Abstract
Selective separation of uranium using bis(2,4,4-trimethylpentyl)phosphinic acid in xylene and C8mimNTf2 was investigated. For ionic liquid based system, the extraction kinetics was found to be slower with the predominance of ion exchange mechanism through [UO2(NO3)·2L]+, while for xylene based system solvation mechanism. The nature of the extracted species was found to be different in both the media as observed in luminescence study. Ionic liquid based system was more radioresistant than that of molecular diluents. Na2CO3 was successfully used for stripping. The selectivity was investigated by processing simulated high level waste of pressurized heavy water reactor origin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For safe handling of radioactive waste and effective utilization of uranium sources, it is required to develop efficient and selective separation procedure for U. Significant effort have been reported in the literature demonstrating the effective extraction/separation behaviour of lanthanides and actinides either for the recovery of valuables from the nuclear waste or for the safe management of the radio-toxic nuclear waste involving selective ligands in molecular diluents [1–7].
On the other hand, in modern era, the world-wide research is going on for the development of ‘green’ separation process without generation of short term or long term risk on the environment. Therefore, the alternative to the use of volatile organic solvents (VOC) need to be explored. Due to the favourable properties like low vapour pressure, wide liquid range, high flash point, high degree of stability towards chemical and radiation etc. ionic liquid finds application in the field of chemical synthesis, electrochemistry, and analytical chemistry including separation science [8–16]. Though ionic liquids have been explored for the processing of nuclear waste [17–25], a comparative evaluation of ionic liquid vis a vis molecular diluents on overall metal ion extraction process was not found in the literature very frequently. Therefore, the main objective of the present investigation was to develop a ‘green’ separation method for uranium from nuclear waste solution. Room temperature ionic liquid in combination with ligand was chosen as ‘green’ solvent and different aspects of separation like mechanism, kinetics, species involve in separation, stripping of U from loaded organic phase, radiolytic stability of the solvent system in presence of gamma irradiation, selectivity etc. were evaluated and compared with the normal molecular diluent based solvent system.
In understanding the extraction mechanism or the interaction between the metal ion and the ligating site, XPS and EXAF studies were found to be very important and have been reported in the literature to enhanced sequestration of selenite in water using nanoscale zero valent iron immobilization on carbon nanotubes, to understand retention mechanisms and microstructure of Eu(III) on manganese dioxide, for the determination of colloidal pyrolusite, Eu(III) and humic substance interaction, the interfacial interaction of nickel(II) on titanate nanotubes and colloidal diatomite, radionickel and humic substance interaction [26–30].
Apart from the management of the radioactive waste in a ‘greener’ way, the chemistry of actinides and lanthanides in ionic liquid was reported to be interesting and different than that in molecular diluents. Moreover, slight structural modification in ionic liquid moieties can be utilized to fine tune the extraction procedure [31–33]. The presence of difference extracted species in ionic liquid and molecular diluent indicated the predominance of different extraction mechanism in both the media [34–36]. Ion exchange mechanism was reported to be common for lower homologue of methyl imidazolium based ionic liquid while for higher homologue, the mechanism converted to solvation mechanism with similar extraction efficiency via same extracted species as reported for molecular diluent [25]. Interesting reports were found in literature on the extraction mechanism of UO2 2+ ion by TBP in ionic liquid [25, 37, 38]. The extraction profiles of U in C10mimNTf2 was found to same as of dodecane, molecular diluents, revealing the predominance of same mechanism, ‘ion exchange’, while differed significantly in C4mimNTf2 and C8mimNTf2. In both these ionic liquids initially ‘cation exchange’ mechanism was reported to be operative while beyond certain feed acidity it was ‘solvation’ mechanism which predominated. In C4mimPF6, initially solvation mechanism was found to be operative where as existance of anion exchange mechanism was reported at higher feed acidity [38]. In recent days, several studies have been reported on extraction and complexation of uranium in ionic liquid medium [39–41].
Based on the above background, a comparative evaluation was carried out on the extraction of uranyl ion using bis(2,4,4-trimethylpentyl)phosphinic acid in C8mimNTf2 and xylene. It included the understanding of extraction mechanism, speciation, kinetics, selectivity, stripping and radiolytic stability. Finally the suitability of the solvent system was demonstrated by processing the simulate high level waste (SHLW) solution of pressurized heavy water reactor (PHWR) origin.
Experimental
Instrument and operating conditions
The analysis was carried out using inductively coupled plasma atomic emission spectroscopy (ICP-AES) with capacitively coupled device (CCD) as detector system. Operating conditions and instrumental specifications are listed in Supplementary Table 1.
Reagents and standard solutions
Standard solutions for all the elements were prepared from CertiPUR® ICP standard reference material solution of individual elements (E-Merck, Darmstadt, Germany) by proper dilution. Supra pure HNO3 (E-Merck, Darmstadt, Germany) and quartz double distilled water were used throughout the study. Multi-point standardization was carried out using standard in the range of 0.05–500 mg/L for each analytical line after proper peak search, auto attenuation etc. For the analyses of each sample 5 replicated measurements were carried.
Uranyl stock solution was prepared by dissolving spec pure U3O8 in concentrated HNO3. Xylene was procured Prabhat Chemicals, Gujrat Mumbai, whereas oxalic acid and Na2CO3 were produced from Thomas Baker Chemical limited and Qualigens fine Chemicals, Mumbai, India, respectively. Bis(2,4,4-trimethylpentyl)phosphinic acid (L) was procured from Anhui Jinao Chemical Co., Ltd., China while xylene was procured from A.B.Enterprise, Mumbai, India C8mimNTf2 has been procured from Global Nanotech, India with purity more than 99 % and was used for extraction process without further purification. bis(trifluoromethane)sulfanilamide lithium salt (LiNTf2) has been procured from Aldrich Chemistry, USA. The structures of bis(2,4,4-trimethylpentyl)phosphinic acid and C8mimNTf2 have been shown in Fig. 1.
Method
All the solvent extraction experiment was carried out using aqueous phase and organic phase in the ratio 1:1 respectively. Then it was shaken for 2 h at room temperature and allowed to achieve complete equilibration. Then it was allowed to settle for 10 min followed by centrifugation around 300 s and suitable aliquots were taken for subsequent study. Since in most cases, two-phase system is of analytical interest, an organic solvent and aqueous are involved, D can be expressed as
The subscripts org and aq refer to the organic and aqueous phases respectively. The separation factor was evaluated as
All the experiments were carried out in five replicates and the average of these replicates was reported to coat any value. The error associated with any value was shown as error bar in most of the figures (in Figs. 8, 9, data were presented with an error of less than 5 %). In Table the data were presented in the format below
The 3σ was chosen to report the true value to exsit in the range with 99.8 % confidence level. Similar approach was also observed in the literature for the presentation of the data point in the Figures and Tables [42–45]. In these above literatures a detailed study was reported on the sorption behaviour of Eu3+, arsenate and Cu using technologically important muliwalled carbon nano tube or graphene oxide with appropriate modifications.
Results and discussion
More surface physical nature such as porosity characteristics (surface area, pore volume and pore diameter) and surface chemical nature such as acidic sites and basic sites (Boehm titration) of the materials are very useful to explain experimental results and possible mechanisms in case of solid–liquid equilibrium while in the present case where liquid–liquid equilibrium is concerned, a very simple strategy is followed in understanding the extraction mechanism and the species involved in separation.
Extraction profile of uranium in xylene and ionic liquid: extraction mechanism
The distribution ratio values of uranyl ion were varied as a function of feed nitric acid concentration and the patterns were found to be entirely different, i.e. for ionic liquid based systems the D U values were found to decrease with increase in feed acidity while for xylene based solvent system, the D U values initially decreased to a minimum value at 0.5 M HNO3 followed by gradual increase and a plateau (Fig. 2). These facts revealed that for ionic liquid based solvent system, ‘ion exchange’ mechanism predominated [46–50] whereas in case of xylene based system, though ‘ion exchange’ mechanism predominated initially, beyond 0.5 M HNO3, ‘solvation mechanism’ predominated [25, 49]. If the reaction proceeded through cation exchange mechanism, then the cationic extracted species could be exchanged with the cations of ionic liquid i.e. C8mim+ as shown by Eq. 1; if the overall extracted species was anionic then NTf −2 ion from ionic liquid phase came to the aqueous phase for maintaining the overall charge neutrality as expressed in Eq. 2. In solvation mechanism, the neutral species was to get extracted (Eq. 3).
where ‘aq’ and ‘IL’ referred to the aqueous and ionic liquid phase, ‘m’ and ‘n’ referred to the number of nitrate ion and ligand molecules attached to the thorium atom. At higher feed acidity, H+ ion also competed with the UO2 2+ ion which led to decrease in D U value in ‘cation exchange’ mechanism. As shown in Eq. 3, NO −3 ion was required for the formation of neutral species in ‘solvation mechanism’, and was the main reason for increase in D U value with feed acidity, the plateau beyond that was the fate of competition of H+ with UO2 2+. The acid profiles only gave the indication about the predominate mechanism while to ascertain it through the species involved, variation in NO −3 , NTf −2 , C8mim+ etc. in the aqueous phase were required to investigate.
Effect of ligand variation on D U: determination of metal–ligand stoichiometry
The D U values were found to increase with increase in bis(2,4,4-trimethylpentyl)phosphinic acid concentration indicating the direct participation of the ligand in the extracted species. If the equilibrium constants of the above equations were considered as k ex, then it can be expressed as
For cation exchange mechanism
For anion exchange mechanism
For solvation mechanism
If the free ligand concentration was varied at a particular feed acidity and at particular temperature then equations could be simplified as below, where \(k_{\text{ex}}^{{\prime }}\) is conditional extraction constant. It was assumed that at a particular temperature the partition coefficients for C8mim+ and NTf −2 were constants.
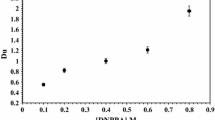
So a plot of log D U versus the logarithm of the concentration of ligand should resulted straight line (Fig. 3) with slope ‘n’ i.e. the number of ligand molecules attached with each UO2 2+ ion. The present investigation revealed the formation of 1:2 metal ligand stoichiometry in both molecular diluents and ionic liquid (the extraction was carried out from 3 M HNO3 and in ionic liquid ion exchange predominated while in xylene solvation predominated). The conditional extraction constant (\(k_{\text{ex}}^{{\prime }}\)) and the Gibb’s energy change (∆G, Eq. 9) [46, 48] during extraction were summarized in Table 1, revealing the spontaneity of the extraction process. The extraction of uranyl ion in ionic liquid was found to be thermodynamically more favored than that in xylene.
The metal ligand stoichiometry was again evaluated for the xylene system in 0.1 M HNO3 feed acidity, where ‘ion exchange’ mechanism was found to be predominating and it was also found to be 1:2 (Supplementary Fig. 1).
Effect of nitrate variation on extraction of uranium
To ascertain the nature of the species involved in extraction it was required to know the participation of NO −3 in the extracted species which could also throw some light on the extraction mechanism. Figure 4 depicted the variation of D U values as a function of NO −3 concentration in aqueous phase (at a particular ligand concentration, Eqs. 4, 5, 6 could be simplified as Eq. 10). In both the case, the NO −3 concentration in aqueous phase was found to favour the complexation, revealing the presence of NO −3 in the extracted species. But in ionic liquid based separation one NO −3 was found to be associated with single uranyl ion whereas presence of two NO −3 in the extracted uranyl complex was observed for xylene based separation (Supplementary Table 2). This fact also suggested that the overall extracted species was neutral for xylene based system while singly charged cation for ionic liquid based system provided there was no participation of other ion in the extracted species.
Effect of C8mim+ ion variation in aqueous phase on extraction of uranium
If the extraction proceeded via Eq. 1, i.e. C8mim+ would come to the aqueous phase from the organic phase in place of uranium complex, then the presence of C8mim+ in aqueous phase would lead to move the equilibrium in the backward direction i.e. the D U value should decrease. Otherwise, i.e. if the reaction proceeded through Eqs. 2 or 3 no such effect would be expected on the D U values. In view of these D U values were varied as a function of C8mim+ ion in the aqueous phase (in the form of C8mimBr), which revealed that one C8mim+ got exchanged by per uranium extracted species (slope = −1), i.e. the overall charge of the extracted species was one positive ([UO2(NO3)·2L]+) for ionic liquid based system. For xylene case, the D U value was found to be insensitive towards the variation of C8mim+ concentration in the aqueous phase, which indicated probably the complex getting extracted was [UO2(NO3)2·2L]. Figure 5 showed the variation of logD U versus log[C8mim+] in the aqueous phase.
To ascertain the cation exchange nature of the extraction system in case of ionic liquid based system, effect of NTf −2 anion in the aqueous phase (in the form of aqueous soluble salt, LiNTf2), on the extraction efficiency of U by bis(2,4,4-trimethylpentyl)phosphinic acid was investigated (Supplementary Fig. 2). The results revealed that there was no participation of NTf −2 anion in the extracted species.
Photoluminescence investigation of the extracted uranyl-bis(2,4,4-trimethylpentyl)phosphinic acid ligand complex
Photoluminescence study was routinely used for probing the local environment around metal ion in the complex [51–54]. Uranyl complexes in room-temperature ionic liquids and molecular diluents were investigated through luminescence spectroscopy to understand the nature of the complex in a detail manner. Figure 6 showed the emission profiles of the bare uranyl ion in HNO3 and uranyl complex in xylene and C8mimNTf2 whereas the decay profiles were shown in Supplementary Fig. 3. This investigation revealed that the nature of the extracted species in xylene and C8mimNTf2 was entirely different and both the extracted species were found differ from the bare uranyl ion. The mono exponential nature of the decay profile revealed the existence of single species in organic phase while the lifetime of the complex in ionic liquid and xylene was found to be different (τ xylene = 221 µs and τ ionic liquid = 346 µs) which again suggesting the different nature of the complex. In case of uranyl ion, the difference between two nearby humps in the emission spectra was a measure of the symmetric stretching frequency of O=U=O moiety. On complexation if there was any perturbation in the U–O stretching, that would be reflected in the luminescence spectra of uranyl ion. The symmetric stretching frequency for U in 3 M HNO3 was found to be 822.44 cm−1 which was similar with that of UO3, xH2O complex (805 cm−1) [54]. On complexation with bis(2,4,4-trimethylpentyl)phosphinic acid, the starching frequency of O=U=O bond decreased to 627.71 cm−1 in ionic liquid while it further decreased in xylene medium (330 cm−1). This fact suggested that on the approach of ligand, the U–O bond strength decreased and this bond became weaker might be due to steric effect. The data also revealed that in xylene, the ligand molecules came closer to the uranyl moieties than in ionic liquid which led to the weakening of U–O bond and was reflected in the emission profile. The ground state frequency of symmetric stretching vibration of UO2 2+ in C10mimBr and C4mimNTf2 were reported to be 750 cm−1 [55] and 825 cm−1, respectively [56]. The uranyl-TOPO complex in ionic liquid and molecular diluents was also reported to have different stretching frequency [57].
Extraction kinetic
Time required to attain the equilibrium distribution ratio value was one of the important aspect for any separation procedure. In the present investigation, the D U value was found to increase gradually up to 15 min followed by a plateau at D U = 3.8 in xylene based separation system whereas for ionic liquid based separation the D U value was found to increase gradually with the time of equilibration up to 60 min followed by a plateau at D U = 5. Comparatively slower kinetics was observed for ionic liquid based solvent system which can be attributed to the higher viscosity coefficient the ionic liquid compared to the molecular diluents, xylene. Figure 7 presented the extraction kinetic behaviour of uranyl using bis(2,4,4-trimethylpentyl)phosphinic acid into C8mimNTf2 and xylene. Similar slower kinetics was also observed for the extraction of Am3+ by diglycolamide functionalized ligands in ionic liquid [47, 48] and even for the extraction of Sr2+ by substituted crown ether in different ionic liquid [49].
Stripping of UO2 2+ from organic phase
The stripping behavior of uranyl ion from ionic liquid and molecular diluents was investigated thoroughly. Lowering of aqueous phase acidity was not at all successful as the D U value at lower acidity was also found to be very high. Therefore, aqueous phase complexation was found to be necessary for the quantitative back extraction of uranium from organic phase. 0.05 M oxalic acid, Na2CO3 and EDTA solutions were used for the present investigation. It was found that from ionic liquid phase only ~78, 97 and 40 % uranium can be back extracted by oxalic acid, Na2CO3 and EDTA in a single step with a phase ratio of 1, whereas from xylene they were ~85, 99.5 and 65 %, respectively (Fig. 8). Na2CO3 was found to be the most effective strippant. Similar observation was also seen for stripping of hexavalent actinides from diglycolamide functionalized ionic liquid phase [58, 59]. The investigation was further extended for quantitative stripping of uranium (more than 99.9 %) and it was found that, two contacts of Na2CO3 was found to be successful for ionic liquid as well as molecular diluents based systems while using oxalic acid as strippant, five contacts were required for ionic liquid based system and four for xylene. Even after employing five contacts of EDTA, 40 and 99 % of uranium can be back extracted from C8mimNTf2 and xylene, respectively using EDTA solution. % of U back extracted after each contact of the strippant was shown in Supplementary Fig. 4.
Radiolytic stability of the solvent system
One of the criteria for choosing the solvent system for the processing of radioactive waste solution is the radiolytic stability. The solvent systems had to expose continuous radioactive exposure during processing of radio toxic elements. For the present study the bis(2,4,4-trimethylpentyl)phosphinic acid in ionic liquid and in xylene were exposed to gamma irradiation up to 1500 kGy and using the irradiated solvent system the efficiency for the extraction of U was monitored and was found to decrease gradually. It was observed that after 500 kGy the Du became 92 and 81 % of their original values for C8mimNTf2 and xylene, respectively while after 1000 kGy they came down to 80 and 54 %, respectively. The ionic liquid based solvent system was found to be more radio resistant than the molecular diluents based system. After an exposure of 1500 kGy, the the D U values became 70 and 30 % of the original for C8mimNTf2 and xylene, respectively. Figure 9 summarized the radiolytic stability of bis(2,4,4-trimethylpentyl)phosphinic acid-C8mimNTf2 and bis(2,4,4-trimethylpentyl)phosphinic acid -xylene systems after exposure of different amount of gamma dose.
Application of solvent system for processing of SHLW of PHWR origin
The success of a developed solvent system lies in processing the nuclear waste solution. In view of this bis(2,4,4-trimethylpentyl)phosphinic acid in xylene and C8mimNTf2 was applied for processing of simulated high level waste (SHLW) solution of pressurized heavy water reactor (PHWR) origin. Both the systems were found to extract Y, Zr and Ru from the aqueous phase partially i.e. more than 10 % but less than 50 % while such partial extraction was observed for La, Ce, Pr, Nd and Sm in case of molecular diluents based system. Sr, Mo, Pd, Ba, Cr, Mn, Fe, Ni and Na were found to be almost unextracted by both the solvent systems with separation factor more than 100 compared to uranium. Bis(2,4,4-trimethylpentyl)phosphinic acid in C8mimNTf2 was found to be more selective for U than that in xylene. Table 2 summarized the analytical results obtained by feeding the raffinate into the plasma after extraction of SHLW with bis(2,4,4-trimethylpentyl)phosphinic acid in xylene and C8mimNTf2 alonwith the distribution ratio values and separation factors of these metal ions.
Conclusions
A systematic study was carried out to evaluate bis(2,4,4-trimethylpentyl)phosphinic acid in C8mimNTf2 and xylene for the extraction of uranium. ‘Cation exchange’ mechanism was found to predominate for ionic liquid based system while for xylene though initially ion exchange mechanism was found to be operative, salvation mechanism predominates at higher feed acidity. The metal ligand stoichiometry was evaluated as 1:2 with participation of two NO −3 for xylene and one for C8mimNTf2. Luminescence investigation revealed that the nature of the extracted species in ionic liquid and in molecular diluents was not same. The mono exponential nature of the decay profile revealed the presence of single species in the extracted complex. The extraction in ionic liquid was found to follow slower kinetics compared to molecular diluents system can be attributed to the viscosity effect. 0.05 M Na2CO3 was found to be suitable for the almost quantitative stripping of uranium from the organic phase. Ionic liquid based solvent system was found to be more radio resistance than that of molecular diluents based system. Y, Zr and Ru were found to be co extracted by these solvent systems while processing SHLW of PHWR origin.
References
Quach DL, Mincher BJ, Wai CM (2014) Supercritical fluid extraction and separation of uranium from other actinides. J Hazard Mater 274:360–366
Ansari SA, Mohapatra PK, Manchanda VK (2009) A novel malonamide grafted polystyrene-divinyl benzene resin for extraction, pre-concentration and separation of actinides. J Hazard Mater 161:1323–1329
Mahanty BN, Raut DR, Mohapatra PK, Das DK, Behere PG, Afzal Md (2014) Comparative evaluation of actinide ion uptake by polymer inclusion membranes containing TODGA as the carrier extractant. J Hazard Mater 275:146–153
Raju ChSK, Subramanian MS (2007) Sequential separation of lanthanides, thorium and uranium using novel solid phase extraction method from high acidic nuclear wastes. J Hazard Mater 145:315–322
Bhattacharyya A, Mohapatra PK, Gadly T, Raut DR, Ghosh SK, Manchanda VK (2011) Liquid–liquid extraction and flat sheet supported liquid membrane studies on Am(III) and Eu(III) separation using 2,6-bis(5,6-dipropyl-1,2,4-triazin-3-yl)pyridine as the extractant. J Hazard Mater 195:238–244
Yantasee W, Fryxell GE, Addleman RS, Wiacek RJ, Koonsiripaiboon V, Pattamakomsan K, Sukwarotwat V, Xu J, Raymond KN (2009) Selective removal of lanthanides from natural waters, acidic streams and dialysate. J Hazard Mater 168:1233–1238
Ansari SA, Pathak PN, Manchanda VK (2005) N,N,N′,N′-Tetraoctyl diglycolamide (TODGA): a promising extractant for actinide-partitioning from high-level waste (HLW). Solv Extr Ion Exch 23:463–479
Rout A, Venkatesan KA, Srinivasan TG, Rao PRV (2012) Liquid–liquid extraction of Pu(IV), U(VI) and Am(III) using malonamide in room temperature ionic liquid as diluent. J Hazard Mater 221–222:62–67
Gao S, Sun T, Chen Q, Shen X (2013) Improvement of the cloud point extraction of uranyl ions by the addition of ionic liquids. J Hazard Mater 263:562–568
Zeng X, Li J (2014) Innovative application of ionic liquid to separate Al and cathode materials from spent high-power lithium-ion batteries. J Hazard Mater 271:50–56
Zhu P, Chen Y, Wang LY, Qian GY, Zhou M, Zhou J (2012) A new technology for separation and recovery of materials from waste printed circuit boards by dissolving bromine epoxy resins using ionic liquid. J Hazard Mater 239–240:270–278
Escudero LB, Wuilloud RG, Olsina RA (2013) Sensitive determination of thallium species in drinking and natural water by ionic liquid-assisted ion-pairing liquid–liquid microextraction and inductively coupled plasma mass spectrometry. J Hazard Mater 244–245:380–386
Sun X, Ji Y, Zhang L, Chen J, Li D (2010) Separation of cobalt and nickel using inner synergistic extraction from bifunctional ionic liquid extractant (Bif-ILE). J Hazard Mater 182:447–452
Ge HL, Liu SS, Su BX, Qin LT (2014) Predicting synergistic toxicity of heavy metals and ionic liquids on photobacterium. J Hazard Mater 268:77–83
Molaakbari E, Mostafavi A, Afzali D (2011) Ionic liquid ultrasound assisted dispersive liquid–liquid microextraction method for preconcentration of trace amounts of rhodium prior to flame atomic absorption spectrometry determination. J Hazard Mater 185:647–652
Kalidhasan S, Santhana Krishna Kumar A, Rajesh V, Rajesh N (2012) An efficient ultrasound assisted approach for the impregnation of room temperature ionic liquid onto Dowex 1 × 8 resin matrix and its application toward the enhanced adsorption of chromium (VI). J Hazard Mater 213–214:249–257
Billard I, Ouadi A, Gaillard C (2011) Liquid–liquid extraction of actinides, lanthanides, and fission products by use of ionic liquids: from discovery to understanding. Anal Bioanal Chem 400:1555–1566
Binnemans K (2007) Lanthanides and actinides in ionic liquids. Chem Rev 107(6):2592–2614
Cocalia VA, Gutowski KE, Rogers RD (2006) The coordination chemistry of actinides in ionic liquids: a review of experiment and simulation. Coord Chem Rev 250:755–764
Mudring AV, Tang S (2010) Ionic liquids for lanthanide and actinide chemistry. Eur J Inorg Chem 18:2569–2581
Sun X, Luo H, Dai S (2011) Ionic liquids-based extraction: a promising strategy for the advanced nuclear fuel cycle. Chem Rev 112:2100–2128
Vasudeva Rao PR, Venkatesan KA, Rout A, Srinivasan TG, Nagarajan K (2012) Potential applications of room temperature ionic liquids for fission products and actinide. Sep Sci Technol 47:204–222
Dietz ML (2006) Ionic liquids as extraction solvents: where do we stand? Sep Sci Technol 41:2047–2063
Nakashima K, Kubota F, Maruyama T, Goto M (2005) Feasibility of ionic liquids as alternative separation media for industrial solvent extraction processes. Ind Eng Chem Res 44:4368–4372
Cocalia VA, Jensen MP, Holbrey JD, Spear SK, Stepinski DC, Rogers RD (2005) Identical extraction behavior and coordination of trivalent or hexavalent f-element cations using ionic liquid and molecular solvents. Dalton Trans 11:1966–1971
Sheng G, Alsaedi A, Shammakh W, Monaquel S, Sheng J, Wang X, Li H, Huang Y (2016) Enhanced sequestration of selenite in water by nanoscale zero valent iron immobilization on carbon nanotubes by a combined batch, xps and xafs investigation. Carbon 99:123–130
Sheng GD, Yang ST, Li YM, Gao X, Huang YY, Hu J, Wang XK (2014) Retention mechanisms and microstructure of Eu(III) on manganese dioxide studied by batch and high resolution EXAFS technique. Radiochim Acta 102:155–167
Sheng G, Yang Q, Peng F, Li H, Gao X, Huang Y (2014) Determination of colloidal pyrolusite, Eu(III) and humic substance interaction: a combined batch and EXAFS approach. Chem Eng J 245:10–16
Sheng G, Ye L, Li Y, Dong H, Li H, Gao X, Huang Y (2014) EXAFS study of the interfacial interaction of nickel(II) on titanate nanotubes: role of contact time, pH and humic substances. Chem Eng J 248:71–78
Sheng G, Shen R, Dong H, Li Y (2013) Colloidal diatomite, radionickel and humic substance interaction: a combined batch, XPS and EXAFS investigation. Environ Sci Pollut Res 20:3708–3717
Dietz ML, Dzielawa JA, Laszak I, Young BA, Jensen MP (2003) Influence of solvent structural variations on the mechanism of facilitated ion transfer into room-temperature ionic liquids. Green Chem 5:682–685
Sengupta A, Mohapatra PK (2012) Extraction of radiostrontium from nuclear waste solution using crown ethers in room temperature ionic liquids. Supramol Chem 24:771–778
Dietz ML, Stepinski DC (2005) A ternary mechanism for the facilitated transfer of metal ions into room-temperature ionic liquids (RTILs): implications for the “greenness” of RTILs as extraction solvents. Green Chem 7:747–750
Giridhar P, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2005) Extraction of uranium(VI) from nitric acid medium by 1.1 M tri-n-butylphosphate in ionic liquid diluent. J Radioanal Nucl Chem 265:31–38
Giridhar P, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2004) Effect of alkyl group in 1-alkyl-3-methylimidazolium hexafluorophosphate ionic liquids on the extraction of uranium by tri-n-butylphosphate diluted in various ionic liquids. J Nucl Radiochem Sci 5:21–26
Giridhar P, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2008) Extraction of Uranium (VI) by 1.1 M Tri-n-butylphosphate/ionic liquid and the feasibility of recovery by direct electrodeposition from organic phase. J Alloys Compd 448:104–108
Dietz ML, Stepinski DC (2008) Anion concentration-dependent partitioning mechanism in the extraction of uranium into room-temperature ionic liquids. Talanta 75:598–603
Billard I, Ouadi A, Jobin E, Champion J, Gaillard C, Georg S (2011) Understanding the extraction mechanism in ionic liquids: HNO3/TBP/C4mimNTf2 as a case study. Solvent Extr Ion Exch 29:577–601
Zhang Y, Liu Z, Fan F, Zhu L, Shen Y (2014) Extraction of uranium and thorium from nitric acid solution by todga in ionic liquids. Sep Sci Technol 49:1895–1902
Shen Y, Wu J, Liu Z, Wu W (2015) Environmentally friendlier approach to nuclear industry: recovery of uranium from carbonate solutions using ionic liquids. Ind Eng Chem Res 54:8624–8628
Yaprak D, Spielberg ET, Cker TB, Richter M, MallickB Klein A, Mudring AV (2014) A roadmap to uranium ionic liquids: anti-crystal engineering. Chem Eur J 20:6482–6493
Sheng G, Li Y, Dong H, Shao D (2012) Environmental condition effects on radionuclide 64Cu(II) sequestration to a novel composite: polyaniline grafted multiwalled carbon nanotubes. J Radioanal Nucl Chem 293:797–806
Li Y, Sheng G, Sheng J (2014) Magnetite decorated graphene oxide for the highly efficient immobilization of Eu(III) from aqueous solution, J Mol. Liquids 199:474–480
Sheng G, Li Y, Yang X, Ren X, Yang S, Hub J, Wang X (2012) Efficient removal of arsenate by versatile magnetic graphene oxide composites. RSC Adv 2:12400–12407
Li J, Chen S, Sheng G, Hu J, Tan X, Wang X (2011) Effect of surfactants on Pb(II) adsorption from aqueous solutions using oxidized multiwall carbon nanotubes. Chem Eng J 166:551–558
Shen Y, Tan X, Wang L, Wu W (2011) Extraction of the uranyl ion from the aqueous phase into an ionic liquid by diglycolamide. Sep Purif Technol 78:298–302
Mohapatra PK, Sengupta A, Iqbal M, Huskens J, Verboom W (2013) Diglycolamide-functionalized calix[4]arenes showing unusual complexation of actinide ions in room temperature ionic liquids: role of ligand structure, radiolytic stability, emission spectroscopy, and thermodynamic studies. Inorg Chem 52(5):2533–2541
Sengupta A, Mohapatra PK, Iqbal M, Huskens J, Verboom W (2012) A highly efficient solvent system containing functionalized diglycolamides and an ionic liquid for americium recovery from radioactive wastes. Dalton Trans 41(23):6970–6979
Sengupta A, Mohapatra PK, Iqbal M, Verboom W, Huskens J, Godbole SV (2012) Extraction of Am(III) using novel solvent systems containing a tripodal diglycolamide ligand in room temperature ionic liquids: a ‘green’ approach for radioactive waste processing. RSc Adv 2:7492–7500
Sengupta A, Mohapatra PK, Iqbal M, Huskens J, Verboom W (2013) Role of organic diluent on actinide ion extraction using a both-side diglycolamide-functionalized calix[4]arene. Supramol Chem 25(9–11):688–695
Sengupta A, Godbole SV, Mohapatra PK, Iqbal M, Huskens J, Verboom W (2014) Judd-Ofelt parameters of diglycolamide-functionalized calix[4]arene Eu3+ complexes in room temperature ionic liquid for structural analysis: effects of solvents and ligand stereochemistry. J Lumin 148:174–180
Sengupta A, Wu L, Feng W, Yuan L, Natarajan V (2015) Luminescence investigation on Eu—Pillar[5]arene-based diglycolamide (DGA) complexes: nature of the complex, Judd—Ofelt calculations and effect of ligand structure. J Lumin 158:356–364
Sengupta A, Fang Y, Yuan X, Yuan L (2015) Probing of the local environment and calculation of J.O. parameters for Eu3+ CMPO functionalized pillararene complexes by time resolved fluorescence spectroscopy. J Lumin 166:187–194
Sengupta A, Mohapatra PK, Iqbal M, Huskens J, Verboom W (2014) Spectroscopic investigation of Eu3+—complexes with ligands containing multiple diglycolamide pendant arms in a room temperature ionic liquid. J Lumin 154:392–401
Azenha MEDG, Miguel MDG, Formisinho SJ, Burrows HD (2001) The characterisation by luminescence spectroscopy of uranium(VI) incorporated into zeolites and aluminas. J Mol Struct 563–564:439–442
Sengupta A, Murali MS, Mohapatra PK (2012) Role of alkyl substituent in room temperature ionic liquid on the electrochemical behavior of uranium ion and its local environment. J Radioanal Nucl Chem. doi:10.1007/s10967-012-2334-5
Nockemann P, Servaes K, Deun RV, Hecke KV, Meervelt LV, Binnemann K, Walrand C (2007) Speciation of uranyl complexes in ionic liquids by optical spectroscopy. Inorg Chem 46:11335–11344
Mohapatra PK, Raut DR, Sengupta A (2014) Extraction of uranyl ion from nitric acid medium using solvent containing TOPO and its mixture with D2EHPA in room temperature ionic liquids. Sep Purif Technol 133:69–75
Sengupta A, Mohapatra PK, Kadam RM, Manna D, Ghanty TK, Iqbal M, Huskens J, Verboom W (2014) Diglycolamide-functionalized task specific ionic liquids for nuclear waste remediation: extraction, luminescence, theoretical and EPR investigations. RSC Adv 4:46613–46623
Acknowledgments
The authors wish to acknowledge Dr. P.K. Pujari, Head, Radiochemistry Division and Dr. P.K. Mohapatra, Head, Actinide Chemistry Section, Radiochemistry Division from Bhabha Atomic Research Centre, Mumbai, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, M., Sengupta, A., Murali, M.S. et al. Selective separation of uranium from nuclear waste solution by bis(2,4,4-trimethylpentyl)phosphinic acid in ionic liquid and molecular diluents: a comparative study. J Radioanal Nucl Chem 309, 1199–1208 (2016). https://doi.org/10.1007/s10967-016-4691-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4691-y