R (1961b) Infrared spectra of layer-lattice silicates. J Am Ceram Soc 44(12):625
Sucha V, Elsass F, Eberi DD, Kuchta L, Madejova J, Gates WP, Komadel P (1998) Hydrothermal synthesis of ammonium illite. Am Mineral 83:58–67
Tsai C, Li KH, Sarathy J, Shih S, Campbell JC, Hance BK, White JM (1991) Thermal treatment studies of the photoluminescence intensity of porous Silicon. Appl Phys Letrs 59(22):2814–2816
Tuddenham WM, Lyon RJP (1960) Infrared techniques in the identification and measurement of minerals. Anal Chem 32(12):1630–1634
Venkatachalapathy R, Sridharan T, Dhanapandian S, Manoharan C (2002) Determination of firing temperature of ancient potteries by means of infrared and mossbauer studies. Spectrosc Lett 35(6):769–779
White JL, Roth CB (1996) Infrared spectrometry. In: Klute A (ed) Methods of soil analysis Part I–Physical and mineralogical methods, 2nd edn. SSSA Book Ser. No. 5, SSSA and ASA, Madison, WI, pp. 291–330
Wilson MJ (1987) A handbook of determinative methods in clay mineralogy, 1st edn. Chapman and Hall Publications, New York 133
YuMing L, PeiHuo L, ChingJiang C, ChingChung H (1987) Measurement of Terrestrial Gamma radiation in Taiwan, Republic of China. Health Phys 52:805–811
Acknowledgments
The authors gratefully acknowledged Prof & Head, Dr. K. Ganesan, (Retd), TBML College, Porayar, Tamil Nadu, India. Prof. V. Ramasamy, Dept of Physics, Annamalai University, Annamalainagar, Tamil Nadu, India, inspired in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mullainathan, S., Nithiyanantham, S. FTIR spectroscopic studies of rock sediments in Namakkal, Tamil Nadu, South India, for vegetations . Environ Earth Sci 75, 692 (2016). https://doi.org/10.1007/s12665-016-5384-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5384-0