Abstract
The p-tert-butyl calix[4]arene symmetrical sulfide derivatives (3a and 3b) were successfully synthesized using p-tert-butyl calix[4]arene as the raw material and were characterized by FT-IR. The effects of pH value, temperature, and contact time on the U(VI) extraction with compounds 3a and 3b from aqueous solution were systematically investigated. The results showed that the optimum pH value for uranium extraction was 4.0 and the extraction equilibration time was 90 min. It is also found that the pseudo-second-order kinetic model (R 2 > 0.999) was better fitted to the extraction process. The thermodynamic parameters of the enthalpy (ΔH θ) were all negative, indicating that the extraction reaction were exothermic reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, nuclear energy as a clean and efficient energy has been accelerated development and utilization, and widely used in military, aerospace, the chemical energy and other fields [1]. The demand for uranium as an important raw material for nuclear industry is increasing, and the pollution of water caused by uranium is more and more [2]. Uranium is one of the most important heavy metals with chemical toxicity and radioactivity. And uranium is through inhalation and ingestion or penetrating wound, soluble uranium compounds can also enter the body through the skin without injury, resulting in the internal irradiation and chemical toxicity [3,4,5], will produce damage to human nervous system, immune system, kidney, and fertility, and may lead to gene mutation and cancer [6,7,8]. Therefore, development of efficient and economically viable methods for removal of uranium from waste water is a very urgent and formidable task.
At present, the methods for separating heavy metals include solid-phase separation [9, 10], cloud-point separation [11], liquid–liquid extraction [12], ion exchange [13] and liquid-phase microextraction [14], etc. Among them, Solvent extraction has been widely used in the removal because of its advantages of high efficiency, easy operation, and high selectivity. And it has also some advantages in the separation and purification of heavy metals and waste water treatment [15]. Thus, the further research on suitable and ideal extractant and its industrial application has become a hot topic of research [16, 17].
The calix[n] arene is made up of the phenol unit by the ortho connection of methylene in the phenol hydroxyl group [18], and its upper and lower rims are easy to be chemical modified [19, 20].
The cesium and strontium in the material are separated by the modified calixarene [21]. Because them have higher complexing ability and is easier to be used as extractant [22, 23]. The modification of the lower rim of calixarene is mainly based on the intermolecular hydrogen bond between phenolic hydroxyl groups of calixarene [24], by controlling the reaction conditions, the phenolic hydroxyl groups will be converted into ethers, esters, amides and other groups, and the calixarene derivatives, which have certain selectivity to heavy metal ions are obtained [25,26,27].However, there are few reports about the calixarene modified by heteroatom containing groups with such strong ligands as heavy metal groups, such as alkoxy groups.
In this work, the p-tert-butyl calix[4]arene symmetrical sulfide derivatives (3a or 3b) were successfully synthesized for the extraction of U(VI) from aqueous solution by chemical modifying of calix[4]arene as raw material. The different factors affecting the extraction behavior such as the effects of pH value, the temperature and the contact time were investigated. The extraction kinetics, isotherms and thermodynamic properties of the extraction process for U(VI) were evaluated. The parameters are important for scaling up of the process before investigating the extraction properties in continuous systems.
Experimental
Reagents
The aqueous solution containing U(VI) ions was prepared by dissolving an appropriate amount of UO2(NO3)2·6H2O, which was provided by the Key Discipline Laboratory for National Defence for Biotechnology in Uranium Mining and Hydrometallurgy, University of South China. All the solvents, 4-tert-butylphenol, formalin, sodium hydroxide, ethyl acetate, acetic acid, diphenyl ether, 1,3-dibromopropane, potassium carbonate, acetonitrile, dichloromethane, tetrabutylammonium iodide, sodium sulfide nonahydrate, ethanol, magnesium sulphate, methanol, arsenazo III, were obtained from Tianjin Damao Chemical Reagent Co. Ltd., China, which are of analytical grade.
Apparatus
UV–Vis 8500 (Shanghai sky Scientific Instrument Co., Ltd.) was used to determine the concentration of UO22 + in the aqueous solution containing uranium ions. PHS-3C Model pH meter (Shanghai Peng Shun Scientific Instrument Co., Ltd.) was used for measuring the pH of the aqueous solutions containing uranium ions. The extraction experiments were conducted in a THZ-82 thermostated shaker bath (Jintan Analytical Instrument Co., Ltd.). Fourie transform infrared (FT-IR) spectroscopy was recorded on a IR Prestige-21 (Shimadzu,Japan) spectrometer using KBr pellets.
Synthesis experiment
The experimental procedure for preparation of 3a or 3b was described as follows (as shown in Scheme 1).
Firstly, 1.25 g of p-tert-butyl calix[4]arene, 4 mL 1,2- dibromethane, 0.7231 g of K2CO3 (dry and weight) and 20 mL absolute acetonitrile were added into the 50 mL flask and stirred for 48 h at 70 °C. After the reaction, rotary evaporation to eliminate solvent and unreacted 1,2-dibromethane, and the hydrochloric acid and CHCl3 were added into residue. Then washing the organic layer to neutral, adding MgSO4 to dry, filtering, and white crystalline powder (2a or 2b) was obtained by adding methanol.
Afterward, 0.1033 g of 2a or 2b, 0.1373 g of four butyl ammonium iodide and 0.0855 g sodium sulfide (dry weight) were dissolved in 5 mL water and 25 mL absolute ethanol, and then stirred for 12 h at 55 °C.
Finally, the organic layer was obtained by rotating evaporation and adding saturated sodium chloride and ethyl acetate. Then, adding MgSO4 to dry, filtering, and the product(3a or 3b) was extracted by adding methanol.
Characterization of the samples
The p-tert-butyl calix[4]arene symmetrical sulfide derivatives (3a or 3b) were analyzed by FT-IR (IR Prestige-21, Shimadzu, Japan) using KBr pellets in the 4000–500 cm−1 region.
Extraction experiment
The chloroform solution of 3a or 3b was used as extractant of U(VI), and the standard solution of 2 × 10−4 mol L−1 was used. The solution of 5 mg L−1 U(VI) was distilled with two distilled water. According to the experimental requirements, the chloroform solution of 3a or 3b of the 10 mL ligand was removed in the 50 mL conical flask and U(VI) solutions with different concentrations (0.05, 0.01, 0.1 mol L−1) of HCl and NaOH have been adjusted for pH of the solution, then it was placed at constant temperature (298 K) in the water bath shaker and subjected to constant temperature oscillation of 90 min. After the shaking was stopped, the appropriate pipette tip was selected to remove a certain volume of aqueous phase. The absorbance was measured by UV–Vis spectrophotometer, and the extraction rate (E%) was calculated as.
where C 0 and C e are the initial and the final concentration of U(VI) in solution phase, respectively.
Effect of pH
At the temperature of 25 °C, the initial concentration of the chloroform solution of 3a or 3b was 2 × 10−4 mol L−1, and the initial concentration of U(VI) was 2.1 × 10−5 mol L−1, and the contact time was 90 min, the effect of the extraction of U(VI) on the chloroform solution of 3a or 3b was discussed when the initial pH value of the aqueous solution containing U(VI) ions was 2–8.
Effect of temperature
The complete extraction isotherms of U(VI) was obtained by placing 10 mL the chloroform solution of 3a or 3b chloroform solution into a series of conical flasks containing 50 mL of the aqueous solution containing U(VI) ions with the initial concentration of 2.1 × 10−5 mol L−1 and pH 4.0 [28]. The flasks were agitated for 90 min, while keeping the temperature at 25, 30, 35, 40 or 45 °C. After the equilibration, the residual concentration of U(VI) was determined.
Effect of contact time
The chloroform solution of 3a or 3b was added to the aqueous solution containing U(VI) ions with the initial concentration of 2.1 × 10−5 mol L−1, adjusted pH 4.0 under the experimental conditions. At the constant temperature of 25 °C, controling the shaker oscillation time were 5, 10, 20, 30, 60, 90, 120, 180 min, and the residual concentration of U(VI) ions was measured.
Results and discussion
Characterization
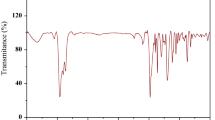
The results are shown in Fig. 1. From the spectra in Fig. 1(3a), it can be seen that antisymmetric stretching vibration peaks at 1234.4 cm−1 for the –C–O–C and skeleton vibration peaks at 1 581.6 and 1 479.4 cm−1 for the aromatic ring. It also can be found that antisymmetric stretching vibration absorption peaks (wide and strong) at 2 951.1 and 2 862.4 cm−1 for the –CH3, the antisymmetric bending vibration peaks at 1 361.7 cm−1 for the C–H in the tertiary butyl group, and the symmetric stretching vibration absorption peaks at 1 020.3 cm−1 for the C–S. In Fig. 1(3a), the characteristic peaks for the –(CH2)3– appeared at 728.9 cm−1 and the peaks at 742.6 cm−1 for the –(CH2)2– in Fig. 1(3b), which implies two target compounds in Scheme l.
Effect of pH
Figure 2 shows the relationship between pH and extraction rate. In this extraction experiment, it is clear that pH has a great effect on the extraction of U(VI) by the chloroform solution of 3a or 3b. As pH increases (2.0 < pH < 4.0), the extraction percentage of U(VI) increased greatly from 2.0 to 4.0, which could be due to at low pH, the protonation affect the binding ability of –OH or –S– functional groups. When pH from 5.0 to 8.0, the extraction rate was reversed, which was due to at high pH, the hydrolysis may be the reason of decreasing the extraction properties [29]. It was unfavorable for the extraction of metal ions by 3a or 3b. It was clear that the highest uptake value was observed at pH 4.0 for both 3a or 3b. Therefore, pH 4.0 was chosen for extraction of U(VI) in the experiment.
Extraction thermodynamics
From the spectra in Fig. 3, it can be seen that with the temperature increased, the extraction rate of U(VI) were decreased by the chloroform solution of 3a or 3b. As can be seen from Fig. 4a, the extraction rate of U(VI) decreased with the increase of temperature, indicating that the extraction reaction is an exothermic reaction, and the increase of temperature is unfavorable to the extraction [16].
After drawing a linear fit lg D versus 1000/T (Fig. 4b, c), we obtained a straight slope for calculating the enthalpy change (ΔH θ). The results shown that ΔH θ for the extraction of U(VI) with the chloroform solution of 3a or 3b are − 56.75 and − 63.57 kJ/mol−1, respectively, which means the extraction process is exothermic.
Extraction kinetics
The extraction kinetics were studied for describing the rate of extraction process, and the removal of U(VI) ions changing with contact time is shown in Fig. 5. The results show that as the shaking time increased, the extraction rate of extractant U(VI) increased with the increase of time and the concentrations remained relatively unchanged after 90 min,which indicated that the process of extraction had reached equilibrium. Therefore, the best extraction time of the chloroform solution of 3a or 3b in this experiment was 90 min. This may be due to the hydroxyl functional groups and the sulfur groups in the extractant were in contact with U(VI), and the binding sites were many and the rate was fast.
With the contact time prolonged, the binding site on the extractant functional groups was close to saturation, and the change of extraction amount was not obvious. In order to further study the relationship between the extraction amount and time of extractant U(VI), the experimental data were fitted by pseudo-first-order kinetic model and pseudo-second-order kinetic model. The fitting parameters of the model were shown in Table 1.
As can be seen from Table 1, the pesudo-second-order kinetic equation was more relevant than the pesudo-first-order kinetic equation, so the extraction process of U(VI) is in line with the pesudo-second-order kinetic equation. The formulas for the pesudo-first-order kinetic equation and the pesudo-second-order kinetic equation were (2) and (3),
where q e represents the equilibrium extraction capacity (mg L−1), q t represents the extraction capacity at t moments (mg L−1), k 1 represents the pesudo-first-order extraction rate constant (min−1), k 2 represents the pesudo-second-order extraction rate constant [g(mg min)−1], t (min).
Conclusion
-
(1)
By means of the infrared spectroscopy analysis of the p-tert-butyl calix [4] arene symmetrical sulfide derivatives (3a or 3b), its were found that 3a or 3b provided a large surface area and more extraction sites for the extraction of U(VI).
-
(2)
The chloroform solution of 3a or 3b all have a better extraction effect on U(VI). When the optimum pH value is 4.0, the temperature is 25 °C, and the initial concentration of U(VI) is 2.1 × 10− 5 mol L−1. The extraction process has the characteristics of stage extraction, which is characterized by rapid surface extraction. At 60 min, the extraction rates of U(VI) on 3a or 3b was respectively 76.39 and 76.29%, and reached equilibrium at 90 min.
-
(3)
The extraction of U(VI) from the aqueous solution containing U(VI) ions on the chloroform solution of 3a or 3b is consistented with the pesudo-second order kinetic model s. The process of extraction U(VI) is consistented with the pesudo-second order kinetic model (R 2 > 0.999), and the process of extraction is chemical extraction. The enthalpy of extraction reactiona is less than zero, indicating that it is an exothermic reaction.
References
Guang Y, Wen JH (2010) The status quo of China’s nuclear power and the uranium gap solution. Energy Policy 38(2):966–975
Xiao FZ, Peng GW, Ding DX, Dai YM (2015) Preparation of a novel biosorbent ISCB and its adsorption and desorption properties of uranium ions in aqueous solution. J Radioanal Unclear Chem 306(2):349–356
Peng GW, Ding DX, Xiao FZ, Wang XL, HuN Wang YD, Dai YM, Cao Z (2014) Adsorption of uranium ions from aqueous solution by aminegroup functionalized magnetic Fe3O4 nanoparticle. J Radioanal Unclear Chem 301:781–788
Peng GW, Ding DX (2011) Biosorption behavior of U(VI) in wastewater containing U(VI) by immobilized saccharomyces cerevisiae. Adv Mater Res 335–336:1489–1492
Aurélie S, Céline BC, Guillaume P, François R, Elias F (2010) Calixarene-entrapped nanoemulsion for uranium extraction from contaminated solutions. J Pharm Sci 99(3):1375–1383
Lopez R, DiazSylvester PL, Ubios AM, Cabrini RL (2000) Percutaneous toxicity of uranyl nitrate: its effect in terms of exposure area and time. Health Phys 78(4):434–437
Fukuda S (2005) Chelating agents used for plutonium and uranium removal in radiation emergency medicine. Curr Med Chem 12(23):2765–2770
Taylor DM, Taylor SK (1997) Environmental uranium and human health. Rev Environ Health 12(3):147–157
Zhu L, Xiao CL, Dai X, Li J, Sheng DP (2017) Exceptional perrhenate/pertechnetate uptake and subsequent immobilization by a low-dimensional cationic coordination polymer: overcoming the hofmeister bias selectivity. Environ Sci Technol 4(7):316–322
Mladenova E, Dakova I, Karadjova I, Karadjov M (2012) Column solid phase extraction and determination of ultra-trace Au, Pd and Pt in environmental and geological samples. Microchem J 101:59–64
Dalali N, Javadi N, Agrawal YK (2008) On-line incorporation of cloud point extraction in flame atomic absorption spectrometric determination of silver. Turk J Chem 32(5):561–570
Liang P, Zhang L, Zhao E (2010) Displacement-dispersive liquid–liquid microextraction coupled with graphite furnace atomic absorption spectrometry for the selective determination of trace silver in environmental and geological samples. Talanta 82(3):993–996
Al-Merey R, Hariri Z, Abu HJ (2003) Selective separation of gold from iron ore samples using ion exchange resin. Microchem J 75(3):169–177
Kagaya S, Takata D, Yoshimori T, Kanbara T, Tohda K (2010) A sensitive and selective method for determination of gold(III) based on electrothermal atomic absorption spectrometry in combination with dispersive liquid-liquid microextraction using dicyclohexylamine. Talanta 80(3):1364–1370
Rao A, Rathod NV, Malkhede DD, Raut VV, Ramakumar KL (2013) Supercritical carbon dioxide extraction of uranium from acidic medium employing calixarenes. Sep Sci Technol 48(4):644–651
Hu PZ, Qian LJ, Zhou X, Pan DF, Wu WS (2013) Solvent extraction of uranyl by N, N, N′,N′-tetraoctylsuccinylamide from nitric acid solution. J Radioanal Nucl Chem 295(2):1209–1213
Hu PZ, Qian LJ, He YX, Wang HL, Wu WS (2013) Solvent extraction of uranium(VI) and thorium(IV) by N, N′-di-p-tolylpyridine-2,6-dicarboxamide from nitric acid solution. J Radioanal Nucl Chem 297(1):133–137
Gutsche CD, Iqbal M, Nam KS, See K, Alam I (1988) Conformational and complexational characteristics of calixarenes. Pure Appl Chem 60(4):483–488
Sutariya PG, Pandya A, Modi NR, Menon SK (2013) A highly efficient PET switch on-off-on fluorescence receptor based on calix[4]arene for the selective recognition of Cd2+ and Sr2+. Analyst 138(8):2244–2248
Cinthia CQ, Horacio GM, Carolina J (2014) Study by fluorescence of calix[4]arenes bearing heterocycles with divalent metals: highly selective detection of Pb2+. J Incl Phenom Macrocycl Chem 79(1–2):161–169
Xiao CL, Zhang AY (2016) Synthesis and characterization of a cesium-selective macroporous silica-based supramolecular recognition material with high stability. J Radioanal Nucl Chem 307(1):713–723
Kang DE, Lee EK, Bartsch RA (2016) Cone di-ionisable calix[4]arene-1,3-crown-5 ligands with elongated pendant side arms: synthesis and metal ion extraction. Supramol Chem 28(5–6):551–556
Nilesh VR, Ankita R, Pradeep K, Karanam LR, Dipalee D (2015) Studies on complexation and supercritical fluid extraction of Cd2+ with calixarenes. Ind Eng Chem Res 54(15):3933–3941
Gutsche CD (1983) Calixarenes. Acc Chem Res 16:161–170
Maria K, Françoise AN, Véronique HB, Igor S, Vitaly K (2010) Novel phosphorylated calixarenes for the recognition of f-elements. J Incl Phenom Macrocycl Chem 66(1–2):113–123
Aurélie S, Céline BC, Marc A, Guillaume P, Franois R (2010) Quick and efficient extraction of uranium from a contaminated solution by a calixarene nanoemulsion. Int J Pharm 398(1–2):179–184
Zhang P, Dong LF, Zhang DX (2017) Efficient extraction of Nd(III) by calix[4] arene derivatives containing diethyl phosphite. Hydrometallurgy 169:47–58
Zhou LM, Shang C, Liu ZR, Huang GL, Adesina AA (2012) Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J Colloid Interface Sci 366:165–172
Yang JB, Volesky B (1999) Biosorption of uranium on sargassum biomass. Water Res 33(12):3357–3363
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51574152, 11705085), the China postdoctoral Science Foundation (No. 2016M602418), the Natural Science Foundation of Hunan Province (Nos. 2017JJ2232, 2017JJ3262, 2017JJ4009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pu, Yq., Xiao, F., He, S. et al. Synthesis of the p-tert-butyl calix[4] arene symmetrical sulfide derivatives and its extraction properties towards U(VI) from aqueous solution. J Radioanal Nucl Chem 314, 2137–2143 (2017). https://doi.org/10.1007/s10967-017-5608-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5608-0