Abstract
Sorption of Np(V) on illite, shale and MX-80 under oxidizing conditions were first studied in two types of high ionic strength solutions: (i) a reference brine solution (SR-270-PW) with an ionic strength of 6.0 M, and (ii) Na–Ca–Cl solutions. The effects of pHc, Na/Ca ratio, and ionic strength on Np(V) sorption in Na–Ca–Cl solutions were investigated. The K d values and the sorption isotherms in SR-270-PW and Na–Ca–Cl solutions were also evaluated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sedimentary rocks in Canada are being considered as potential host rocks for a deep geologic repository (DGR) for radioactive waste. Some of these rocks contain Na–Ca–Cl brine solutions with total dissolved solids (TDS) concentrations up to 200–400 g/L, for example, groundwaters in Southern Ontario, Michigan Basin [1]. Sorption of radionuclides, including actinides and their daughter products onto host rock surrounding a DGR and onto materials comprising the engineered barrier system is considered an important mechanism for retarding sub-surface transport to the surface environment [2–7]. Therefore, it is important to elucidate the sorption behaviour of radionuclides not only on host rocks, but also on engineered barrier materials such as MX-80 bentonite [2].
The Nuclear Waste Management Organization (NWMO) has been developing a sorption database of sorption coefficient values for Canadian sedimentary rocks and bentonite [2, 8]. The principal objective of this study is to investigate the sorption behavior of Np(V) in highly saline solutions.
Under anaerobic conditions, such as in the deep groundwater systems, tetravalent Np(IV) (e.g. Np4+) is the dominant oxidation state. Under aerobic conditions, such as in shallow groundwaters, pentavalent Np(V) (e.g. NpO2 +) is the dominant oxidation state. In general, the solubility of Np(IV) is very low (e.g. approximately 10−10 M in a diluted neutral pH solution [9] ) and its interaction with bentonite and rocks is strong [3, 10, 11]. On the other hand, Np(V) is more soluble (e.g. the solubility of Np(V) is approximately 10−4 M in a diluted neutral pH solution [12] ) and has moderate interaction with bentonite and rocks [3, 10, 11, 13]. This indicates that due to the different chemical behaviors of Np under oxidizing and reducing conditions, the sorption of Np must be studied by strictly controlling its oxidation state. Marsac et al. [14] observed partial reduction of Np(V) to Np(IV) on the illite surface although the measurement of redox potentials suggested the predominance of Np(V). This indicates that the redox reaction of Np on the solid/liquid interface is critical in the sorption behavior of Np on some minerals.
Recently, several extensive reviews on the aqueous chemistry of actinides have been published [3, 15–20]. Many studies have been conducted to investigate the influence of salinity on the sorption of radionuclides, mainly in the ionic strength range from dilute to saline waters [3, 21–26]. Note that fresh water has a TDS < 2 g/L, saline water has a TDS of 10–100 g/L (keeping in mind that seawater has a TDS of 33–37 g/L), and brine water has a TDS > 100 g/L. As mentioned in Ref. [8], some sorption experiments [27–33] have been carried out in solutions with higher ionic strengths. However, there are no published sorption measurements of Np(V) on Canadian sedimentary rocks and MX-80 in brine solutions.
This paper presents the results of a study conducted of the sorption behavior of Np(V) on shale, illite, which is the main mineral of shale [2], and MX-80 bentonite in brine solutions under oxidizing conditions. This work includes: (1) measurement of the sorption distribution coefficient, K d [mL g−1], of Np(V) on illite, shale and MX-80 in the SR-270-PW reference brine solution with ionic strength (I) of 6.0 M and the Na–Ca–Cl solution (I = 4.6 M); (2) investigation of the pHc dependence of Np(V) sorption at 6 < pHc < 10; (3) investigation of ionic strengths effects on Np(V) sorption at 0.10 M ≤ I ≤ 4.6 M; (4) investigation of the dependence of Np(V) sorption on Na/Ca molar concentration ratio (pure CaCl2 solution, Ca–Na–Cl solution, Na–Ca–Cl solution, and pure NaCl solution); and (5) measurement of Np(V) sorption isotherm with initial Np(V) concentrations ranging from 1.0 × 10−4 M to 1.0 × 10−8 M.
This is the first experimental research which systematically studied the sorption behavior of Np(V) under a wide range of high ionic strength conditions. The sorption distribution coefficient values determined in this work will contribute to the NWMO’s database of sorption values for Canadian sedimentary rocks and bentonite [2, 8].
Experimental
Chemicals and soils
All chemicals used were reagent grade and supplied from Fisher Scientific. Deionized water was prepared using a Milli-Q Direct 8. The shale sample was from Queenston Formation of the Michigan Basin. The mineralogy of the shale is described in [2]. Illite (Silver Hill Montana USA, Cambrian Shale) was purchased from the Clay Minerals Society, USA, and was characterized by Hower et al. [34]. Shale and illite samples were crushed using a super hard cylinder of Nichika Inc., and sieved using stainless steel sieves in deionized water. Grain sizes between 150 and 75 μm were used in the sorption experiments. The MX-80 bentonite sample was supplied by the American Colloid Company, and was used as received. A Precise Controlled Atmosphere Glove Box (GB) supplied by Labconco was filled with N2 gas (>99.999 %). The GB was used to exclude CO2, but not O2.
Preparation of Np(V) stock solution
The Np-237 solution was purchased from Stuart Hunt & Associates Ltd. Np in HNO3 solutions may contain different oxidation states: Np(IV), Np(V) and Np(VI). A 0.01 M HClO4 solution containing only Np(V) was prepared using established procedures [35, 36].
The oxidation state of the prepared Np stock solution was confirmed to be pentavalent by UV–VIS–NIR spectroscopy (Agilent 8453 UV–Vis spectrometer). The Np(V) stock solution was stored in a N2 gas filled GB. The concentration of the Np(V) stock solution was determined to be 1.0 × 10−3 M from the molar absorption coefficient of Np(V) at 981 nm, ε = 385 L/mol·cm [36]. Based on the detection efficiency of germanium (Ge) detection (Canberra) at McMaster University, the concentration of the Np(V) stock solution was also confirmed to be (1.0 ± 0.1) × 10−3 M.
pHc evaluation and Eh measurement
The pH values indicated on the pH meter (pHmeasure) should be considered as operational values [37]. The relationship between the operational pHmeasure and the molar H+ concentrations (pHc = −log c H+) or the molal H+ concentration (pHm = −log m H+) were discussed in detail by Altmaier et al. [38, 39]. In this study, the relationship between pHmeasure and pHc in solutions were determined by titration (Metrohm Ti-Touch 916), and the pHmeasure values were converted to the pHc values.
The redox potentials of the solutions were measured using a Pt combined electrode with Ag/AgCl reference electrode (Fisher Scientific Accumet AB 150/Accumet ORP electrode), and converted into Eh versus standard hydrogen electrode by the calibration of the electrode with a sanderd ORP solution. In this study, the Eh value was used as a relative indicator, by which we confirmed that the experiments were conducted under the oxidizing conditions. The measured Eh values are summarized in the Supplementary Information.
Preparation of solutions
SR-270-PW reference brine solution
The SR-270-PW reference brine solution (water type Na–Ca–Cl) has an Eh of −200 mV and pH of 6.0 [28]. Given that in this research sorption experiments were performed under oxidizing conditions, the experimental SR-270-PW reference brine solution was prepared under oxidizing conditions using the chemical compounds given in Table 1, excluding the minor ions of Li, F, I, Br, and Si. No visible precipitates were observed during the preparation of the reference brine solution. The prepared reference brine solution was stored in a GB, and was decanted and centrifuged before use in order to ensure that any precipitates were not present. The prepared experimental solution has the ionic strength of 6.0 M, and is referred to as SR-270-PW reference brine solution in this paper.
Na–Ca–Cl (I = 4.6 M) solution
A Na–Ca–Cl solution was prepared using the same weights of NaCl and CaCl2·2H2O used to prepare 1 L of SR-270-PW reference brine solution (Table 2), and was stored in the GB. The prepared Na–Ca–Cl solution has an ionic strength of 4.6 M and a Na/Ca molar concentration ratio of 2.7. This established Na–Ca–Cl solution is referred to as Na–Ca–Cl (I = 4.6 M) brine solution in this paper.
Na–Ca–Cl solutions with various ionic strengths
Six Na–Ca–Cl solutions with the same Na/Ca molar concentration ratio of 2.7 as above were prepared to study the ionic strength effect on the Np(V) sorption. The weights of NaCl and CaCl2·2H2O for preparation of 1 L of the six Na–Ca–Cl solutions were: (NaCl (g), CaCl2·2H2O (g)) = (2.777, 2.572), (13.884, 12.861), (27.767, 25.722), (55.534, 51.444), (83.301, 77.166), and (111.068, 102.888). These Na–Ca–Cl solutions have ionic strengths of 0.10, 0.50, 1.0, 2.0, 3.0, and 4.0 M, respectively.
Na–Ca–Cl solutions with various Na/Ca ratios
Four Na–Ca–Cl/Ca–Na-Cl solutions with the ionic strength of 4.6 M but with different Na/Ca molar concentration ratios, as well as pure CaCl2 and NaCl solutions with the ionic strength of 4.6 M were prepared. The weights of NaCl and CaCl2·2H2O for preparation of 1 L of pure CaCl2, Ca–Na–Cl, Na–Ca–Cl and pure NaCl solutions are: (NaCl (g), CaCl2·2H2O (g)) = (0, 223.65), (8.603, 216.430), (66.675, 167.735), (166.687, 83.8675), (205.153, 51.611), and (266.70, 0). The prepared CaCl2, Ca–Na–Cl, Na–Ca–Cl, and NaCl solutions have Na/Ca molar concentration ratios of 0.0, 0.10, 1.0, 5.0, 10, and infinite, respectively.
Sorption experiments
All sorption experiments were carried out in triplicate at 25 °C. In preliminary tests, the sorption of Np(V) on the wall of a polycarbonate reaction vessel was found to be negligible when the initial Np(V) concentration was 1 × 10−6 M and above. In the experiments with initial Np(V) concentrations below 1 × 10−6 M, the sorption of Np(V) on the reaction vessel wall was measured in blank tests. Assuming that the Np(V) sorption on the reaction vessel wall follows ideal sorption in the presence and in the absence of solids, the sorption on the wall was taken into account in the estimation of the sorption amounts of Np(V) on the solid.
In order to investigate the sorption mechanisms in detail, not only sorption experiments, but also desorption experiments are important. Because this is the first systematic study on the sorption behavior of Np(V) on illite, shale and MX-80 in high ionic strength brine solutions, the sorption behavior of Np(V) was studied in this work; the desorption behavior will be investigated in future research.
Kinetics of Np(V) sorption
Sorption kinetics of Np(V) on illite, shale and MX-80 in the SR-270-PW reference brine solution and the Na–Ca–Cl (I = 4.6 M) brine solution were measured. The sorption kinetic measurements were conducted at two solid/liquid (S/L) ratios of 0.50 g/5.0 mL and 0.10 g/5.0 mL. The procedures for conducting sorption kinetics experiments are described below and will be used for the subsequent sorption experiments.
In the GB, 0.50 or 0.10 g of illite, shale or MX-80 was added into a polycarbonate reaction vessel and 5.0 mL of SR-270-PW or Na–Ca–Cl (I = 4.6 M) brine solution was added into the reaction vessel. The suspensions were kept in the GB for 3–4 days for pre-equilibration (the pHc and Eh of solutions in contact with illite, shale and MX-80 had stabilized within 1 day in the preliminary tests). The reaction vessels were removed from the GB and the liquid and solid were separated by centrifugation for 30 min at 12,000 rpm (Beckman Coulter, Allegra X-30R). The reactions vessels were then transferred back into the GB and the liquid was removed by pipette. SR-270-PW or the Na–Ca–Cl (I = 4.6 M) brine solution was then added into the reaction vessel again, and a portion of the Np(V) stock solution was spiked into the reaction vessel. The initial Np(V) concentration in the liquid of the reaction vessel was 1.0 × 10−5 M. The final volume of the liquid was 5.0 mL. After one day, the pHc and Eh (Fisher Scientific Accumet AB 150/Accumet pH/ORP electrodes) of the liquid in the reaction vessel were measured (also in the GB) and the reaction vessel was then tightly re-sealed.
The reaction vessels were transferred from the GB to an incubator at 25 °C (Infors HT Ectron) and were shaken for pre-decided periods. After each pre-decided period, the liquid was separated from the solid by centrifugation for 30 min at 12,000 rpm at 25 °C. The pHc and Eh of the liquid were measured in the GB, and an aliquot was sampled from the liquid phase of each reaction vessel. The concentration of Np in the aliquot was measured using the Ge detector. The concentration of Np sorbed on the solid was evaluated, and the percent sorption of Np(V) was calculated by:
As discussed in the “Results and Discussion” section below, the sorption equilibrium for Np(V) on illite, shale and MX-80 (i.e. the kinetics of Np(V) sorption) was achieved within 3 days. Hence, the sorption reaction time was set to one week or longer in subsequent experiments.
Effect of the solid/liquid (S/L) ratio on Np(V) sorption
The S/L ratio dependence of Np(V) sorption onto illite, shale and MX-80 in the SR-270-PW and the Na–Ca–Cl (I = 4.6 M) brine solutions was measured at S/L ratios of 0.20 g/5.0 mL, 0.30 g/5.0 mL, and 0.40 g/5.0 mL.
Sorption results were expressed as K d (mL g−1), which is calculated as follows:
where C 0 (M) is the initial concentration of Np(V) in the liquid phase, C e (M) the equilibrium concentration of Np(V) in the liquid phase, V (mL) the volume of liquid phase, and W (g) the mass of the solid phase.
As discussed in the “Results and Discussion” section below, the K d values were found to be independent of the S/L ratio at S/L ratios above 0.30 g/5.0 mL. Therefore, the S/L ratio was set to 0.50 g/5.0 mL in the subsequent experiments.
In general, it is useful to normalize the sorption amount using the cation exchange capacity (CEC) for solid on which the ion exchange reaction is dominant and using the specific surface area for non-interlayered solid. In this research, the sorption of Np(V) could be normalized by the specific surface area because of high ionic strengths (except for several experiments) in the experimental conditions. However, since, under the experimental conditions, N2 can contribute to the sorption on the interlayer of clay minerals in BET (Brunauer–Emmett–Teller) measurements, it may not be adequate to use the specific surface area determined by the BET method. It may be better to normalize the sorption amount using the sorption site density or the specific surface area of the relevant edge faces. However, since the principal objective of this study is to investigate the sorption behavior of Np(V) in highly saline solutions, and this is the first research which systematically studied the sorption behavior of Np(V) under a wide range of high ionic strength conditions, the K d value was used to express the sorption amount in this work. In the future study, not only the sorption site density or the specific surface area of the edge faces in each solid but also the amount of each mineral in solid and the fractionation of sorption sites available to the sorption in the mineral will be taken into consideration to study the sorption bahavior of Np(V) on illite, shale and MX-80.
K d measurements in reference brine and Na–Ca–Cl solutions
The K d values of Np(V) on illite, shale and MX-80 in the SR-270-PW reference brine solution and the Na–Ca–Cl (I = 4.6 M) brine solution were measured.
Effect of pHc on K d value
The pHc dependence of the K d value of Np(V) on illite, shale and MX-80 in the Na–Ca–Cl (I = 4.6 M) brine solution was measured. The pHc of the liquid in reaction vessels was adjusted to values between 6 < pHc < 10 by addition of 0.01 M HClO4 or 0.01 M NaOH solution in the GB. The pHc in the reaction vessel was measured twice a day, and the pHc was re-adjusted to the original pHc value. In this experiment, the sorption reaction time was set to two weeks.
Effect of the ionic strength on K d value
The ionic strength dependence of the K d value of Np(V) on illite, shale and MX-80 in the Na–Ca–Cl solutions with various ionic strengths was measured. In the preliminary test, the reproducibility of the solid/liquid separation by centrifugation for 30 min at 12,000 rpm was confirmed, but colloids might be produced and present in the separated solutions in MX-80 series at lower ionic strengths.
Effect of the Na/Ca ratio on K d value
The Na/Ca molar concentration ratio dependence of the K d value of Np(V) on illite, shale and MX-80 in CaCl2, Ca–Na–Cl, Na–Ca–Cl and NaCl solutions with various Na/Ca ratios was investigated.
Sorption isotherms in SR-270-PW and Na–Ca–Cl solutions
The sorption isotherms of Np(V) on illite, shale and MX-80 in the SR-270-PW reference brine solution and the Na–Ca–Cl (I = 4.6 M) brine solution were measured. The initial Np(V) concentrations ranged from 1.0 × 10−4 M to 1.0 × 10−8 M. The concentration of Np(V) in the liquid phase after centrifugation was measured by ICP-MS (Agilent ICP-MS 8800). For the initial Np(V) concentration below 1 × 10−6 M, the sorption of Np(V) on the wall was taken into account to calculate the K d values.
Results and discussion
Kinetics of Np(V) sorption
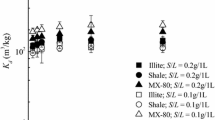
The percent sorption of Np(V) measured on illite, shale and MX-80 at the S/L ratio of 0.10 g/5.0 mL and 0.50 g/5.0 mL in the SR-270-PW reference brine solution and the Na–Ca–Cl (I = 4.6 M) brine solution is shown in Figs. 1 and 2, respectively. The values of pHc and Eh at the sorption equilibrium are shown in the Supplementary Information (S/L = 0.10 g/5.0 mL and 0.50 g/5.0 mL in Table 1). These results illustrate that sorption equilibrium for Np(V) on illite, shale and MX-80 was achieved within 3 days. The time to attain the sorption equilibrium was independent of the studied solution compositions, solid types and the solid/liquid ratios.
Sorption kinetics in the SR-270-PW reference brine solution at S/L of 0.10 g/5.0 mL(filled square illite; filled circle shale; filled triangle MX-80) and 0.50 g/5.0 mL (open square illite; open circle shale; open triangle MX-80). The initial concentration of Np(V) was 1.0 × 10−5 M. pHc and Eh at sorption equilibrium are summarized in the Supplementary Information (Table 1)
Sorption kinetics in the Na–Ca–Cl (I = 4.6 M) brine solution at S/L of 0.10 g/5.0 mL (filled square illite; filled circle shale; filled triangle MX-80) and 0.50 g/5.0 mL (open square illite; open circle shale; open triangle MX-80). The initial concentration of Np(V) was 1.0 × 10−5 M. pHc and Eh at sorption equilibrium are summarized in the Supplementary Information (Table 1)
Nagasaki et al. [40] measured the sorption kinetics of Np(V) on poorly crystallized ferric oxide and found that sorption equilibrium was achieved in 1 h. Snow et al. [41] found that no significant changes in the Np(V) sorption on goethite were observed from 1 to 8 days, suggesting that the sorption equilibrium was achieved in 1 day. On the other hand, Nagasaki et al. [42, 43] measured the sorption kinetics of Np(V) on illite and Na-montmorillonite, and found that more than 1 day was required to reach sorption equilibrium. This difference in equilibration times may be due to the difference in structure between clay minerals and Fe(III) oxides. The solid structures of illite, shale and MX-80 and the brine conditions considered in this current study may contribute to the slightly longer times required to reach sorption equilibrium.
Effect of the solid/liquid (S/L) ratio on K d value
The dependence of the K d value of Np(V) on the solid/liquid (S/L) ratio in the SR-270-PW and the Na–Ca–Cl (I = 4.6 M) brine solutions is illustrated in Fig. 3. The data at the S/L ratio of 0.10 g/5.0 mL and 0.50 g/5.0 mL in Fig. 3 were taken from the results of sorption kinetics experiments. The pHc and Eh values of solutions at sorption equilibrium are summarized in the Supplementary Information (Table 1). It was found that the pHc value was dependent on the solid phase, but was independent of the S/L ratio.
With the exception of MX-80 in the SR-270-PW reference brine solution, it was found that K d values of Np(V) on illite, shale and MX-80 increased as the S/L ratio increased from 0.10 g/5.0 mL to 0.20 g/5.0 mL, and reached plateaus at the S/L ratios above 0.30 g/5.0 mL. For MX-80 in the SR-270-PW, the K d values were independent of the S/L ratios considered in this study. Namely, Fig. 3 illustrates that the ideal sorption range under the selected experimental conditions is reached at S/L ≧ 0.30 g/5.0 mL.
Several researchers have reported a decrease of the K d value with the S/L ratio for some elements (e.g. [44] ); whereas, others reported that the K d value was independent of the S/L ratio (e.g. [45] ). An increase of the K d value with the S/L ratio as observed in this study was also reported in the studies of Th sorption on graphene oxide [46] and on attapulgite [47]. It was discussed that with increasing solid content in the suspension, the number of sites on the solids which participated in the sorption of Th increased and thereby the removal of Th increased [46, 47]. The effect of the S/L ratio on Np(V) sorption will be further investigated in future research.
K d values in SR-270-PW and Na–Ca–Cl solutions
The K d values of Np(V) on illite, shale, and MX-80 in the SR-270-PW and the Na–Ca–Cl (I = 4.6 M) brine solutions are summarized in Table 3. Table 3 also lists the K d values calculated from the kinetics experiment conducted at a S/L ratio of 0.50 g/5.0 mL. The average K d values on illite and shale in the SR-270-PW reference brine solution are slightly larger than those in the Na–Ca–Cl (I = 4.6 M) brine solution, and the average K d values on MX-80 in the SR-270-PW reference brine solution are slightly smaller than those in the Na–Ca–Cl (I = 4.6 M) brine solution. However, considering the experimental errors in the K d values and the difference of pHc, there may be no marked differences in the K d values on illite and MX-80 between these two solutions. On the other hand, the K d value on shale in the SR-270-PW reference brine solution is appreciably larger than those in the Na–Ca–Cl (I = 4.6 M) brine solution, even when the experimental errors in the K d values and the difference of pHc are taken into account.
The content of illite in the Queenston shale used in this study is 60 % [2]. Assuming that the simple mineral additivity is appropriate for the evaluation of K d values and that illite dominates the sorption of Np(V) on shale [2, 8], the K d value of Np(V) on illite can be used to estimate the K d value on shale [8]. However, it was found that the K d values on shale obtained in this work were much larger than 60 % of the K d values on illite. This suggests that Np(V) also sorbs onto other minerals in shale (e.g. chlorite and dolomite) in brine conditions, or that the simple mineral additivity is questionable. It is important to normalize the sorption amounts on illite and shale using the specific surface area or the sorption site density, and estimate the K d value using the normalized sorption amount.
Marsac et al. [14] found the reduction of Np(V) on the purified illite surface in 0.1 M NaCl solution under Ar atmosphere. In this work, we did not examine whether Np(V) was reduced to Np(IV). However, because our K d values of Np(V) on the illite are similar to those reported in the previous researches [33, 48, 49] which will be discussed later, and our K d values are much smaller than those reported by Marsac et al. [14], we think the possibility of reduction of Np(V) to Np(IV) in the experiments of this work is very small.
Bradbury and Baeyens [50, 51] proposed a methodology to predict the K d values of actinides and some heavy metals on montmorillonite and illite. The model and the methodology of Bradbury and Baeyens [50, 51] were not applied to this work bcause (1) our experimental conditions, such as the solution compositions (Na–Ca–Cl brine solutions with an ionic strength of 4.6 and 6.0 M were used in this work) and the pre-conditioning procedures of the solids, were not identical to those of what Bradbury and Baeyens used, and (2) the Pitzer model parameters which should be used for the model calculation are not fully compiled. We appreciate that it would be interesting to investigate the applicability of the model of Bradbury and Baeyens at the higher ionic conditions and to develop a sorption model and methodology available under the wider brine and solid conditions.
Sorption in Na–Ca–Cl solutions
Effect of pHc on K d value
The pHc dependence of K d value of Np(V) in the Na–Ca–Cl (I = 4.6 M) brine solution is illustrated in Fig. 4. The values of pHc and Eh at the sorption equilibrium are shown in the Supplementary Information (Table 2). Figure 4 shows that the K d values of Np(V) on illite, shale and MX-80 increase with pHc. This tendency is consistent with the results of the previous studies [8, 33, 48, 50–53], although many of those K d data were not measured under brine conditions. It was also found that the slope of K d value vs. pHc for illite was similar to that for shale, and slightly smaller than that for MX-80. We will continue to examine the physical and chemical meanings of the similarity and the difference in the slope of K d value vs. pHc.
In April 2014, the Japan Atomic Energy Agency (JAEA) updated their Thermodynamic Database and the SIT (Specific Ion Interaction Theory) model parameters [54]. Using simulations conducted with the geochemical code PHREEQC (version 3.1.2) [55] and JAEA’s SIT thermodynamic database for Na–Ca–Cl solutions with an ionic strength of less than 4 M, it was predicted that NpO2 + is dominant at pH values from 6 to 9. If NpO2 + is the dominant species in both the SR-270-PW reference brine solution and the Na–Ca–Cl (I = 4.6 M) brine solution, this pHc dependence of the K d value can be explained by deprotonation of the surface complexation sites and then formation of surface complexes with NpO2 + as pHc increases. This pHc dependence is also predicted by the stoichiometry of sorption equations which were formulated by Bradbury and Baeyens [50, 51].
Mucciardi et al. [33] measured the sorption of Np(V) on illite in CaCl2 solution (I = 5.2 M) at pH of 7, and reported the K d values of 13–44 mL g−1. Gorgeon [48] reported the K d values of 60–195 mL g−1 on illite in NaClO4 solution (I = 1.0 M) at pH of 6–7. There are some reported K d values measured under low ionic strength conditions. Torstenfelt et al. [49] observed the K d values of 80–90 mL g−1 in Na–Ca–HCO3–SO4 solution (I = 0.004 M) at pH of 8.5. Mucciardi et al. [33] also reported the the K d values of 150–460 mL g−1 in NaHCO3 solution (I = 0.025 M) at pH 8–9, and 13–74 mL g−1 in NaCl solution (I = 0.03 M) at pH of 7. Considering that the K d value of Np(V) on illite is independent of ionic strength as discussed below, the K d values obtained in this work agree with those reported by previous researches.
Mucciardi et al. [33] also studied the sorption of Np(V) on montmorillonite, and reported the K d values of 18–24 mL g−1 in CaCl2 solution (I = 5.2 M) at pH of 7–8. Gorgeon [48] reported the K d values of 25–130 mL g−1 on smectite in NaClO4 solution (I = 1.0 M) at pH of 6–7. Kitamura and Tomura [52] measured the K d values of 2–21 mL g−1 on smectite in NaCl solution (I = 1 M) at pH of 8–9. Considering that the K d value of Np(V) on MX-80 is not dependent on the ionic strength when the ionic strength is over 1 M as discussed below, the K d values obtained in this work were consistent with the study of Gorgeon [48], but were larger than the the K d values by Mucciardi et al. [33] and Kitamura and Tomura. [52].
Vilks [8] conducted an extensive review of the K d values of Np(V) onto shale, limestone and bentonite published in scientific papers, reports and international sorption databases, discussed the validity of these K d values in brine conditions, and recommended a range of K d values for brine solutions. The recommended K d values for shale were 7.8–120 mL g−1 at 6.1 ≤ pH ≤ 7.1. The recommended K d values for bentonite were 2–130 mL g−1 at 6 ≤ pH ≤ 8. The measured K d values on shale at pHc = 6.3 (26 mL g−1) and on MX-80 at pHc = 6.2 and 7.2 (46 and 70 mL g−1, respectively) in the Na–Ca–Cl (I = 4.6 M) solution (Fig. 4) are within the recommended K d values by Vilks [8].
Effect of the ionic strength on K d value
The ionic strength dependence of the K d value of Np(V) in Na–Ca–Cl solutions is illustrated in Fig. 5. The pHc and Eh values at sorption equilibrium are summarized in the Supplementary Information (Table 3).
The pHc value changed slightly over a series of sorption experiments on illite (pHc = 7.1–7.8), shale (pHc = 7.6–8.1) and MX-80 (pHc = 8.5–9.1). Considering the variation of pHc of the Na–Ca–Cl solutions with different ionic strengths and the pHc dependence of K d values (Fig. 4), the K d values on illite and shale can be considered to be independent of the ionic strength in the range of 0.10–4.6 M being investigated. On the other hand, the K d value on MX-80 decreased with increasing the ionic strength from 0.10 to 1.0 M, but was independent of the ionic strength when the ionic strength was over 1 M. The difference in the sorption on illite and MX-80 may be explained by (i) the difference in the structures of interlayer of illite and MX-80, and (ii) sorption by ionic exchange reactions at ionic strengths below 1 M. Furthermore, as Schnurr et al. [27] pointed out, the coagulation effects of MX-80 might also be a possibility for the decrease in sorption with increasing ionic strength. However, the coagulation effects are marked at relatively low salt concentration such as 0.01–0.1 M.
Gorgeon [48] measured the sorption of Np(V) on Na-smectite in 0.025 and 0.1 M NaClO4 solutions and reported the K d value of 60–160 mL g−1 at pH of 7–9. Turner et al. [56] reported the K d value of 50–200 mL g−1 on Na-montmorillonite in 0.1 M NaNO3 solution at pH of 7.5–9. Sakamoto et al. [57] reported the K d value of 15–44 mL g−1 on bentonite in 0.01 M NaClO4. Higgo et al. [58] reported the K d value of 1100–12,400 mL g−1 on smectite in sea water. Morgan et al. [59] studied the sorption of Np(V) on bentonite in deionized water and reported the K d value of 110–4000 mL g−1. As discussed above, the K d values on illite and shale (I = 0.10–4.6 M) and those on MX-80 (I ≧ 1.0 M) obtained in this work are consistent with the previous researches. However, the K d values on MX-80 (I = 0.10 and 0.50 M) are higher than those reported by Gorgeon [48], Turner et al. [56], and Sakamoto et al. [57], but much smaller than those by Higgo et al. [58], and Morgan et al. [59]. As explained previously, the reproducibility of the solid/liquid separation by centrifugation for 30 min at 12,000 rpm was confirmed, but colloids might be produced and present in the separated solutions in MX-80 series at I = 0.10 and 0.50 M. It will be useful to further investigate the different Np(V) sorption behavior on illite, shale and MX-80 over a wide range of high ionic strength conditions, including the influence of smaller size colloids.
Effect of the Na/Ca ratio on K d value
The effect of the Na/Ca molar concentration ratio on the K d value of Np(V) in CaCl2, Ca–Na–Cl, Na–Ca–Cl, and NaCl solutions with an ionic strength of 4.6 M is summarized in Table 4. The pHc and Eh values of the solutions at the sorption equilibrium are shown in the Supplementary Information (Table 4).
Although the pHc value of the solution varied over a series of sorption experiments on illite (pHc = 6.9–8.6), shale (pHc = 7.4–9.2) and MX-80 (pHc = 8.5–10.2), the K d value seemed to increase as the Na/Ca ratio increased in experiments conducted with all three solids, suggesting that the presence of Ca in solution reduces the sorption of Np(V) on illite, shale and MX-80. Possible explanations may be the competition of Ca with Np(V) for sorption sites, or the change in the aqueous speciation of Np(V) induced by high concentrations of Ca. However, these cannot explain the ionic strength dependence and the Na/Ca ratio dependence simultaneously. Schnurr et al. [27] observed a similar effect in the Eu sorption on illite and smectite, and considered that the competition for the same sorption sites might not be a sufficient explanation. Bradbury and Baeyens [50] found that the K d value of Eu(III) on smectite in NaCl solution is more than one order of magnitude higher than that in CaCl2 solution. Activity coefficients in the solution containing Ca might be different from those in the solution containing Na, but this effect has not yet been understood. The effect of Na/Ca ratio on Np(V) sorption including the effect of pHc will be further investigated in the future research.
Isotherms of Np(V) sorption in SR-270-PW and Na–Ca–Cl solutions
Sorption isotherms in the SR-270-PW reference brine solution and the Na–Ca–Cl (I = 4.6 M) brine solution are illustrated in Figs. 6 and 7, respectively. The data at the initial Np(V) concentration of 1.0 × 10−5 M were from the experimental results of K d value measurement in the SR-270-PW and Na–Ca–Cl (I = 4.6 M) brine solutions. The pHc and Eh values of the solutions at the sorption equilibrium are shown in the Supplementary Information (Table 5).
Sorption isotherms in the SR-270-PW reference brine solution. The data at the initial concentration of 1.0 × 10−5 M was from the K d value measurement in the SR-270-PW. A solid line represents a sorption isotherm on MX-80 with the slope of 1. pHc and Eh at sorption equilibrium are summarized in the Supplementary Information (Table 5)
Sorption isotherms in the Na–Ca–Cl (I = 4.6 M) brine solution. The data at the initial concentration of 1.0 × 10−5 M was from the K d value measurement in the Na–Ca–Cl (I = 4.6 M). A solid line represents a sorption isotherm on MX-80 with the slope of 1. pHc and Eh at sorption equilibrium are summarized in the Supplementary Information (Table 5)
In both solutions, the measured isotherms are observed to be linear on log–log plots with slopes of approximately 1 at a Np(V) equilibrium concentration in the liquid less than 2 × 10−6 M (the initial Np(V) concentration was 1.0 × 10−5 M). The data points at a Np(V) equilibrium concentrations of 1 × 10−5 to 2 × 10−5 M in the liquid (the initial Np(V) concentration was 1.0 × 10−4 M) slightly deviate from a linear relationship (Figs. 6 and 7). Figures 6 and 7 illustrate that the ideal sorption range under the selected experimental conditions is reached at the Np(V) concentration in the liquid of less than 2 × 10−6 M.
Conclusions
This is the first research systematically studying the sorption behavior of Np(V) under a wide range of high ionic strength. The K d values of Np(V) on illite, shale and MX-80 in the SR-270-PW reference brine solution and in the Na–Ca–Cl (I = 4.6 M) brine solution were evaluated using batch sorption experiments. Considering the experimental errors in the K d values and the difference of pHc, there may be no marked difference in the K d values on illite and MX-80 between these two solutions. On the other hand, the K d value on shale in the SR-270-PW reference brine solution is observed to be larger than those in the Na–Ca–Cl brine solution.
The sorption equilibrium of Np(V) on illite, shale and MX-80 was achieved within 3 days and was independent of the compositions of the studied solutions. The K d values of Np(V) on MX-80 in the reference SR-270-PW and Na–Ca–Cl (I = 4.6 M) brines were independent of the S/L ratios. Those on illite and shale in the reference SR-270-PW and Na–Ca–Cl (I = 4.6 M) brine solutions as well as those on MX-80 in the Na–Ca–Cl (I = 4.6 M) solution increased with the S/L ratio and reached plateaus at S/L ratios above 0.30 g/5.0 mL. The K d value of Np(V) on the 3 solids (illite, shale and MX-80) in the Na–Ca–Cl (I = 4.6 M) brine solution increased with pHc. The K d values of Np(V) on shale and MX-80 obtained in this work fall into the range of recommended Np(V) K d values for brine solutions recommended by Vilks [8]. The K d values on illite and shale in Na–Ca–Cl solutions were independent of the ionic strength in the range of 0.10–4.6 M. The K d values on MX-80 in Na–Ca–Cl solutions decreased with increasing the ionic strength from 0.10 M to 1.0 M and became independent of the ionic strength of 1.0–4.6 M. The K d values on 3 solids were all observed to increase with the Na/Ca molar concentration ratio of solutions. The slopes of the sorption isotherms for the minerals in both the reference SR-270-PW brine and Na–Ca–Cl (I = 4.6 M) solutions were approximately 1 at the Np(V) equilibrium concentration of less than 2 × 10−6 M.
References
Hobbs M Y, Frape S K, Shouakar-Stash O, Kennel LR (2011) Regional hydrogeochemistry—Southern Ontario, NWMO DGR-TR-2011-12, Toronto, Canada
Vilks P (2011) Sorption of selected radionuclides on sedimentary rocks in saline conditions—literature review, Nuclear Waste Management Organization technical report NWMO TR-2011-12, Toronto, Canada
Geckeis H, Lützenkirchen J, Polly R, Rabung T, Schmidt M (2013) Mineral-water interface reactions of actinides. Chem Rev 113:1016–1062
Zavarin M, Powell BA, Bourbin M, Zhao PH, Kersting AB (2012) Np(V) and Pu(V) ion exchange and surface-mediated reduction mechanisms on montmorillonite. Environ Sci Technol 46:2692–2698
Chapman N, Apted M, Aspinall W, Berryman K, Cloos M, Connor C, Connor L, Jaquet O, Kiyosugi K, Scourse E, Sparks S, Stirling M, Wallace L, Goto J (2012) TOPAZ Project long-term tectonic hazard to geological repositories. Nuclear Waste Management Organization of Japan technical report NUMO-TR-12-05, Tokyo, Japan
Ahn T, Ikeda T, Ohe T, Kanno T, Sakamoto Y, Chiba T, Tsukamoto M, Nakayama S, Nagasaki S, Banno K, Fujita T (1995) Quantitative performance allocation of multi-barrier system for HLW disposal. J At Energy Soc Jpn 37:59–77 (in Japanese)
National Academy of Science (1983) A study of the isolation for geologic disposal of radioactive wastes, Waste Isolation Systems Panels, Board on Radioactive Waste Management, Washington DC, USA
Vilks P (in preparation) Sorption of selected radionuclides on sedimentary rocks in saline conditions—updated sorption values. Nuclear Waste Management Organization technical report, Toronto, Canada
Neck V, Kim JI (2001) Solubility and hydrolysis of tetravalent actinides. Radiochim Acta 89:1–16
Japan Atomic Energy Agency (2000) H12: Project to establish the scientific and technical basis for HLW disposal in Japan, Supporting report 3: Safety assessment of the geological disposal system. The Japan Nuclear Cycle Development Institute technical note, JNC TN 1410 2000-004, Tokyo, Japan
Choppin GR (2006) Environmental behavior of actinides. Czechoslovak J Phys 56:D13–D21
Itagaki H, Nakayama S, Tanaka S, Yamawaki M (1992) Effect of ionic strength on the solubility of neptunium(V) hydroxide. Radiochim Acta 58(59):61–66
Nagasaki S, Tanaka S, Suzuki A (1998) Geochemical behavior of actinides in high-level radioactive waste disposal. Prog Nucl Energy 32:141–161
Marsac R, Lal Banik N, Lützenkirchen J, Marquardt CM, Dardenne K, Schild D, Rothe J, Diascorn A, Kupcik T, Schäfer T, Geckeis H (2015) Neptunium redox speciation at the illite surface. Geochim et Cosmochim Acta 152:39–51
Altmaier M, Gaona X, Fanghänel Th (2013) Recent advances in aqueous actinide chemistry and thermodynamics. Chem Rev 113:901–943
Knope KE, Soderholm L (2013) Solution and solid-state structural chemistry of actinide hydrates and their hydrolysis and condensation product. Chem Rev 113:944–994
Walther C, Denecke A (2013) Actinide colloids and particles of environmental concern. Chem Rev 113:995–1015
Yoshida Z, Johnson SG, Kimura T, Krsul JR (2010) Neptunium. In: Morss LR, Edelstein NM, Fuger J (eds) The chemistry of the actinide and transactinide elements, vol 2, 4th edn. Springer, Dordrecht, pp 699–812
Choppin GR, Jensen MP (2010) Actinides in solution: complexation and kinetics. In: Morss LR, Edelstein NM, Fuger J (eds) The chemistry of the actinide and transactinide elements, vol 4, 4th edn. Springer, Dordrecht, pp 2524–2621
Runde W, Neu MP (2010) Actinides in the geosphere. In: Morss LR, Edelstein NM, Fuger J (eds) The chemistry of the actinide and transactinide elements, vol 6, 4th edn. Springer, Dordrecht, pp 3475–3593
Kar AS, Kumar S, Tomar BS (2012) U(VI) sorption by silica: effect of complexing anions. Colloid Surf A 395:240–247
Zhu WB, Liu ZJ, Chen L, Dong YH (2012) Sorption of uranium(VI) on Na-attapulgite as a function of contact time, solid content, pH, ionic strength, temperature and humic acid. J Radioanal Nucl Chem 289:781–788
Tertre E, Pret D, Ferrage E (2011) Influence on the ionic strength and solid/solution ratio on Ca(II)-for-Na+ exchange on montmorillonite. Part 1: chemical measurements, thermodynamic modeling and potential implications for trace elements geochemistry. J Colloid Interface Sci 353:248–256
Amayri S, Jermolajev A, Reich T (2011) Neptunium(V) sorption on kaolinite. Radiochim Acta 99:349–357
Schmeide K, Bernhard G (2010) Sorption of Np(V) and Np(VI) onto kaolinite: effects of pH, ionic strength, carbonate and humic acid. Appl Geochem 25:1238–1247
Schlegel ML, Descostes M (2009) Uranium uptake by hectorite and montmorillonite: a solution chemistry and polarized EXAFS study. Environ Sci Technol 43:8593–8598
Schnurr A, Marsac R, Rabung Th, Lutzenkirchen J, Geckeis H (2015) Sorption of Cm(III) and Eu(III) onto clay minerals under saline conditions: batch adsorption, laser-fluorescence spectroscopy and modeling. Geochim et Cosmochim Acta 151:192–202
Vilks P, Miller NH (2013) Sorption studies with sedimentary rock under saline conditions. Nuclear Waste Management Organization technical report, NWMO TR-2013-23, Toronto, Canada
U. S. Environmental Protection Agency (1998) Assessment of K ds used in the CCA, Technical support document for Section 194.14: DOCKET NO: A-93-02 V-B-4, Washington DC, USA
Warnecke E, Hollmann A, Tittel G, Brennecke P (1994) Gorleben radionuclide migration experiments: more than 10 years of experience. Radiochim Acta 66(67):821–827
Lieser KH, Muhlenweg U (1988) Neptunium in the hydrosphere and in the geosphere. Radiochim Acta 44(45):129–133
Laul JC, Smith MR, Hubbard N (1985) Behaviour of natural uranium, thorium, and radium isotopes in the Wolfcamp brine aquifers, Palo Doro Basin. Mater Res Soc Symp Proc 44(Scientific Basis for Nuclear Waste Management VIII):475–482
Mucciardi AN, Johnson TC, Saunier J (1979) Statistical investigation of the mechanics controlling radionuclide sorption. Annual report, Battelle-Pacific Northwest Laboratories, ADI Ref. 548, Richland, USA
Hower J, Mowatt TC (1966) The mineralogy of illites and mixed layer illite-montmorillonite. Am Miner 51:825–854
Kirishima A, Tochiyama O, Tanaka K, Niibori Y, Mitsugashira T (2003) Redox speciation method for neptunium in a wide range of concentrations. Radiochim Acta 91:191–196
Kirishima A (2014) Private communication
Fanghänel Th, Neck V, Kim JI (1996) The ion product of H2O, dissociation constants of H2CO3 and Pitzer parameters in the system Na+/H+/OH−/HCO3 −/CO3 2−/ClO4 −/H2O at 25 °C. J Sol Chem 25:327–343
Altmaier M, Metz V, Neck V, Müller R, Fanghänel Th (2003) Solid-liquid equilibria of Mg(OH)2(cr) and Mg2(OH)3Cl·4H2O(cr) in the system Mg–Na–H–OH–Cl–H2O at 25 & #xB0;C. Geochim et Cosmochim Acta 67:3595–3601
Altmaier M, Neck V, Fanghänel Th (2008) Solubility of Zr(IV), Th(IV) and Pu(IV) hydrous oxides in CaCl2 solutions and the formation of ternary Ca–M(IV)–OH complexes. Radiochim Acta 96:541–550
Nagasaki S, Tanaka S, Todoriki M, Suzuki A (1998) Surface sorption and surface diffusion of NpO2 + with poorly crystallized ferric oxide. J Alloy Compd 271–273:252–256
Snow MS, Zhao P, Dai Z, Kersting AB, Zavarin M (2013) Neptunium(V) sorption to goethite at attomolar to micromolar concentrations. J Colloid Interface Sci 390:176–182
Nagasaki S, Tanaka S (1998) Sorption equilibrium and kinetics of NpO2 + uptake onto illite. Radiochim Acta 82:263–267
Nagasaki S, Tanaka S (2000) Sorption equilibrium and kinetics of NpO2 + on dispersed particles of Na-montmorillonite. Radiochim Acta 88:705–709
Tachi Y, Shibutani T, Sato H, Shibata M (1999) Sorption and diffusion behavior of palladium in bentonite, granodiorite and tuff. The Japan Nuclear Cycle Development Institute technical note, JNC TN 8400, Tokyo, Japan
Wang XK, Rabung Th, Geckeis H, Panak PJ, Klenze R, Fanghäenel Th (2004) Effect of humic acid on the sorption of Cm(III) onto γ-Al2O3 studied by the time resolved laser fluorescence spectroscopy. Radiochim Acta 92:691–695
Li Y, Wang C, Guo Z, Liu C, Wu W (2014) Sorption of thorium(IV) from aqueous solutions by graphene oxide. J Radioanal Nucl Chem 299:1683–1691
Wu W, Fan Q, Xu J, Niu Z, Lu S (2007) Adsorption of Th(IV) on attapulgite: effects of pH, ionic strength, and temperature. Appl Radiat Isot 65:1108–1114
Gorgeon L (1994) Contribution à la modélisation physico-chimique de la rétention de radioéléments à vie longue par des matériaux argileux, Ph.D. Dessertation, Université Paris 6
Torstenfelt B, Rundberg RS, Mitchell AJ (1988) Actinide sorption on granites and minerals as a function of pH and colloids/psuedocolloids. Radiochim Acta 44(45):111–117
Bradbury MH, Baeyens B (2005) Modelling the sorption of Mn(II), Co(II), Ni(II), Zn(II), Cd(II), Eu(III), Am(III), Sn(IV), Th(IV), Np(V) and U(VI) on montrmorillonite: linear free energy relationships and estimates of surface binding constants for some selected heavy metals and actinides. Geochim et Cosmochim Acta 69:875–892
Bradbury MH, Baeyens B (2009) Sorption modeling on illite. Part II: actinide sorption and linear free energy relationships. Geochim et Cosmochim Acta 73:1004–1013
Kitamura A, Tomura T (2003) Sorption behaviour of neptunium onto smectite under reducing conditions in carbonate media. Japan Nuclear Cycle Development Institute technical note, JNC TN8400 2003-25 (in Japanese)
Stammose D, Ly J, Pitsch H, Dolo JM (1992) Sorption mechanisms of three actinides on a clayey mineral. Appl Clay Sci 7:225–238
Kitamura A, Doi R, Yoshida Y (2014) Update of JAEA-TDB: update of thermodynamic data for palladium and tin, refinement of thermodynamic data for protactinium, and preparation of PHREEQC database for use of the Brønsted-Guggenheim-Scatchard model. Japan Atomic Energy Agency, JAEA-Data/Code 2014-009, Tokai, Japan
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations, U.S. Geological Survey, Water Resources Investigations Report 99-4259
Turner DR, Pablan RT, Bertetti FP (1998) Neptunium(V) sorption on montrmorillonite: an experimental and surface complexation modeling study. Clays Clay Miner 46:256–269
Sakamoto Y, Konishi M, Shirahashi K, Senoo M, Moriyama N (1990) Adsorption behavior of neptunium for soil. Radiact Waste Manage Nucl Fuel Cycle 15:13–25
Higgo JJW, Rees LVC, Cronan DS (1983) Sorption of americium and neptunium by deep-sea sediments. Radiact Waste Manag Nucl Fuel Cycle 4:73–102
Morgan RD, Pryke DC, Rees JH (1988) Data for the sorption of actinides on candidate materials for use in repository. UK Department of Environment Report, DOE/RW/87.094
Acknowledgments
This work is funded by the Nuclear Waste Management Organization and the Natural Science and Engineering Research Council of Canada, Discovery Grant Program (RGPIN-2014-05732). The authors wish to acknowledge Dr. Akira Kirishima (Tohoku University) for his valuable comments on the spectroscopic measurement, the molar absorption coefficient of Np(V), and the Np oxidation state adjustment to Np(V). The authors would like to thank Dr. Monique Hobbs of NWMO for valuable discussion and review of this manuscript. The constructive and valuable reviews by two anonymous referees are highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nagasaki, S., Saito, T. & Yang, T.T. Sorption behavior of Np(V) on illite, shale and MX-80 in high ionic strength solutions. J Radioanal Nucl Chem 308, 143–153 (2016). https://doi.org/10.1007/s10967-015-4332-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4332-x