Abstract
The dependence of sorption distribution coefficient (K d) of Np(IV) for illite, shale and MX-80 was investigated as a function of pHc and ionic strength (I) under high ionic strength, reducing conditions. The overall trends of K d on three solids were independent of pHc at 5 ≤ pHc ≤ 10 and I at 0.5 M ≤ I ≤ 6 M. The surface complexation constants of Np(IV) sorption on illite and MX-80 were estimated by the 2 SPNE SC/CE model. The sorption model well predicted the pHc dependence of K d, but could not completely describe the ionic strength dependence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sedimentary rocks in Canada are being considered as potential host rocks for a deep geologic repository (DGR) for radioactive waste. Some of these rocks in Canada (for example, Southern Ontario, Michigan Basin [1]) contain Na–Ca–Cl brine solutions with total dissolved solids (TDS) concentration up to 350 g/L. Sorption of radionuclides, including actinides and their daughter products onto host rocks surrounding a DGR and onto materials comprising the engineered barrier system is considered an important mechanism for retarding their subsurface transport from the repository to the biosphere [2,3,4,5,6]. Therefore, it is important to elucidate the sorption behaviour of radionuclides not only on host rocks, but also on engineered barrier materials such as MX-80 montmorillonite [2]. The Nuclear Waste Management Organization of Canada (NWMO) has been maintaining a sorption database of distribution coefficients, K d, for Canadian sedimentary rocks (shale and limestone) and bentonite [2].
The aqueous chemistry of actinides including their complexation, redox and sorption has been extensively reviewed [3, 7,8,9,10,11,12]. Several studies have also been conducted to investigate the influence of salinity on the sorption of radionuclides, mainly in the ionic strength range from fresh to saline waters [3, 13,14,15,16,17,18]. For Np(IV) sorption on clay minerals, a few investigations have been carried out [19,20,21,22,23,24,25]. However, the research reported by Tanaka et al. [19], Baston et al. [20], and Nagasaki et al. [21] was conducted under the diluted solution conditions. Marsac et al. [22] investigated the sorption of Np(V) onto a purified illite in 0.1 M NaCl solution under an Ar atmosphere and found a possibility of surface mediated reduction of Np(V) to Np(IV). Kitamura and Tomura [23] and Ashida et al. [24] studied the sorption of Np(IV) on smectite at ionic strength (I) of 1.0 M. Marsac et al. [26] investigated the sorption of Pu on illite under anaerobic conditions at the molal concentration of NaCl, m NaCl, of 1.0 and 3.2 molal. Marsac et al. [27] also examined Pu sorption on kaolinite by surface complexation model using the literature sorption data of Am(III), Th(IV), Np(V) and U(VI) as analogues. The results of Pu sorption under reducing conditions by Marsac et al. [26, 27] are valuable to estimate the sorption of Np(IV) on illite and other clay minerals under reducing conditions. Recently, Bertetti [25] has investigated the sorption of Se, As, Tc, U, Pu and Np on Canadian sedimentary rocks (shale and limestone) and MX-80 in diluted (I = 0.01 M) and brine solutions (I = 6.0 M) under low O2, reducing conditions. It was discussed that with the exception of Np, it was likely that the experiments adequately evaluated the sorption of target valence state for the radioelements tested (Se(–II), As(III), Tc(IV), U(IV) and Pu(III/IV)) [25]. Further study is required to investigate the sorption behavior of Np(IV) in high ionic strength solutions under strictly controlled reducing conditions.
Sorption modelling improves the understanding of sorption processes. The 2-site protolysis non-electrostatic surface complexation and cation exchange model (2 SPNE SC/CE model) [28, 29] has been successfully applied to simulate the sorption of tetravalent actinides such as Th(IV), Np(IV) and Pu(IV) on illite and montmorillonite [22, 26, 28, 29]. The simulated K d values contributed to explaining and extrapolating the K d values measured experimentally. To our knowledge, however, only Marsac et al. [22] estimated the surface complexation constants for Np(IV) sorption on illite (Illite du Puy) and demonstrated the reliability of the 2 SPNE SC/CE model in prediction of K d values in 0.1 M NaCl solution. It is important to confirm whether the surface complexation constants estimated by Marsac et al. [22] are also appropriate for Np(IV) sorption on illite in the high ionic strength solutions. The surface complexation constants for Np(IV) sorption on montmorillonite were estimated by the linear free energy relationship (LFER) [28]. However, the reliability of such estimates has not yet been demonstrated by directly applying the 2 SPNE SC/CE model to the real sorption data of Np(IV) on montmorillonite.
The principal objectives of this study are (i) to investigate the sorption behaviour of Np(IV), which is dominant under expected reducing repository conditions, on illite, shale, and MX-80 in highly saline solutions, and (ii) to apply the 2 SPNE SC/CE model and the surface complexation constants estimated for Np(IV) sorption on illite and montmorillonite [22, 28] to the pHc and I dependence of the Np(IV) K d values on illite and MX-80 measured in this work and to discuss the appropriateness of 2 SPNE SC/CE model in the wide range of pHc and I. This was the first research which systematically studied the sorption behaviour of Np(IV) under the wide range of pHc and ionic strength conditions by the sorption experiments and the 2 SPNE SC/CE model.
This paper follows our previous work on Np(V) sorption on illite (Silver Hill, USA), Queenston shale and MX-80 under oxidizing conditions [13] and presents the experimental results of the sorption of Np(IV) on illite, shale and MX-80 in brine solutions under reducing conditions. This work includes: (1) measurement of the K d value of Np(IV) for illite, shale, and MX-80 in the SR-270-PW reference brine solution (Na–Ca–Cl type) with I = 6 M; (2) investigation of the pHc dependence of Np(IV) sorption at 3 ≤ pHc ≤ 10 (in NaCl–CaCl2 solution with I = 0.1 M and 4 M, and NaCl–CaCl2–NaClO4 solution with I = 6 M); (3) investigation of the ionic strength effects on Np(IV) sorption at 0.1 M ≤ I ≤ 6 M (NaCl–CaCl2 solution with I ≤ 4 M, and NaCl–CaCl2–NaClO4 solution with I = 6 M); and (4) sorption modelling of the pHc and I dependence of Np(IV) K d values on illite and MX-80 by 2 SPNE SC/CE model and estimation of surface complexation constants for Np(IV) sorption on these solids. The K d values for Np(IV) determined in this work will contribute to the NWMO’s database of K d values for Canadian sedimentary rocks and bentonite [2].
Experimental
Chemicals and solids
All chemicals used were reagent grade and supplied from Fisher Scientific. Deionized water from a Milli-Q Direct 8 was used after being contacted with 10% H2+ 90% N2 gas in the presence of iron (electrolytic powder) for three days. After the iron powder was removed by centrifugation, 1 × 10−2 M Na2S2O4 was added to the deionized water, and that was then stored in a glove box described below. The Eh of the water just after three-day treatment was around −250 to −170 mV (versus standard hydrogen electrode (SHE)). Eh value of the water was found to be stable in the range of −250 to −170 mV (vs. SHE) for 30 days. Just before use, the water was treated with 10% H2 + 90% N2 gas again for several hours without iron powder addition. The solids used were illite (Silver Hill, USA), Queenston shale (from southern Ontario, Michigan Basin) and MX-80. The specific surface area of illite (Silver Hill), Queenston shale and MX-80 are 70, 10.3 and 26.2 m2 g−1, respectively [25, 30]. Other information including their mineralogy are described elsewhere [2, 13, 25]. A precise controlled atmosphere glove box (GB) supplied by Labconco was filled with N2 gas (>99.999%) to exclude CO2, and the N2 gas was left running through the GB. The concentration of O2 in the running N2 gas was confirmed to be less than 2 ppm by oxygen sensor (Inert Technology).
Preparation of Np(IV) solution
The Np-237 solution was purchased from Stuart Hunt & Associates Ltd. Np(IV) solution was prepared using the established procedures [31,32,33,34]. At first, a Np(V) solution was prepared [13], and then it was treated with 10% H2 + 90% N2 gas for 6 h in the presence of platinum black catalyst. Since some Np(V) might be reduced to Np(III), the Np solution was contacted with air for 30 min. The oxidation state of the prepared Np solution was confirmed to be tetravalent by UV–Vis spectroscopy (Agilent 8453 UV–Vis spectrometer) (Fig. S1 in Supplementary Information). In order to make sure of the complete reduction to Np(IV), we applied 0.1 M TTA-xylene extraction to the Np solution [35], and confirmed that the concentration of Np(V) was less than the detection limit of Np-237 (1 × 10−13 M) by ICP-MS (Agilent ICP-MS 8800). We also confirmed that the oxidation of Np(IV) to Np(V) was negligibly small at least for 30 days after the Np(IV) stock solution was prepared. Np(IV) stock solution was prepared for use just before sorption experiments. The concentration of the Np(IV) stock solution was 1.0 × 10−7 M in 0.01 M HClO4 solution.
pHc evaluation and Eh measurement
The pH values indicated on the pH meter (pHmeasure) should be considered as operational values [36]. The relationship between the operational pHmeasure and the molar H+ concentrations (pHc = −log c H+) or the molal H+ concentration (pHm = −log m H+) were discussed in detail by Altmaier et al. [37, 38]. In this study, the relationship between pHmeasure (Fisher Scientific Accumet AB 150/micro accupHast pH combination electrode) and pHc in solutions was determined by acid–base titration (Metrohm Ti-Touch 916) in desired media, and the pHmeasure values were converted to the pHc values.
The redox potentials in the solutions were measured using a Pt combined electrode with Ag/AgCl reference electrode (Fisher Scientific Accumet AB 150/Accumet ORP electrode), and converted into Eh versus SHE. As noted by Marsac et al. [26], there was no ionic strength dependent correction to apply to the experimental Eh values. In this work, we did not consider any ionic strength dependent correction to the Eh values experimentally measured. A commercial redox buffer (+220 mV vs. SHE) was used for calibration. An equilibrium time of 15–20 min was allowed for all Eh measurements. According to the calibration results and references [26, 39], we considered that the uncertainties of Eh measurements in this study were ±50 mV. As shown in Supplementary Information, Np(IV) was considered to be a thermodynamically dominant species under all experimental conditions used in this work.
Preparation of solutions
SR-270-PW reference brine solution
The SR-270-PW reference brine solution (Na–Ca–Cl water type) has an Eh of −200 mV and pH of 6.0 [2]. The chemical composition of SR-270-PW reference brine solution used in this work is described elsewhere [13]. Bertetti [25] successfully established a recipe for the SR-270-PW reference brine solution with low Eh (smaller than −100 mV (vs. SHE)). In this work, we followed the established procedures [25] to prepare the SR-270-PW reference brine solution, and stored it in the GB. The prepared reference brine solution had an ionic strength of 6 M, and was decanted and centrifuged before use in order to ensure that any precipitates were not present.
NaCl–CaCl2 (I ≤ 4 M) and NaCl–CaCl2–NaClO4 (I = 6 M) solutions
NaCl–CaCl2 and NaCl–CaCl2–NaClO4 solutions (same water type as the SR-270-PW reference brine but with simpler chemical compositions) were also used in this work to study the effect of pHc and ionic strength on Np(IV) sorption. Because of solubility limits of NaCl and CaCl2·2H2O compounds, NaCl–CaCl2 solution was used for the experiments at I ≤ 4 M and NaCl–CaCl2–NaClO4 solution was used for the experiments at I = 6 M.
The molar concentration ratio of Na+ to Ca2+ (Na/Ca ratio) in SR-270-PW reference brine solution is 2.7. Therefore, NaCl–CaCl2 solutions with various ionic strengths (I = 0.1, 0.5, 1, 2, 3 and 4 M) were prepared at Na/Ca = 2.7 using NaCl and CaCl2·2H2O compounds. NaCl–CaCl2–NaClO4 solution with I = 6 M and Na/Ca ratio = 2.7 was prepared with NaCl, CaCl2·2H2O and NaClO4·H2O compounds. The recipe of preparing the NaCl–CaCl2–NaClO4 solution is shown in Supplementary Information (Table S1). We did not observe any precipitate in the prepared solutions.
Sorption experiments
All sorption experiments were carried out at 25 °C in triplicate. Since the basic procedures of sorption experiments were the same as those we used in the sorption experiments of Np(V) [13], only the differences in the procedure and conditions from the previous ones are described in this paper. In this paper, the value of K d (m3 kg−1) is used to express the sorption results. The definition of K d and the limitation of K d concept are also stated in the Ref. [13]. The S/L ratio was set to 0.2 g/1 L or 0.1 g/1 L for all the sorption experiments.
In this work, the liquid was separated from the solid by filtration (Vivaspin 6 (3000 MWCO) for 120 min at 10,000 rpm at 25 °C; Beckman Coulter, Allegra X-30R) or centrifugation (Nalgene Oak Ridge tubes for 120 min at 18,000 rpm at 25 °C). Nitsche [40] described the procedure to minimize the effect of sorption of elements on filter. In the present work, we followed Nitsche’s procedure [40] in the filtration. We conducted the preconditioning of the filter by washing it with Np(IV) solution with the same pHc and Eh as those of the sample for solid/liquid separation. It was confirmed that the sorption of Np(IV) on the filter was negligible at I = 1 M to 6 M solutions, but 0–5% of Np(IV) added was found to be sorbed on the filter at I = 0.1 M and 0.5 M solutions. Therefore, the liquid was separated from the solid by centrifugation for I = 0.1 M and 0.5 M and by filtration for I = 1 M to 6 M solution systems. In the preliminary test, by comparing the solid/liquid separation by centrifugation with that by filtration, we confirmed that we could successfully separate the liquid from the solid by centrifugation.
Kinetics of Np(IV) sorption
Sorption kinetics of Np(IV) for illite, shale, and MX-80 in NaCl–CaCl2–NaClO4 solution (I = 6 M) was measured with the solid/liquid (S/L) ratios of 0.2 g/1 L and 0.1 g/1 L.
During the solid/liquid pre-equilibration period of 3–4 days, 10% H2 + 90% N2 gas passed through the solid/liquid mixture for 30 min once a day. The solid/liquid mixture was then removed from the GB, the liquid and solid were separated by centrifugation for 30 min at 12,000 rpm. pHc and Eh of the solution were measured in the GB to confirm the low Eh values (smaller than −100 mV (vs. SHE)). NaCl–CaCl2–NaClO4 solution (I = 6 M) was added to the pre-equilibrated solid in the polycarbonate reaction vessel in the GB and the reaction vessel was fully shaken. The solid/liquid mixture was then quantitatively transferred to different reaction vessels for the kinetics sorption experiments, and a portion of the Np(IV) stock solution was spiked into the reaction vessels in the GB. The initial Np(IV) concentrations in the vessels were 1.0 × 10−11 M.
The reaction vessels were tightly sealed in the GB, transferred from the GB to an incubator at 25 °C (Infors HT Ectron), and were gently shaken for pre-decided period of time. Once a day, all reaction vessels were transferred to the GB and 10% H2 + 90% N2 gas passed through the vessels for 30 min to 1 h to keep the low Eh condition, and then returned to the incubator. After each pre-decided period of time, the pHc and Eh of the solution were measured in the GB. A portion of the mixture was sampled from the reaction vessel, and the liquid was separated from the solid by filtration. The concentration of Np in the liquid was measured using the ICP-MS.
As discussed in the Results section below, the sorption equilibrium of Np(IV) for illite, shale, and MX-80 in NaCl–CaCl2–NaClO4 solution (I = 6 M) was achieved within 3–7 days for both S/L ratios. In the previous study [21], the sorption equilibrium of Np(IV) on bentonite in 0.01 M NaClO4 solution was confirmed to be achieved within 7–10 days. Hence, the sorption reaction time was set to 7–10 days in the subsequent experiments.
K d measurements in SR-270-PW reference brine solution
The K d values of Np(IV) sorption for illite, shale, and MX-80 in the SR-270-PW reference brine solution were measured. The initial Np(IV) concentrations in the samples were 1.0 × 10−11 M. The S/L ratio was 0.1 g/1 L.
Effect of pHc on K d value
The pHc dependence of the K d value of Np(IV) sorption for illite, shale, and MX-80 in NaCl–CaCl2 solution (I = 4 M) and NaCl–CaCl2–NaClO4 solution (I = 6 M) was measured. The pHc of the solution was adjusted to the pre-decided values between 3 ≤ pHc ≤ 10 by addition of 0.01 M HCl, 0.1 M HCl, 0.1 M NaOH or 0.01 M NaOH solution in the GB. For illite and MX-80, the sorption of Np(IV) at I = 0.1 M was also measured at 3 ≤ pHc ≤ 10. The pHc of the solution was measured once a day in the GB and re-adjusted to the original pHc value if the pHc changed by more than ± 0.3 from the original value. The initial Np(IV) concentrations in the mixtures were 1.0 × 10−11 M. The S/L ratio was 0.2 g/1 L.
Effect of the ionic strength on Kd value
The ionic strength dependence of the K d value of Np(IV) sorption for illite, shale, and MX-80 in NaCl–CaCl2 solutions with I = 0.1 M to 4 M and in NaCl–CaCl2–NaClO4 solution with I = 6 M was investigated. During the sorption experiments, the pHc of the solution was measured once a day, and adjusted to 8.0 ± 0.3 (illite), 8.2 ± 0.3 (shale) and 8.8 ± 0.3 (MX-80). The values of 8.0, 8.2, and 8.8 were the equilibrium pHc values of the illite-, shale- and MX 80-equilibrated NaCl–CaCl2–NaClO4 solutions (I = 6 M), respectively, which were measured in the preliminary tests. In this work, the ionic strength was adjusted to I = 0.1, 0.5 1, 2, 3, 4 and 6 M. The experiments at I = 0.1, 2, 3, 4 and 6 M were carried out twice to confirm the reproducibility. The initial Np(IV) concentrations in the samples were 1.0 × 10−11 M. The S/L ratio was 0.1 g/1 L for MX-80 at I = 0.1 M and 0.5 M. For all the other systems 0.2 g/L was used.
Sorption model
In this work, the 2 SPNE SC/CE model [28, 29] was applied to simulate the pHc dependence of K d values of Np(IV) on illite and MX-80 at I = 0.1 M and 4 M. The specific ion interaction theory (SIT [41]) was used to calculate activity coefficients of aqueous species. Because Pitzer paramters for Np(IV) in NaCl–CaCl2–NaClO4 solution are not completely compiled, the 2 SPNE SC/CE model was not applied to the K d values measured at I = 6 M. Thermodynamic constants for Np(IV) aqueous species were taken from the NEA thermodynamic database [42]. When the SIT parameters required for the calculation were not reported in the NEA thermodynamic database, the parameters in the SIT database provided with PHREEQC [43] were used.
For illite du Puy, Bradbury and Baeyens [29, 44] reported the specific surface area of 97 m2 g−1, the sorption site capacity of 2.0 × 10−3 mol kg−1 (strong site: ≡SsOH) and 4.0 × 10−2 mol kg−1 (weak site: ≡Sw1OH), and the CEC of 225 ± 15 meq kg−1. The illite (Silver Hill) used in this work has the specific surface area of 70 m2 g−1 and the CEC of 150 meq kg−1 [30], but the sorption site capacity has not been measured. Hence, assuming that the sorption site capacity is proportional to the specific surface area, we adopted 1.4 × 10−3 mol kg−1 (strong site). We used the equilibrium constants for surface protolysis reactions estimated by Bradbury and Baeyens [29] and the surface complexation constants estimated for Np(IV) by Marsac et al. [22]. As shown in the discussion section later, the simulation using the surface complexation constants by Marsac et al. [22] was found to overestimate the sorption of Np(IV). Hence, in the present work, we also estimated the surface complexation constants which provided the best fit of simulation to the experimental K d values. At the fitting, the surface complexation constants estimated for Pu(IV) [45] were used as a starting point. This was because the Np(IV) K d values between 5 ≤ pHc ≤ 10 at I = 0.1 and 4 M in the present work agreed with the simulation results of 2 SPNE SC/CE model using the surface complexation constants estimated for Pu(IV) [26]. Like Marsac et al. [22], a weight of 1 was applied to all data (I = 0.1 M and 4 M), and the error between experimental and simulated K d values was estimated by the root mean squared deviation.
For MX-80, we used the surface complexation constants which Bradbury and Baeyens [28] estimated for Np(IV) on montmorillonite by the LFER. However, the surface complexation constant for the reaction
was not reported, although the surface complexation constant for similar reaction of Th(IV) was estimated as log s K = −16.9. This is because the hydrolysis constant for Np(OH) −5 is required for the estimation with LFER but it is not compiled in the NEA database [41]. Hence, the surface complexation constant for reaction (1) was estimated by fitting to the K d values measured in this work. In addition, we also estimated the surface complexation constants for other reactions which provided the best fit of simulation to the experimental K d values. For the fitting, the surface complexation constants for Np(IV) estimated by using LFER by Bradbury and Baeyens [28] were used as a starting point. For the starting value for reaction (1), log s K = −16.9 was used although this was estimated for Th(IV). As explained above, a weight of 1 was applied to all data (I = 0.1 M and 4 M), and the error was estimated by the root mean squared deviation.
In this work, the 2 SPNE SC/CE model [28, 29] was not applied for the K d values on shale. This was because (i) there was a possibility that Np(IV) sorbed not only on illite contained in Queenston shale but also on other minerals such as chlorite, calcite and dolomite, and the investigation on the reliability of 2 SPNE SC/CE model for various types of solid surface was beyond the purpose of this work, (ii) the composition of minerals contained in shale significantly depends on its origin, and (iii) the LFER for shale has not yet been developed, and therefore it was not easy to discuss the validity of surface complexation constants of Np(IV) for shale.
Results
Kinetics of Np(IV) sorption
The results of the sorption kinetics of Np(IV) for illite (Silver Hill), Queenston shale, and MX-80 in NaCl–CaCl2–NaClO4 solution (I = 6 M) at S/L = 0.2 g/1 L and 0.1 g/1 L are illustrated in Fig. 1. The values of pHc and Eh at each sorption time are shown in Supplementary Information (Table S2). These results illustrated that sorption equilibrium of Np(IV) for three solids used were achieved within 3–7 days for both S/L ratios. Figure 1 also shows that there was no marked difference in the K d values of Np(IV) for each solid between S/L = 0.2 g/1 L and 0.1 g/1 L, considering the uncertainties of the K d values.
K d values in SR-270-PW reference brine solution
The K d values of Np(IV) sorption for illite (Silver Hill), Queenston shale, and MX-80 in the SR-270-PW reference brine solution are summarized in Table 1. The initial and final pHc and Eh values of solutions are in Supplementary Information (Table S3). Compared with the K d values of Np(V) for these solids [13], those of Np(IV) were found to be three orders of magnitude larger.
The retardation factor R (R = 1 + ρK d/ε, ρ is the rock density, ε rock porosity) provides an indication of how much slower a sorbing contaminant will be transported compared to the average groundwater pore velocity [47]. For example, Np(IV) would be transported 3 × 106 times slower than groundwater in Queenston shale (ρ = 2.608 g cm−3, ε = 0.0663) [48]. It indicates that Np(IV) is very strongly retarded in illite, shale and MX-80, and essentially immobile.
K d values in NaCl–CaCl2 and NaCl–CaCl2–NaClO4 solutions
Effect of pHc on K d value
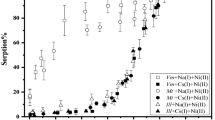
The pHc dependence of K d value of Np(IV) sorption in NaCl–CaCl2 solutions (I = 0.1 M and 4 M) and NaCl–CaCl2–NaClO4 solution (I = 6 M) is illustrated in Fig. 2. The K d values of Np(IV) on illite (Rochester, USA) and montmorillonite (Tsukinuno, Japan) in 0.1 M NaClO4 solution estimated from the migration curves of Np(IV) which had been experimentally observed in the presence of clay [46] are also plotted (around pH 6 and 8) in Fig. 2 for reference. These were found to be consistent with the K d values measured in the present work at I = 0.1 M. The values of pHc and Eh at the sorption equilibrium measured in the present work are shown in Supplementary Information (Table S4).
pHc dependence of the K d values in NaCl–CaCl2 solution (I = 0.1 M and 4 M) and NaCl–CaCl2–NaClO4 solution (I = 6 M): a illite (Silver Hill); b Queenston shale; c MX-80. The initial concentration of Np(IV) was 1.0 × 10−11 M. pHc and Eh values in sorption experiment are summarized in Supplementary Information (Table S4). The K d values on illite (Rochester) and montmorillonite (Tsukinuno) in 0.1 M NaClO4 solution estimated from the previous work [46] are also plotted for reference. Solid lines in (a) and (c) are the simulation results using 2 SPNE SC/CE model. SC constant represents “surface complexation constant”
Figure 2 shows that the K d values of Np(IV) for illite (Silver Hill) and MX-80 were independent of pHc between 5 and 10 at I = 0.1, 4 and 6 M. The K d values on Queenston shale were also independent of pHc between 5 and 10 at I = 4 M. However, the K d values on the shale at pHc = 9 and 10 obtained at I = 6 M were slightly smaller than those at 5 ≤ pHc ≤ 8. We considered that the overall trend of pHc dependence of the K d values at I = 0.1, 4 and 6 M for all three solids did not depend on pHc at 5 ≤ pHc ≤ 10. The K d values of Np(IV) for illite (Silver Hill), Queenston shale, and MX-80 at I = 6 M were 120 ± 6.4 m3 kg−1 (pHc = 8.1), 170 ± 37 m3 kg−1 (pHc = 8.1) and 170 ± 7.1 m3 kg−1 (pHc = 9.1), respectively (Fig. 2). The K d values of Np(IV) for illite (Silver Hill), Queenston shale, and MX-80 at I = 6 M measured in the sorption kinetics experiments were 150 ± 22, 130 ± 12 and 180 ± 31 m3 kg−1 at S/L ratio = 0.2 g/1 L and 130 ± 18, 110 ± 11 and 220 ± 39 m3 kg−1 at S/L ratio = 0.1 g/1 L, respectively (Fig. 1). Considering the uncertainties of the K d values in both media, we considered the variation in K d values was not significant.
For the illite, the presence of edge faces is essential for the sorption of tetravalent actinides [22, 26, 45]. Although the specific surface areas of solids used are different, the amounts of the edge faces are likely similar. Hence, the overall sorption is more or less unaffected. Compared with the sorption on illite for Th(IV) [29] and Pu(IV) [26, 45], it was considered that oxidation of Np(IV) did not take place during the sorption experiments.
Effect of the ionic strength on K d value
The ionic strength dependence of the K d value of Np(IV) sorption in NaCl–CaCl2 solutions (I = 0.1 M–4 M) and NaCl–CaCl2–NaClO4 solution (I = 6 M) is illustrated in Fig. 3. The pHc and Eh values at sorption equilibrium are summarized in Supplementary Information (Table S5).
The pHc of the solution was adjusted to 8.0 ± 0.3 for illite (Silver Hill), 8.2 ± 0.3 for Queenston shale and 8.8 ± 0.3 for MX-80. Considering the pHc dependence of Np(IV) K d values (5 ≤ pHc ≤ 10) and the uncertainties of the K d values, the K d values for illite (Silver Hill), Queenston shale and MX-80 were found to be independent of the ionic strength in the range of 0.5 M–6 M. On the other hand, the K d values on all three solids at I = 0.1 M were slightly larger than those at I ≥ 0.5 M. As illustrated in Fig. 2 (a) and (c), the K d values on illite (Silver Hill) and MX-80 at 5 ≤ pHc ≤ 10 at I = 0.1 M were a bit larger than at I = 4 and 6 M.
Discussion
There are several previous studies on Np(IV) sorption on clay minerals and shale [21,22,23,24]. Nagasaki et al. [21] studied Np(IV) sorption on bentonite in 0.01 M NaClO4 solution, and reported that the K d values of Np(IV) were constant in the pH range of 6.3–8.2. Marsac et al. [22] performed the predictive simulation of Np(IV) sorption onto illite in 0.1 M NaCl solution, indicating the pH independence of K d values in the pH range of 6–11. Kitamura and Tomura [23] investigated the pH dependence of K d values of Np(IV) sorption for smectite in 1.0 M NaCl solution in the pH range of 7.4–8.5. They reported that the K d values decreased with pH. However, in their experiments, carbonates (0.09–1.0 M) were included and Np(V) and Np(IV) coexisted. Ashida et al. [24] studied the effect of carbonate ions on Np(IV) sorption in 1 M NaCl solution on smectite. The K d values for illite (Silver Hill) at I = 0.1 M measured in the present work were approximately 230–270 m3 kg−1. These values were found to be consistent with those predicted by Marsac et al. [22] within a factor of 3–4. Since the concentration of Ca2+ was very low at I = 0.1 M, the effect of Ca2+ on Np(IV) sorption could be considered small. There is no previous work studying the pHc and ionic strength dependences of K d values of Np(IV) sorption in high ionic strength solutions (ionic strength up to 6 M) for illite, shale, and montmorillonite like MX-80. Hence, the present work is considered to be the first systematic study of the effects of pHc and ionic strength on Np(IV) sorption for illite, shale, and montmorillonite in high ionic strength solutions.
Vilks [2] extensively reviewed the K d values of Np(IV) sorption onto shale, bentonite and limestone published in scientific papers and reports, compiled the K d values under saline conditions, and recommended a range of K d values of Np(IV) for saline solutions. The recommended K d values are 0.15–2.3 m3 kg−1 for shale and 0.84 –23 m3 kg−1 for bentonite [2]. However, as pointed out by Vilks [2], the recommended K d values for shale based on the sorption of Np(IV) on mudstones [49] which were measured under the extremely low ionic strength conditions was only a rough estimate. The K d values for bentonite were recommended based on the sorption data by Kitamura and Tomura [23]. As mentioned above, in their work carbonates were included and Np(V) and Np(IV) coexisted. Hence, the recommended K d values by Vilks [2] are not comparable to the K d values observed in this work.
Recently, Marsac et al. [26] investigated Pu sorption on illite under anaerobic conditions at 3 < pHm < 10 and m NaCl = 1.0 and 3.2 molal. They found that the overall Pu uptake at pHm < 6 is mainly attributed to the presence of Pu(III) and its competition with Na+ and that the overall Pu uptake at pHm > 6 is largely insensitive to m NaCl due to the prevalence of strongly adsorbing Pu(IV). The pHc dependence of K d value of Np(IV) on illite (Silver Hill), Queenston shale and MX-80 observed in the present work at pHc > 5 is qualitatively the same as that of Pu observed by Marsac et al. at pHm > 6 [26]. Furthermore, the absence of ionic strength dependence of Np(IV) sorption on illite (Silver Hill), Queenston shale and MX-80 at I ≥ 0.5 M observed in this work also agrees well with that of Pu(IV) sorption observed by Marsac et al. [26].
According to Marsac et al. [26] and Banik et al. [45], the K d values of Pu(IV) on illite du Puy are independent of ionic strenght (m NaCl = 0.1, 1.0 and 3.2 molal) at pHm > 6. The K d values on illite (Silver Hill) and MX-80 at I = 0.1 M were slightly but clearly larger than those at I ≥ 0.5 M in the present work (Figs. 2, 3). The K d values of Th(IV) on montmorillonite (Na-SWy-1) experimentally measured at I = 0.1 M are smaller than those measured at I = 1 M between 3 ≤ pH ≤ 8 [28]. Zhao et al. [50] studied the effect of pH, ionic strength and temperature on Th(IV) sorption for MX-80. They found that the sorption decreased with the concenration of KNO3 (up to 0.3 M) at pH = 1.86 ± 0.02 and concluded that cation exchange partly contributed to the sorption of Th(IV) on MX-80.
The simulation results by 2 SPNE SC/CE model are also presented in Fig. 2a and c. As shown in Fig. 2a and c, the simulation results in the pHc range between 6 and 10 at I = 4 M was identical to that at I = 0.1 M. This trend is the same as the results shown in the previous research [26, 28, 29]. Furthermore, the best fits using the surface complexation constants obtained in this work (lines ① and ② in Fig. 2a and c) were significantly different from the simulation results using the reported surface complexation constants [22, 28] (lines ③ and ④ in Fig. 2a and c).
For illite, it was found that the K d values simulated using the surface complexation constants of Np(IV) [22] (lines ③ and ④ in Fig. 2a) were larger than the measured K d values at pHc ≥ 4. The reason is not clear at the present. However, a possible reason is that the surface complexation constants were estimated by extrapolation and a different illite was used in the research by Marsac et al. [22]. Banik et al. [45] reported that the K d values in 0.1 M NaCl solution by 2 SPNE SC/CE model using the surface complexation constants of Np(IV) [22] were larger at pH ≥ 4 and smaller at pH ≤ 4 than those using the surface complexation constants of Pu(IV) [45]. The surface complexation constants of Pu(IV) were fully discussed in the sorption experiments at m NaCl = 0.1, 1.0 and 3.2 molal [26, 45], while those of Np(IV) were estimated only from the sorption experiments at m NaCl = 0.1 M [22]. Furthermore, Marsac et al. [22] compared the predicted Np(IV)-illite pH-edge with the experimental pH-edge of Th(IV) and Sn(IV). The predicted K d values were larger than the experimental values of Th(IV) and Sn(IV) at pH ≥ 5. Hence, the model using the surface complexation constants of Np(IV) by Marsac et al. [22] might overestimate the sorption of Np(IV) on illite (Silver Hill).
Table 2 summarizes the surface complexation constants for Np(IV) sorption on illite (Silver Hill) estimated in the present work. The estimated surface complexation constants for Np(IV) agreed well with those estimated for Pu(IV) sorption on illite du Puy [45]. The K d values of Np(IV) on illite (Silver Hill) were qualitatively simulated using these estimated surface complexation constants by the 2 SPNE SC/CE model in the wide range of pHc (lines ① and ② in Fig. 2a). Although there were several assumptions in the simulation as mentioned before, the Np(IV) sorption on illite (Silver Hill) was found to be explained by the surface complexation reactions that Bradbury and Baeyens considered for illite du Puy [29] and the surface complexation constants estimated by the fitting to Np(IV) sorption on illite (Silver Hill) measured in this study.
For MX-80, the surface complexation constant of the reaction (1) was estimated as log s K = –7.8 ± 0.3 (I = 0 M). Using this value for reaction (1) and the surface complexation constants of Np(IV) on montmorillonite estimated by using the LFER by Bradbury and Baeyens [28] for other reactions, it was found that the simulation results by 2 SPNE SC/CE model were qualitatively consistent with the experimental K d values at pHc ≥ 6, but underestimated the experimental data at pHc ≤ 5 (lines ③ and ④ in Fig. 2c).
In the present work, we estimated the values of surface complexation constants for Np(IV) on MX-80 which gave the best fit to the pHc dependence of K d values measured in the present work (lines ① and ② in Fig. 2c), as given in Table 3. The values estimated by Bradbury and Baeyens [28] based on the LFER are also shown in Table 3. In this estimation, we neglected the surface complexation reactions on weak sites, because the concentration of Np(IV) is very small and the reactions “≡SsOH + Np4+ ↔ ≡ SsONp3+ + H+” and “≡Sw1OH + Np4+ ↔ ≡ Sw1ONp3+ + H+” did not contribute to the simulation practically.
The surface complexation constants for surface species “≡SsONp(OH) +2 ” and “≡SsONp(OH) 03 ”, log s K = 5.7 ± 0.2 and log s K = 0.10 ± 0.1, respectively, are well consistent with those estimated by LFER. On the other hand, the surface complexation constant for surface species “≡SsONpOH2+” estimated in this work was larger than that by LFER.
The 2 SPNE SC/CE model is a valuable tool to predict the K d values of Np(IV) for illite (Silver Hill) and MX-80 in the wide range of pHc (3 ≤ pHc ≤ 10). However, its capability to describe the K d values over a wide range of ionic strength must be seen with caution. As demonstrated in this study (Fig. 2a, c), we observed ionic strength dependency in the Np(IV) sorption that cannot adequately be reproduced by the 2 SPNE SC/CE model, although the variation was within a factor of 2–3 which may not be so significant considering all the uncertainties in the natural systems. This might be caused by neglecting an electrostatic part of surface complexation reaction in the model. The activity coefficient calculation using SIT for up to I = 4 M solutions in this study should also be questioned.
Conclusion
This is the first research systematically studying the sorption behaviour of Np(IV) in Na–Ca–Cl solutions with high ionic strength. The K d values of Np(IV) sorption for illite (Silver Hill), Queenston shale and MX-80 in the SR-270-PW reference brine (Na–Ca–Cl type water, I = 6 M), NaCl–CaCl2 (I = 0.1 M–4 M) and NaCl–CaCl2–NaClO4 (I = 6 M) solutions were measured.
The sorption equilibrium of Np(IV) for illite (Silver Hill), Queenstone shale and MX-80 in NaCl–CaCl2–NaClO4 solution (I = 6 M) were achieved within 3–7 days. The K d values of Np(IV) for illite (Silver Hill), Queenston shale and MX-80 in the SR-270-PW reference brine solutions (I = 6 M) were 92 ± 15, 98 ± 9.3, and 130 ± 31 m3 kg−1, respectively. The average K d values for illite (Silver Hill), Queenston shale, and MX-80 in NaCl–CaCl2–NaClO4 solution (I = 6 M) measured in sorption kinetics and pHc and ionic strength dependence experiments were in the range of 120–160, 130–180, and 170–220 m3 kg−1, respectively. Considering the uncertainties of the K d values, the variation in the K d values for illite (Silver Hill), Queenston shale and MX-80 between the SR-270-PW reference brine solution and the NaCl–CaCl2–NaClO4 solution was considered to be insignificant.
The overall trend of pHc dependence of Np(IV) on illite (Silver Hill), Queenston shale and MX-80 was that the K d values increased with pHc at pHc ≤ 5 and were independent of pHc at 5 ≤ pHc ≤ 10. The overall trend of ionic strength dependence of Np(IV) on illite (Silver Hill), Queenston shale and MX-80 was that the K d values decreased from I = 0.1 M to 0.5 M, and did not depend on ionic strength at 0.5 M ≤ I ≤ 6 M.
The surface complexation constants for Np(IV) sorption on illite (Silver Hill) and MX-80 were estimated by fitting the 2 SPNE SC/CE model to the measured pHc dependence of the K d values. It was found that the 2 SPNE SC/CE model is a valuable tool to simulate the pHc dependence of the K d values of Np(IV) in high ionic strength solutions (0.5 M ≤ I ≤ 4 M) for illite (Silver Hill) and MX-80 in the wide range of pHc (3 ≤ pHc ≤ 10). However, by neglecting an electrostatic part of surface complexation reaction, the 2 SPNE SC/CE model could not simulate the decrease in the K d values from I = 0.1 M to 0.5 M measured in this work. The contribution of electrostatic interaction will continue to be studied.
References
Hobbs MY, Frape SK, Shouakar-Stash O, Kennel LR (2011) Regional hydrogeochemistry—Southern Ontario. NWMO DGR-TR-2011-12, Toronto, Canada
Vilks P (2011) Sorption of selected radionuclides on sedimentary rocks in saline conditions—literature review. Nuclear Waste Management Organization technical report NWMO TR-2011-12, Toronto, Canada
Geckeis H, Lützenkirchen J, Polly R, Rabung T, Schmidt M (2013) Mineral-water interface reactions of actinides. Chem Rev 113:1016–1062
Zavarin M, Powell BA, Bourbin M, Zhao PH, Kersting AB (2012) Np(V) and Pu(V) ion exchange and surface-mediated reduction mechanisms on montmorillonite. Environ Sci Technol 46:2692–2698
Chapman N, Apted M, Aspinall W, Berryman K, Cloos M, Connor C, Connor L, Jaquet O, Kiyosugi K, Scourse E, Sparks S, Stirling M, Wallace L, Goto J (2012) TOPAZ project long-term tectonic hazard to geological repositories. Nuclear Waste Management Organization of Japan technical report NUMO-TR-12-05, Tokyo, Japan
National Academy of Science (1983) A study of the isolation for geologic disposal of radioactive wastes. Waste Isolation Systems Panels, Board on Radioactive Waste Management, Washington DC, USA
Knope KE, Soderholm L (2013) Solution and solid-state structural chemistry of actinide hydrates and their hydrolysis and condensation product. Chem Rev 113:944–994
Walther C, Denecke A (2013) Actinide colloids and particles of environmental concern. Chem Rev 113:995–1015
Yoshida Z, Johnson SG, Kimura T, Krsul JR (2010) Neptunium. In: Morss LR, Edelstein NM, Fuger J (eds) The chemistry of the actinide and transactinide elements, vol 2, 4th edn. Springer, Dordrecht, pp 699–812
Choppin GR, Jensen MP (2010) Actinides in solution: complexation and kinetics. In: Morss LR, Edelstein NM, Fuger J (eds) The chemistry of the actinide and transactinide elements, vol 4, 4th edn. Springer, Dordrecht, pp 2524–2621
Runde W, Neu MP (2010) Actinides in the geosphere. In: Morss LR, Edelstein NM, Fuger J (eds) The chemistry of the actinide and transactinide elements, vol 4, 4th edn. Springer, Dordrecht, pp 3475–3593
Natrajan LS, Swinburne AN, Andrews MB, Randall S, Heath SL (2014) Redox and environmentally relevant aspects of actinide(IV) coordinate chemistry. Coord Chem Rev 266–267:171–193
Nagasaki S, Saito T, Yang T (2016) Sorption behaviour of Np(V) on illite, shake and MX-80 in high ionic strength solutions. J Radioanal Nucl Chem 308:143–153
Kar AS, Kumar S, Tomar BS (2012) U(VI) sorption by silica: effect of complexing anions. Colloid Surf A 395:240–247
Zhu WB, Liu ZJ, Chen L, Dong YH (2012) Sorption of uranium(VI) on Na-attapulgite as a function of contact time, solid content, pH, ionic strength, temperature and humic acid. J Radioanal Nucl Chem 289:781–788
Tertre E, Pret D, Ferrage E (2011) Influence on the ionic strength and solid/solution ratio on Ca(II)-for-Na+ exchange on montmorillonite. Part 1: chemical measurements, thermodynamic modeling and potential implications for trace elements geochemistry. J Colloid Interface Sci 353:248–256
Amayri S, Jermolajev A, Reich T (2011) Neptunium(V) sorption on kaolinite. Radiochim Acta 99:349–357
Schmeide K, Bernhard G (2010) Sorption of Np(V) and Np(VI) onto kaolinite: effects of pH, ionic strength, carbonate and humic acid. Appl Geochem 25:1238–1247
Tanaka S, Nagasaki S, Suzuki A, Yamaguchi T, Tsushima S, Yamaguchi K, Moriyama Y (1998) The underground environment migration behaviour of TRU elements (3). UTNL-Report 371:64–65, The University of Tokyo, Tokai, Ibaraki, Japan (in Japanese)
Baston GMN, Berry JA, Brownsword M, Heath TG, Ilett DJ, Tweed CJ, Yui M (1997) The effect of temperature on the sorption of technetium, uranium, neptunium and curium on bentonite, tuff and granodionite. Proc Mater Res Soc 465:805–812
Nagasaki S, Tanaka S, Suzuki A (1999) Sorption of neptunium on bentonite and its migration in geosphere. Colloid Surf A 155:137–143
Marsac R, Banik NL, Lützenkirchen J, Marquardt CM, Dardenne K, Schild D, Rothe J, Diascorn A, Kupcik T, Schäfer T, Geckeis H (2015) Neptunium redox speciation at the illite surface. Geochim Cosmochim Acta 152:39–51
Kitamura A, Tomura T (2003) Sorption behaviour of neptunium onto smectite under reducing conditions in carbonate media. JNC TN8400 2003-25, Tokai, Ibaraki, Japan
Ashida T, Shibutani T, Sato H, Tachi Y, Kitamura A, Kawamura K (1999) Nuclide migration study in the QUALITY—data acquisitions for the second progress report. JNC TN8400 99-083, Tokai, Ibaraki, Japan
Bertetti P (2016) Determination of sorption properties for sedimentary rocks under saline, reducing conditions—key radionuclides. Nuclear Waste Management Organization technical report NWMO TR-2-16-08, Toronto, Canada
Marsac R, Banik NL, Lützenkirchen J, Diascorn A, Bender K, Marquadt CM, Geckeis H (2017) Sorption and redox speciation of plutonium at the illite surface under highly saline conditions. J Colloid Interface Sci 485:59–64
Marsac R, Banik NL, Lützenkirchen J, Buda RA, Kratz JV, Marquadt CM (2015) Modeling plutonium sorption to kaolinite: accounting for redox equlibria and the stability of surface species. Chem Geol 400:1–10
Bradbury MH, Baeyens B (2005) Modelling the sorption of Mn(II), Co(II), Ni(II), Zn(II), Cd(II), Eu(III), Am(III), Sn(IV), Th(IV), Np(V) and U(VI) on montmorillonite: linear free energy relationships and estimates of surface binding constants for some selected heavy metals and actinides. Geochim Cosmochim Acta 69:875–892
Bradbury MH, Baeyens B (2009) Sorption modelling on illite. Part II: actinide sorption and linear free energy relationships. Geochim Cosmochim Acta 73:1004–1013
Pivovarov P (2006) Physico-chemical modeling of heavy metals (Cd, Zn, Cu) in natural environments. In: Somasundaran P (ed) Encyclopedia of surface and colloid science, vol 6, 2nd edn. Taylor & Francis, New York, pp 4617–4642
Kirishima A, Tochiyama O, Tanaka K, Niibori Y, Mitsugashira T (2003) Redox speciation method for neptunium in a wide range of concentrations. Radiochim Acta 91:191–196
Kirishima A (2014) Private communication
Nagasaki S (1993) Extraction and colloidal geochemistry of actinides. Ph.D. Thesis, The University of Tokyo, Tokyo, Japan
Inoue Y, Tochiyama O, Shinohara N (1980) The effect of Np concentration on the preparation of Np(III) by hydrogen reduction. J Inorg Nucl Chem 42:757–759
Fujiwara K, Kohara Y (2008) Hydrolysis constants of tetravalent neptunium by using solvent extraction method. Radiochim Acta 96:613–616
Fanghänel Th, Neck V, Kim JI (1996) The ion product of H2O, dissociation constants of H2CO3 and Pitzer parameters in the system Na+/H+/OH−/HCO3 −/CO3 2−/ClO4 −/H2O at 25 °C. J Sol Chem 25:327–343
Altmaier M, Metz V, Neck V, Müller R, Fanghänel Th (2003) Solid-liquid equilibria of Mg(OH)2(cr) and Mg2(OH)3Cl·4H2O(cr) in the system Mg-Na-H-OH-Cl-H2O at 25 °C. Geochim et Cosmochim Acta 67:3595–3601
Altmaier M, Neck V, Fanghänel Th (2008) Solubility of Zr(IV), Th(IV) and Pu(IV) hydrous oxides in CaCl2 solutions and the formation of ternary Ca-M(IV)-OH complexes. Radiochim Acta 96:541–550
Altmaier M, Gaona X, Fellhauser D, Buckau G (eds) (2010) Intercomparison of redox determination methods on designed and near-natural aqueous systems. FP7 EURATOM Collaborative Project “Redox Phenomena Controlling Systems”, Karlsruhe Institute of Technology Scientific Reports 7572
Nitsche H (1991) Solubility studies of transuranium elements for nuclear waste disposal: principles and overview. Radiochim Acta 52(53):3–8
Ciavatta L (1980) The specific interaction theory in the evaluating ionic equilibria. Annali di Chimica (Rome) 70:551–562
Guillaumont R, Fanghänel T, Fuger J, Grenthe I, Neck V, Palmer DA, Rand MH (2003) Update on the chemical thermodynamics of uranium, neptunium, plutonium, americium and technetium. In: Mompean FJ, Domenech-Orti C, Ben-Said K, OECD/NEA Data Bank (eds) chemical thermodynamics, vol 5. Elsevier, Amsterdam
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2)—a computer program for speciation, batch reaction, one-dimensional transport and inverse geochemical calculation. U.S. Geological Survey Water-resources Investigation Report 99-4259, Denver, Colorado, USA
Bradbury MH, Baeyens B (2009) Sorption modelling on illite. Part I: titration measurements and the sorption of Ni, Co, Eu and Sn. Geochim Cosmochim Acta 73:990–1003
Banik N, Marsac R, Lützenkirchen J, Diascorn A, Bender K, Marquardt CM, Geckeis H (2016) Sorption and redox speciation of plutonium at the illite surface. Environ Sci Technol 50:2092–2098
Nagasaki S (1994) Influence of pseudocolloid generation on migration of nuclides and diffusion of charged colloids. The University of Tokyo. UTNL-R-0312, 43-55, Tokai, Japan
Japan Atomic Energy Agency (1999) H12: project to establish the scientific and technical basis for HLW disposal in Japan, supporting report 3 safety assessment of geological disposal system. Japan Atomic Energy Agency. JNC TN 1410 2000-004, Tokai, Japan
Vilks P, Miller N H, Felushko K (2011) Sorption experiments in brine solutions with sedimentary rock and bentonite. Nuclear Waste Management Organization technical report NWMO TR-2011-11, Toronto, Canada
Tachi Y, Tochigi Y, Suyama T, Saito Y, Yui M, Ochs M (2008) Development of the sorption and diffusion database system for safety assessment of geological disposal. Japan Atomic Energy Agency JAEA Data/Code-2008-034 (in Japanese)
Zhao DL, Feng SJ, Chen CL, Chen SH, Xu D, Wang XK (2008) Adsorption of thorium(IV) on MX-80 bentonite: effect of pH, ionic strength and temperature. Appl Clay Sci 41:17–23
Acknowledgements
This work is funded by the Nuclear Waste Management Organization and the Natural Science and Engineering Research Council of Canada, Discovery Grant Program (RGPIN-2014-05732). The authors wish to acknowledge Dr. Akira Kirishima (Tohoku University) for his valuable comments on the Np oxidation state adjustment to Np(IV) and on the confirmation of the oxidation states of Np. The authors would like to thank Dr. Naoki Sugiyama and Mr. Wijdan Malik of Agilent Technologies for helpful discussion on the Np-237 measurement and improvement of detection limit of Np-237 by ICP-MS, thank Drs. Tadao Tanaka, Tetsuji Yamaguchi and Yoshihisa Iida of Japan Atomic Energy Agency (JAEA) for fruitful discussion on Np(IV) sorption under reducing conditions under the Memorandum of Cooperation for Research on Radioactive Waste Disposal between McMaster University and JAEA, and thank Dr. Satoru Tsushima of the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) under the Memorandum of Cooperation for Research on Radioactive Waste Disposal between McMaster University and HZDR and Dr. Peter Vilks of Canadian Nuclear Laboratories for valuable support on the 2 SPNE SC/CE model simulation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nagasaki, S., Riddoch, J., Saito, T. et al. Sorption behaviour of Np(IV) on illite, shale and MX-80 in high ionic strength solutions. J Radioanal Nucl Chem 313, 1–11 (2017). https://doi.org/10.1007/s10967-017-5290-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5290-2