Abstract
The sorption–desorption of Eu(III) on attapulgite was studied using batch technique. The sorption of Eu(III) on attapulgite was strongly dependent on pH and ionic strength at low pH, and independent of ionic strength at high pH. The interaction was dominated by outer-sphere surface complexation/ion exchange at low pH, and by inner-sphere surface complexation at high pH. The irreversible sorption–desorption curves also suggested the formation of strong surface complexes. The high sorption capacity of attapulgite suggested that the attapulgite is a suitable material for the efficient elimination of Eu(III) from aqueous solutions in nuclear wastewater management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of nuclear energy, large amounts of radioactive wastes are produced and the long-lived radionuclides are inevitably released into the natural environment. Also in the processes of uranium mining and spent fuel reprocessing, large volumes of wastewater containing different kinds of radionuclides are released into the natural environment. Although the radioactive wastewater is pretreated to eliminate most radionuclides before it is discharged into the environment, the long-lived radionuclides at trace level concentrations cannot be removed from the solution completely. The radionuclides in the environment can be accumulated through the food chain and at last are deposited in human body, which is dangerous to human health. Thereby, it is crucial to eliminate the radionuclides from the radioactive wastewater as much as possible [1–4]. Among the long-lived trivalent lanthanides and actinides, Eu(III) is usually considered as a homologue for the trivalent lanthanides and actinides because the physicochemical properties of Eu(III) are very similar with those of other trivalent lanthanides and actinides [5, 6]. This is attributed to the similar ionic radius of Eu(III) to those of trivalent actinides and lanthanides. For most actinides, such as Np, Pu, Cm, Am, it is very difficult to carry out the research works on these radionuclides because of the licence of some laboratories. Thereby, the research on the interaction of 152+154Eu(III) with different kinds of oxides, clay minerals or manmade nanomaterials is important to understand the physicochemical properties of other trivalent lanthanides or actinides in the environment. The sorption of 152+154Eu(III) on clay minerals, metal oxides and nanomaterials had been extensively studied in the last decade [7–17], and the results suggested that the sorption of Eu(III) was strongly dependent on ionic strength and pH at low pH values. The sorption was mainly dominated by outer-sphere surface complexation and/or ion exchange at low pH, and by inner-sphere surface complexation and/or surface (co)precipitation at high pH values. Although the sorption of 152+154Eu(III) on clay minerals has been investigated intensively, the desorption property of adsorbed 152+154Eu(III) from clay minerals or oxides is still scarce [18, 19]. Wang et al. studied the kinetic desorption of Eu(III) from humic acid-bound alumina [18] and bentonite [19] by using chelating resin, and found that the Eu(III) ions were adsorbed on the reversible and irreversible sites, which were dependent on pH values, i.e., the surface adsorbed Eu(III) ions could transfer from the reversible sites to irreversible sites with increasing pH values. After the sorption of 152+154Eu(III) on clay minerals, the understanding of the desorption property is crucial to evaluate the secondary pollution of 152+154Eu(III). If the surface adsorbed 152+154Eu(III) cannot be desorbed from clay minerals after the environmental condition changes, then the transformation or the accumulation of 152+154Eu(III) in the environment is negligible. Thereby, it is interesting to study the sorption–desorption behavior of 152+154Eu(III) on clay minerals.
Attapulgite, a hydrated magnesium aluminium silicate, is kind of natural fibrillar mineral present in nature. Generally, it has three kinds of water at room temperature, i.e., the free adsorbed water by physical effect, the weakly bound zeolite water in the micro-channel, and the tightly bound crystalline water that completes the coordination of the (Mg, Al) cations at the borders of each octahedral layer. Besides the three kinds of water, attapulgite also contains structural hydroxyl groups (such as Al–OH and Mg–OH) [20]. There are also many kinds of oxygen-containing functional groups such as Fe–OH, Al–OH, Si–OH, –OH groups on attapulgite surfaces, which can form strong surface complexes with metal ions and thereby can remove heavy metal ions from aqueous solutions efficiently. The sorption of metal ions on attapulgite has been investigated by batch method and spectroscopy techniques, and the results showed that the metal ions can form strong complexes on attapulgite surfaces and can also enter into the interlayer of attapulgite. Fan et al. [20, 21] studied Ni(II) and 152+154Eu(III) sorption on humic acid-bound attapulgte and found that humic acid could act as a “bridge” between metal ions and attapulgite. The presence of humic acid enhanced 152+154Eu(III) and Ni(II) sorption at low pH, but reduced 152+154Eu(III) and Ni(II) sorption at high pH values. The enhanced sorption at low pH was attributed to the formation of strong surface complexes of 152+154Eu(III) and Ni(II) with surface adsorbed humic acid, whereas the reduced sorption at high pH was attributed to the formation of soluble complexes of metal ions with free humic acid molecules in solution [22, 23]. However, the desorption of Eu(III) from attapulgite is still not reported.
In this work, we characterized the attapulgite using SEM, FTIR, XRD and acid–base titrations to understand the surface functional groups, surface properties and structure properties of attapulgite. Then the attapulgite was used as adsorbent to remove 152+154Eu(III) from aqueous solutions to evaluate the sorption properties of 152+154Eu(III) on attapulgite. The interaction mechanism of 152+154Eu(III) with attapulgite was discussed based on the results.
Experimental
Material
All chemicals used in the experiments were analytic purity and used without any purification. The attapulgite sample was achieved from Kaixi Co. (Gansu Province, China). The raw attapulgite sample was treated with 0.5 mol/L hydrochloric acid for 24 h, immersed into 3.3 mol/L NaCl solution for 60 h; and then rinsed with distilled water until no chloride in supernatant was detected by using 0.01 mol/L AgNO3. The sample was then dried at 105 °C for 2 h to eliminate the free adsorbed water, and used in the experiments.
Eu(III) stock solution was prepared from Eu2O3 by dissolution, evaporation and redissolution in 10−3 mol/L perchloric acid. The radiotracer 152+154Eu(III) was used in the sorption experiments. The 152+154Eu(III) concentration in supernatant was analyzed by liquid scintillation counting using Packard 3100 TR/AB Liquid Scintillation analyzer (PerkinElmer) with ULTIMA GOLD AB™ (Packard) Scintillation cocktail.
Sorption experiments
The sorption of radionuclide 152+154Eu(III) on attapulgite was carried out at 25 ± 1 °C in 0.01 mol/L NaClO4 solutions using batch technique in polyethylene tubes. The attapulgite aqueous suspension was mixed with NaClO4 solution firstly to achieve the interaction equilibrium of Na+ with attapulgite, and then the 152+154Eu(III) solution was added into the attapulgite suspension to start the sorption of 152+154Eu(III) on attapulgite. The pH values of the suspension were adjusted with 0.01 mol/L HClO4 or NaOH. The blank experimental results indicated that Eu(III) sorption on the tube walls was negligible. The polyethylene tubes were shaken for 2 days to attain the sorption equilibrium, and then the solid was separated from the liquid phase by centrifugation at 12,000 rpm for 30 min.
For the desorption experiments, after the sorption equilibrium, half volume of the supernatant was pipetted and the same volume solution containing 0.01 M NaClO4 with the same pH as the sorption experiments was added into the system, and then the mixtures were shaken and centrifuged at the same conditions as in the sorption experiments. Finally, the concentration of Eu(III) in the supernatant was measured and the q values were deduced from the difference. All experimental data were the average of determinations of triplicate samples and the error bars (<5 %) were provided.
Characterization
The surface properties of the attapulgite sample were analyzed by Fourier transformed infrared spectroscopy (FTIR). The sample pellet, prepared by mixing sample and KBR followed by pelletization, was mounted on a Bruker EQUINOX55 spectrometer (Nexus). The structure was characterized by X-ray diffraction (XRD), which was obtained from a D/Max-rB equipped with a rotation anode using Cu Kα radiation (λ = 0.15406 nm). The XRD device was operated at 40 kV and 80 mA in the range of 5° ≤ 2θ ≤ 70°.
Results and discussion
Characterization
Figure 1a shows the SEM image of attapulgite sample. It is clear that the attapulgite sample has a fibrous structure and some fibers form the straight parallel aggregates. The straight parallel aggregates can provide some binding sites for the uptake of Eu(III) on its surface.
The potentiometric acid–base titration of the attapulgite suspension (5.0 g/L) was carried out under argon conditions at 25 ± 1 °C with a Mettler-Toledo DL 50 titrator equipped with a combine electrode (glass electrode associated to a reference electrode Ag/AgCl/KCl 3 mol/L). Before the acid–base titration, the attapulgite suspension was titrated up to about pH 3 and purged with argon for 1 h, then the suspension was titrated from pH ~3 to pH ~10 with 0.047 mol/L NaOH. The acid–base titration curve could provide some information about the surface charge of attapulgite and the pH value of point of zero charge (pHpzc). At pH < pHpzc, the surface charge of attapulgite is positive, whereas the attapulgite is negatively charged at pH > pHpzc [24]. As can be seen from the acid–base titration curve (Fig. 1b), the attapulgite has the point of zero charge at pH ~6.0.
The FTIR spectrum is shown in Fig. 1c. The peaks at 3560 and 3620 cm−1 correspond to the stretching vibrations of Fe–OH and Al–OH groups, respectively. The peak at 3420 cm−1 is attributed to the bend vibration of zeolite water, which is also confirmed by the peak at 1660 cm−1. The peaks at 1020 and 474 cm−1 are attributed to the vibration of Si–O–Si bonds [25, 26]. These oxygen-containing functional groups are important to form surface complexes with metal ions on attapulgite surfaces, and thereby can form strong complexes with metal ions on attapulgite particles.
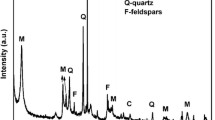
From the XRD pattern (Fig. 1d), one can see that the characteristic peaks (2θ = 8.4°, 19.7°, 27.5°, 34.6° and 42.5°) of attapuligite are present in the XRD pattern. The peak at 2θ = 8.34° has the interplanar distance of d = 1.06 nm, which is attributed to the basal plane of the attapulgite structure [27]. The minerals of montmorillonite and quartz are also found in the attapulgite sample and are marked in Fig. 1d. From the analysis of Fan [20, 28], the contents of different components are 6 % for quartz, 93 % for palygorskite (the main component of the attapulgite sample) and 1 % for montmorillonite.
Effect of contact time
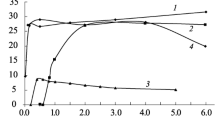
Figure 2 shows the effect of contact time on the sorption of Eu(III) to attapulgite. One can see that the sorption of Eu(III) to attapulgite increases quickly with increasing contact time up to 100 min, and then increases slowly with contact time. The quick sorption of Eu(III) to attapulgite suggests that the sorption of Eu(III) to attapulgite is mainly dominated by chemical sorption or surface complexation rather than physical adsorption [29]. In the following experiments, 48 h of contact time was applied to achieve the sorption equilibration of Eu(III) on attapulgite.
From Fig. 2, one can see that the sorption increases very slowly with increasing contact time after 100 min. This slow increase of sorption should not be attributed to the analytical uncertainty because the sorption increases very slowly with increasing contact time. In order to observe the increase of Eu(III) sorption more clearly, the sorption curve of Eu(III) on attapulgite at a function of contact time is redrawn (the inset figure in Fig. 2), one can see that the sorption increases very slowly with increasing contact time up to 100 min. At the first contact time, the sorption of Eu(III) to attapulgite takes place on the reactive sites of attapulgite which is a rapid uptake process. After the quick sorption of Eu(III) on the reactive sites, the sorption of Eu(III) then takes place on the binding sites which has relatively large activation energies, diffuses into the micropores of attapulgite [30, 31]. After the reactive sites are almost occupied by Eu(III) ions, the subsequent slower sorption of Eu(III) to the less reactive sites and then diffused into the micropore sites can take place on the less reactive sites and diffusion into the micropore sites [32]. This process is much slower as compared to the fast uptake at the initial contact time. Thereby, the sorption of Eu(III) on attapulgite increases very slowly after long contact time, and at last reaches the sorption equilibration.
Effect of ionic strength and pH
Figure 3 shows the sorption of Eu(III) from solution to attapulgite as a function of NaClO4 concentrations at pH = 4.10 ± 0.05 and pH 6.10 ± 0.05, respectively. The sorption percentage of Eu(III) on attapulgite can be defined as:
where C ini is the initial concentration of Eu(III) in the system, C eq is the final concentration of Eu(III) in supernatant after sorption equilibration and centrifugation. From Fig. 3, one can see that the sorption of Eu(III) at pH 4.10 decreases from ~85 to ~20 % with NaClO4 concentration increasing from 0.005 to 0.2 mol/L. With increasing NaClO4 concentration, the competition between Eu(III) and Na+ ions becomes more stronger, and this competition thereby decreases the sorption of Eu(III) on attapulgite if the sorption is mainly dominated by ion exchange or outer-sphere surface complexation. It is necessary to notice that the sorption of Eu(III) at pH 6.10 is independent of NaClO4 concentration under the experimental uncertainties, which suggests that the sorption is mainly dominated by inner-sphere surface complexation rather than outer-sphere surface complexation at high pH values.
The sorption of Eu(III) on attapulgite in 0.01 and 0.1 M NaClO4 solutions at different pH values are shown in Fig. 4. The sorption of Eu(III) on attapulgite is influenced by pH and NaClO4 concentrations obviously at pH <6. The sorption curves can be divided into three pH regions: (1) the Eu(III) sorption increases slowly with pH increasing at pH <3; (2) the sorption increases abruptly with increasing pH at 3 < pH < 6; and (3) the sorption of Eu(III) on attapulgite remains almost constant with increasing pH and does not change with pH increasing at pH >6. At pH <6, the sorption of Eu(III) on attapulgite is strongly dependent on ionic strength. Generally, the outer-sphere surface complexation or ion exchange is affected by ionic strength, whereas the inner-sphere surface complexation is independent of ionic strength. From the batch sorption curves, the interaction mechanism such as outer-sphere surface complexation or inner-sphere surface complexation can be distinguished in different NaClO4 solutions at different pH values. Herein, the strong pH- and ionic strength-dependent sorption at pH <6 suggest that the sorption of Eu(III) is mainly dominated by outer-sphere surface complexation. At pH >6, the sorption is mainly dominated by inner-sphere surface complexation. Fan et al. [28] studied Eu(III) sorption onto attapulgite in different KNO3 solutions, and found that the sorption was dependent on ionic strength at pH <5.5 and independent of pH at pH >5.5. Similar sorption behavior were also found for Eu(III) interaction with other kinds of materials such as carbon nanotube/iron oxide magnetic composites [33], graphene oxides [34, 35], montmorillonite [36] and alumina oxides [37–41].
From Fig. 4, one can also see that the sorption curve in 0.01 M NaClO4 solution shifts to lower pH values as compared to that in 0.1 M NaClO4 solution. In the sorption process, the Na+ ions are firstly equilibrated with attapulgite before the addition of Eu(III) ions. The Na+ ions can alter the surface properties of attapulgite and thereby can affect the sorption of Eu(III) on attapulgite. At high NaClO4 concentration, the cation ions in solution can also compete the interaction of Eu(III) ions on attapulgite surfaces, and thereby decreases Eu(III) sorption to attapulgite. The effect of ionic strength on Eu(III) sorption can be summarized as [42]: (1) the activity coefficients of Eu(III) ions are affected by NaClO4 concentrations, which limits Eu(III) transfer from solution to attapulgite surfaces; (2) the high Na+ concentration can affect the electrical double layer of attapulgite, and then affects the sorption of Eu(III) to attapulgite; and (3) at high NaClO4 concentration, the aggregation of attapulgite particles is aggravated, which reduces the available sites and functional groups for the binding of Eu(III) ions to attapulgite particles [43]. At low pH values, the sorption of Eu(III) to attapulgite can be considered as a competition of Eu(III) with Na+ ions at attapulgite particle surfaces. At pH >6, there is little difference in the sorption curves, which is attributed to the formation of inner-sphere surface complexes or surface (co)precipitates at high pH value [44, 45]. At high pH values, the concentration of Eu(III) adsorbed on attapulgite surface at local area is high enough to form (co)precipitate. The formation of (co)precipitate is difficult to be evidenced from the batch experiments. From the EXAFS analysis, the information (such as bond distances, coordination number) about the microstructures of Eu(III) on attapulgite can be calculated from the EXAFS spectra, and the species of Eu(III) on attapulgite surface can be evaluated. The EXAFS analysis of Eu(III) and U(VI) sorption on materials at high pH values showed the formation of inner-sphere surface complexes and surface (co)precipitation [32, 39]. It is difficult to distinguish the contribution of surface complexes or surface (co)precipitation on metal ion sorption from the EXAFS analysis.
It is well known that the sorption of Eu(III) on attapulgite is also dependent on Eu(III) species at different pH values. The relative distribution of Eu(III) species as a function of pH is shown in Fig. 5. The thermodynamic constants used for the calculation of Eu(III) species are tabulated in Table 1. From Fig. 5, it is clear that Eu3+ is the main species at pH <6, it is reasonable that the sorption of Eu3+ increases with pH increasing and is also affected by ionic strength. From the precipitation constant of Eu(OH)3(s) (K sp = 3.4 × 10−22) [20], the precipitation curve of Eu(OH)3 at Eu(III) initial concentration of 1.4 × 10−6 mol/L was calculated and was also shown in Fig. 4. Without Eu(III) sorption to attapulgite, Eu(III) ions start to form precipitates at pH ~8.8. However, one can see that ~96 % Eu(III) was adsorbed to attapulgite at pH <8.8. Thereby, the contribution of surface precipitation on Eu(III) sorption to attapulgite is negligible at high pH values. The sorption of Eu(III) may be attributed to a series of exchange sites or surface complexation because the sorption edge of Eu(III) is spread over three pH unit [46]. At high pH values, large amounts of Eu(III) is adsorbed on attapulgite surface, and the surface sites are occupied by Eu(III) ions. The surface (co)precipitates may be formed on attapulgite surface at high pH values. For the presence of both surface complexes and surface (co)precipitates, it is very difficult to distinguish them on attapulgite surface. The surface (co)precipitates and inner-sphere surface complexes can be distinguished from the bond distances of Eu–O in the first coordination shell or the bond distances of Eu–O-Eu in the second coordination shell from the EXAFS analysis [47, 48]. From the acid–base titration curve, the surface site concentration of attapulgite was calculated to be 2.425 × 10−3 mol/g [20]. In the sorption experiments, the Eu(III) initial concentration was 1.4 × 10−6 mol/L and the solid content was 0.2 g/L, the amount of Eu(III) adsorbed on attapulgite was about 6.72 × 10−6 mol/g (~96 % Eu(III) was adsorbed to attapulgite at pH <8.8), which was much lower than the site concentration (2.425 × 10−3 mol/g). Thereby, the sorption of Eu(III) at high pH is not mainly dominated by surface (co)precipitation.
Surface complexation modeling
The pH-dependent adsorption of Eu(III) on attapulgite was fitted by diffuse double layer modeling (DDLM) with an aid of Visual MINTEQ v 3.0 codes [49]. The surface acidity constants (log K+, Log K−) of attapulgite were given in the following protonation and deprotonation reactions:
where SOH referred to the amphoteric surface groups of attapulgite. The values of log K+ and log K− were obtained by fitting the potentiometric titration data with an aid of Visual MINTEQ v 3.0 codes.
According to the physiochemical properties of attapulgite, the cation exchange sites (XNa) and surface complexation sites (SOH) were selected to fit the adsorption data. The concentration of total XNa sites and SOH sites (mol/g) can be obtained by fitting the potentiometric data. As shown in Fig. 5, the main Eu3+ and Eu(OH) +2 species were observed at pH <7.0 and pH 7.0–8.0, respectively, therefore the Eu3+ and Eu(OH) +2 species were taken into account in this study. The surface complexation reactions of Eu(III) adsorption on attapulgite can be described by Eqs. (4)–(6):
These adjustable parameters were constantly optimized to match with adsorption data, which were summarized in the Table 2. The fitted results of surface complexation modeling were shown in Fig. 6. It is observed that with the with the aid of FITEQL 3.2 code, the DDLM can satisfactorily simulate the adsorption of Eu(III) on attapulgite with cation exchange (XNa) and surface complexation site (SOH) at 0.01 and 0.1 mol/L NaClO4. The uncertainties on sorption data in the modeling were derived from the value of WOS/DF within in the range of 1–20, suggesting that the surface complexation modeling gave an excellent fits to the experimental data. As shown in Fig. 6, DDLM can give an excellent fit for Eu(III) sorption on attapulgite at pH <6.0, whereas the fitted results are overestimated the experimental data at pH >7.0. The main X3Eu, SOEu2+ and SOEu(OH)2 species are observed at pH <3.0, 3.0–6.0 and 6.0–10.0, respectively. The results of surface complexation modeling indicate that the adsorption mechanism of Eu(III) on attapulgite is cation exchange at low pH conditions, whereas the inner-sphere surface complexation dominates the Eu(III) adsorption at high pH conditions. The results are very similar with those reported by Fan et al. [20]. Fan et al. [20] studied the sorption of Eu(III) on humic acid bound attapulgite and simulated the sorption data using constant capacity model with the aid of FITEQL 3.2 code. The species of Eu(III) on attapulgite are mainly X3Eu0 at low pH and SwOHEu3+ or SsOEu(OH)2 at high pH values. The EXAFS spectroscopy analysis showed that the bond distance of Eu–O decreased from 2.415 to 2.360 Å with pH increasing from 1.76 to 9.50, whereas the coordination number decreased from ~9.94 to ~8.56, suggesting the formation of outer-sphere surface complexes at low pH and inner-sphere surface complexes at high pH values. Sheng et al. [50] studied Eu(III) sorption on MX-80 bentonite and simulated the sorption data using diffuse layer model, and found that the sorption of Eu(III) consisted YOEu2+ species at low pH and XOEu(OH)+ species at high pH. The EXAFS results indicated that there was no difference in the bond distance of Eu–O of Eu(III) adsorbed on MX-80 bentonite at low pH values, suggesting the species of adsorbed Eu(III) at low pH with similar bonding nature. Hu et al. [51] studied the sorption of Eu(III) on Na-rectorite and simulated the sorption data using diffuse layer model with the aid of FITEQL 3.2 code, and found that the species of Eu(III) on Na-rectorite are mainly X3Eu at low pH and SwOHEu3+ or SsOEu(OH) at high pH values. All the results suggest that the sorption of Eu(III) mainly occurs on the exchange sites (i.e., XNa) at low pH values, and on the surface complexation sites (i.e., SOH) at high pH values. The relative contribution of different species on Eu(III) sorption may be different at different pH values for different minerals, the interaction mechanism is very similar under similar experimental conditions.
Sorption–desorption isotherms
The sorption–desorption isotherms of Eu(III) on attapulgite at pH 3.50 ± 0.05 and 4.00 ± 0.05, respectively, are shown in Fig. 7a. The amounts of Eu(III) adsorbed on attapulgite in the sorption and desorption experiments were calculated from the following equations:
Sorption and desorption isotherms of Eu(III) on attapulgite at pH 3.50 ± 0.05 (a) (filled square sorption; open square desorption), pH 4.00 ± 0.05 (a) (filled circle sorption; open circle desorption), and by lowering the pH from 6.10 to different pH values (b, the values in the figure are the pH values after the sorption equilibration and the solution pH after adding HCl for 2 days after desorption equilibration). m/V = 0.2 g/L, I = 0.01 M NaClO4, T = 25 ± 1 °C
where C ini was the initial concentration of Eu(III) in suspension, C eq was the equilibration concentration of Eu(III) in supernatant after sorption experiment, \( C_{\text{eq}}^{{\prime }} \) was the final equilibration concentration of Eu(III) in supernatant after desorption experiment, V was the volume and m was the mass of attapulgite. q sorption was the amount of Eu(III) adsorbed on attapulgite after sorption experiment, and q desorption was the amount of Eu(III) still adsorbed on attapulgite after desorption experiment.
The desorption isotherm is much higher than the sorption isotherm, suggesting that the sorption is irreversible, which indicates that the surface adsorbed Eu(III) cannot be desorbed from the attapulgite after the concentration of Eu(III) in solution is decreased. From the sorption–desorption data, one can see that the q desorption values are a little lower than q sorption values, suggesting that only a small amount of Eu(III) is desorbed from attapulgite when the Eu(III) concentration decreases. The irreversible sorption of Eu(III) on attapulgite suggests that Eu(III) forms strong surface complexes on attapulgite and cannot be easily transferred from attapulgite to solution after the experimental condition changes. Li et al. [52, 53] studied the sorption–desorption of metal ions from carbon nanotubes and found that the oxygen-containing functional groups contributed importantly to the increase of sorption capacity. The sorption of metal ions was not completely reversible for the decrease of the metal ion concentration in the system, but most of adsorbed metal ions could be desorbed by lowering solution pH values. After lowering solution pH, the interaction mechanism of Eu(III) on attapulgite may change from inner-sphere surface complexation to outer-sphere surface complexation. For the outer-sphere surface complexes, the Eu(III) ions are weakly bounded on attapulgite surface and can be desorbed when solution pH decreases. For the inner-sphere surface complexes, the Eu(III) ions are strongly bounded on attapulgite, which is difficult to be desorbed from solid particles.
From Fig. 7a, it is clear that the surface adsorbed 152+154Eu(III) is very difficult to be desorbed from attapulgite by lowering the ionic strength. The desorption of 152+154Eu(III) from attapulgite by lowering the solution pH was carried out to investigate the possible desorption of surface adsorbed 152+154Eu(III) from attapulgite (Fig. 7b). After the sorption equilibration of 152+154Eu(III) on attapulgite at pH 6.10, the suspension pH was lowed to different values by adding small amount of 1.0 or 0.1 mol/L HCl. After 2 days’ shaking time, the concentration of 152+154Eu(III) in the supernatant was measured to evaluate the desorption of surface adsorbed 152+154Eu(III) from attapulgite. After the pH of the system was lowered, part of surface adsorbed Eu(III) could be desorbed from attapulgite. However, comparing to the sorption curve in Fig. 4, it is clear that the sorption percentage is still higher, suggesting that the desorption is not reversible. After lowering the pH values, the attapulgite surface properties were changed and the interaction mechanism between Eu(III) and attapulgite may be changed from inner-sphere surface complexation to outer-sphere surface complexation. It is reasonable that part of surface adsorbed Eu(III) can be desorbed from attapulgite.
In order to understand the interaction mechanism more detail, the sorption isotherms were simulated by Freundlich and Langmuir sorption models, respectively. The Langmuir model and Freundlich model are described as [54]:
For Langmuir model:
For Freundlich model:
where q (mol/g) and q max (mol/g) are the amounts of Eu(III) adsorbed on attapulgite and the maximum sorption capacity, respectively. b is the Langmuir constant related to sorption energy, k F (mol1−n g−1 Ln) represents the sorption capacity when Eu(III) ion equilibrium concentration equals to 1, and n represents the degree of sorption dependence with equilibrium concentration. The linear simulation of Langmuir and Freundlich models are shown in Fig. 8a, b. The relative parameters calculated from the Langmuir and Freundlich models are listed in Table 3. The q max values are 4.00 × 10−3 mol/g at pH 3.50 and 5.41 × 10−3 mol/g at pH 4.00. The sorption isotherms are well simulated by the Langmuir model, suggesting that the sorption of Eu(III) on attapulgite is the monolayer sorption. From the calculation of surface site concentration and the amount of Eu(III) adsorbed on attapulgite surface at monolayer coverage, the surface sites of attapulgite particles are not occupied totally by Eu(III) ions and the formation of surface precipitation may be excluded under the low concentration of Eu(III) used in the experiments. From the results and discussion, the one can get the conclusion about the interaction of Eu(III) with attapulgite, which are dependent on various environmental conditions. At low pH values, the sorption of Eu(III) is mainly dominated by outer-sphere surface complexation. At high pH values, the sorption of Eu(III) increases and the sorption is mainly attributed to inner-sphere surface complexation. Part of Eu(III) ions may form (co)precipitates at solid particle surfaces (Fig. 9). The strong interaction between Eu(III) and attapulgite can reduce the mobility of Eu(III), and thereby can decrease the bioavailability of Eu(III) in the natural environment.
Conclusion
In this manuscript, the sorption–desorption of Eu(III) on attapulgite was studied by batch experiments. From the results, one can draw the following conclusions:
-
(1)
The sorption of Eu(III) on attapulgite is strongly dependent on pH and ionic strength at pH <6, and is independent of ionic strength at pH >6.
-
(2)
The sorption of Eu(III) on attapulgite is mainly dominated by outer-sphere surface complexation or ion exchange at low pH values and by inner-sphere surface complexation or surface (co)precipitation at high pH values. The species of Eu(III) on attapulgite are mainly X3Eu, SOEu2+ and SOEu(OH)2 species at pH <3.0, 3.0–6.0 and 6.0–10.0, respectively.
-
(3)
The sorption of Eu(III) on attapulgite is an irreversible process, the surface adsorbed Eu(III) cannot be easily desorbed from attapulgite when the equilibration system is destroyed. The attapulgite is a suitable material for the immobilization of Eu(III) ions in the natural environment.
-
(4)
The attapulgite has very high sorption capacity for the preconcentration of Eu(III) ions from large volume of aqueous solutions. The high sorption capacity makes the attapulgite a very suitable material as backfill material in nuclear waste repository or as adsorbents to eliminate the radionuclides from aqueous solutions in nuclear wastewater treatment.
References
Tan XL, Ren XM, Chen CL, Wang XK (2014) Analytical approaches to the speciation of lanthanides on solid–water interfaces. Trend Anal Chem (TrAC) 61:107–132
Yang S, Sheng G, Tan X, Hu J, Du J, Montavon G, Wang X (2011) Determination of Ni(II) sorption mechanisms on mordenite surfaces: a combined macroscopic and microscopic approach. Geochim Cosmochim Acta 75:6520–6534
Hu R, Shao D, Wang X (2014) Graphene oxide/polypyrrole composites for highly selective enrichment of U(VI) from aqueous solutions. Polym Chem 5:6207–6215
Wang X, Chen C, Hu W, Ding A, Xu D, Zhou X (2005) Sorption of 243Am(III) to multi-wall carbon nanotubes. Environ Sci Technol 39:2856–2860
Hurel C, Marmier N (2010) Sorption of europium on a MX-80 bentonite sample: experimental and modelling results. J Radioanal Nucl Chem 284:225–230
Lu SS, Xu JZ, Zhang CC, Niu ZW (2011) Adsorption and desorption of radionuclide europium(III) on multiwalled carbon nanotubes studied by batch techniques. J Radioanal Nucl Chem 287:893–898
Shao DD, Fan QH, Li JX, Niu ZW, Wu WS, Chen YX, Wang XK (2009) Removal of Eu(III) from aqueous solution using ZSM-5 zeolite. Microporous Mesoporous Mater 123:1–9
Hu J, Xie Z, He B, Sheng G, Chen C, Li J, Chen Y, Wang X (2010) Sorption of Eu(III) on GMZ bentonite in the absence/presence of humic acid studied by batch and XAFS techniques. Sci China B 53:1420–1428
Das DK, Pathak PN, Kumar S, Manchanda VK (2009) Sorption behavior of Am 3 + on suspended pyrite. J Radioanal Nucl Chem 281:449–455
Palagyi S, Vodickova H, Landa J, Palagyiova J, Laciok A (2009) Migration and sorption of Cs-137 and Eu-152, Eu-154 in crushed crystalline rocks under dynamic conditions. J Radioanal Nucl Chem 279:431–441
Yang S, Zong P, Ren X, Wang Q, Wang X (2012) Rapid and high-efficient preconcentration of Eu(III) by core-shell structured humic acid@Fe3O4 magnetic nanoparticles. ACS Appl Mater Interface 4:6891–6900
Yang S, Sheng G, Montavon G, Guo Z, Tan X, Grambow B, Wang X (2013) Investigation of Eu(III) immobilization on γ-Al2O3 surfaces by combining batch technique and EXAFS analysis: role of contact time and humic acid. Geochim Cosmochim Acta 121:84–104
Rabung Th, Geckeis H, Kim J, Beck HP (1998) The influence of anionic ligands on the sorption behavior of Eu(III) on natural hematite. Radiochim Acta 82:243–248
Sheng G, Yang S, Li Y, Gao X, Huang Y, Hu J, Wang X (2014) Retention mechanisms and microstructure of Eu(III) on manganese dioxide studied by batch and high resolution EXAFS technique. Radiochim Acta 102:155–167
Janot N, Benedetti MF, Reille PE (2011) Colloidal alpha–Al2O3, Europium (III) and humic substances interactions: a macroscopic and spectroscopic study. Environ Sci Technol 45:3224–3230
Tan XL, Fan QH, Wang XK, Grambow B (2009) Eu(III) sorption to TiO2 (anatase and rutile): batch, XPS, and EXAFS study. Environ Sci Technol 43:3115–3121
Rabung T, Pierret MC, Bauer A, Geckeis H, Bradbury MH, Baeyens B (2005) Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 1: batch sorption and time-resolved laser fluorescence spectroscopy experiments. Geochim Cosmochim Acta 69:5393–5402
Wang X, Zhou X, Du J, Hu W, Chen C, Chen Y (2006) Using of chelating resin to study the kinetic desorption of Eu(III) from humic acid–Al2O3 colloid surfaces. Surf Sci 600:478–483
Wang X, Sun Y, Wang X (2015) Interaction mechanism of Eu(III) with MX-80 bentonite studied by batch, TRLFS and kinetic desorption techniques. Chem Eng J 264:570–576
Fan Q, Tan X, Li J, Wang X, Wu W, Montavon G (2009) Sorption of Eu(III) on attapulgite studied by batch, XPS and EXAFS techniques. Environ Sci Technol 43:5776–5782
Fan Q, Shao D, Wu W, Wang X (2009) Effect of pH, ionic strength, temperature and humic substances on the sorption of Ni(II) to Na-attapulgite. Chem Eng J 150:188–195
Chen C, Yang X, Wei J, Tan X, Wang X (2013) Eu(III) uptake on rectorite in the presence of humic acid: a macroscopic and spectroscopic study. J Colloid Interface Sci 393:249–256
Montavon G, Rabung T, Geckeis H, Grambow B (2004) Interaction of Eu(III)/Cm(III) with alumina-bound poly(acrylic acid): sorption, desorption, and spectroscopic studies. Environ Sci Technol 38:4312–4318
Ren X, Li J, Tan X, Shi W, Chen C, Shao D, Wen T, Wang L, Zhao G, Sheng G, Wang X (2014) Impact of Al2O3 on the aggregation and deposition of graphene oxide. Environ Sci Technol 48:5493–5500
Giustetto R, Xamena FXL, Ricchiardi G, Bordiga S, Damin A, Gobetto R, Chierotti MR (2005) Maya blue: a computational and spectroscope study. J Phys Chem B 109:19360–19368
Li A, Wang A (2005) Synthesis and properties of clay-based superabsorbent composite. Eur Polym J 41:1630–1637
Wu WS, Fan QH, Xu JZ, Niu ZW, Lu SS (2007) Sorption–desorption of Th(IV) on attapulgite: effects of pH, ionic strength and temperature. Appl Radiat Isot 65:1108–1114
Fan QH, Zhang ML, Zhang YY, Ding KF, Yang ZQ, Wu WS (2010) Sorption of Eu(III) and Am(III) on attapulgite: effect of pH, ionic strength and fulvic acid. Radiochim Acta 98:19–25
Sheng G, Yang S, Sheng J, Hu J, Tan X, Wang X (2011) Macroscopic and microscopic investigation of Ni(II) sequestration on diatomite by batch, XPS and EXAFS techniques. Environ Sci Technol 45:7718–7726
Lee S, Anderson PR, Bunker GB, Karanfil C (2004) EXAFS study of Zn sorption mechanisms on montmorillonite. Environ Sci Technol 38:5426–5432
Kumar S, Kasar SU, Bajpai RK, Kaushik CP, Guin R, Das SK, Tomar BS (2014) Kinetics of Pu(IV) sorption by smectite-rich natural clay. J Radioanal Nucl Chem 300:45–59
Sun Y, Li J, Wang X (2014) The retention of uranium and europium onto sepiolite investigated by macroscopic, spectroscopic and modeling techniques. Geochim Cosmochim Acta 140:621–643
Chen C, Wang X, Nagatsu M (2009) Europium adsorption on multiwall carbon nanotube/iron oxide magnetic composite in the presence of polyacrylic acid. Environ Sci Technol 43:2362–2367
Sun Y, Wang Q, Chen C, Tan X, Wang X (2012) Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ Sci Technol 46:6020–6207
Sun Y, Shao D, Chen C, Yang S, Wang X (2013) Highly efficient enrichment of radionuclides on graphene oxide supported polyaniline. Environ Sci Technol 47:9904–9910
Takahashi Y, Kimura T, Kato Y, Minai Y (1999) Speciation of europium(III) sorbed on a montmorillonite surface in the presence of polycarboxylic acid by laser-induced fluorence spectroscopy. Environ Sci Technol 33:4016–4021
Montavon G, Hennig C, Janvier C, Grambow B (2006) Comparison of complexed species of Eu in alumina-bound and free polyacrylic acid: a spectroscopic study. J Colloid Inteface Sci 300:482–490
Tan X, Wang X, Geckeis H, Rabung T (2008) Sorption of Eu(III) on humic acid or fulvic acid bound to alumina studied by SEM-EDS, XPS, TRLFS and batch techniques. Environ Sci Technol 42:6532–6537
Yang ST, Zong PF, Sheng GD, Ren XM, Huang YY, Wang XK (2014) New insight into Eu(III) sorption mechanism at alumina/water interface by batch technique and EXAFS analysis. Radiochim Acta 102:143–153
Janot N, Benedetti MF, Reiller PE (2011) Colloidal α-Al2O3, europium(III) and humic substances interactions: a macroscopic and spectroscopic study. Environ Sci Technol 45:3224–3230
Sun Y, Chen C, Tan X, Shao D, Li J, Zhao G, Yang S, Wang Q, Wang X (2012) Enhanced adsorption of Eu(III) on mesoporous Al2O3/expanded graphite composites investigated by macroscopic and microscopic techniques. Dalton Trans 41:13388–13394
Song W, Wang X, Wang Q, Shao D, Wang X (2015) Plasma induced grafting polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Phys Chem Chem Phys 17:398–406
Yang ST, Sheng GD, Guo ZQ, Tan XL, Xu JZ, Wang XK (2012) Investigation of radionuclide 63Ni(II) sequestration mechanisms on mordenite by batch and EXAFS spectroscopy study. Science China Chem 55:632–642
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462
Shao DD, Li JX, Wang XK (2014) Poly(amidoxime)-reduced graphene oxide composites as adsorbents for the enrichment of uranium from seawater. Science China Chem 57:1449–1458
Schlegel ML, Pointeau I, Coreau N, Reiller P (2004) Mechanism of europium retention by calcium silicate hydrates: an EXAFS study. Environ Sci Technol 38:4423–4431
Stumpf T, Curtis H, Walther C, Dardenne K, Ufer K, Fanghanel T (2007) Incorporation of Eu(III) into hydrotalcite: a TRLFS and EXAFS study. Environ Sci Technol 41:3186–3191
Sun Y, Yang S, Chen Y, Ding C, Cheng W, Wang X (2015) Adsorption and desorption of U(VI) on functionalized graphene oxides: a combined experimental and theoretical study. Environ Sci Technol 49:4255–4262
Gustafsson JP (2010) Visual MINTEQ ver. 3.0. http://www2.lwr.kth.se/English/OurSoftware/vminteq/index.htm
Sheng GD, Shao DD, Fan QH, Xu D, Chen YX, Wang XK (2009) Effect of pH and ionic strength on sorption of Eu(III) to MX-80 bentonite: batch and XAFS study. Radiochim Acta 97:621–630
Hu J, Chen C, Sheng G, Li J, Chen Y, Wang X (2010) Adsorption of Sr(II) and Eu(III) on Na-rectorite: effect of pH, ionic strength, concentration and modelling. Radiochim Acta 98:421–429
Li J, Chen C, Zhang S, Wang X (2014) Comparison of adsorption–desorption hysteresis of metal cation and anion ions from carbon nanotubes. Environ Sci 1:488–495
Li J, Chen C, Zhang S, Ren X, Tan X, Wang X (2014) Critical evaluation of adsorption–desorption hysteresis of heavy metal ions from carbon nanotubes: influence of wall number and surface functionalization. Chem Asian J 9:1144–1151
Song WC, Shao DD, Lu SS, Wang XK (2014) Simultaneous removal of uranium and humic acid by cyclodextrin modified graphene oxide nanosheets. Sci China Chem 57:1291–1299
Geckeis H, Rabung Th, Ngomanh T, Kim JI, Beck HP (2002) Humic colloid-borne natural polyvalent metal ions: dissociation experiment. Environ Sci Technol 36:2946–2952
Bradbury MH, Baeyens B (2002) Sorption of Eu on Na- and Ca-montmorillonites: experimental investigations and modelling with cation exchange and surface complexation. Geochim Cosmochim Acta 66:2325–2334
Acknowledgments
Financial support from National Natural Science Foundation of China (21375148) and the Fundamental Research Funds for the Central Universities are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Z., He, J., Chen, L. et al. Sorption and desorption properties of Eu(III) on attapulgite. J Radioanal Nucl Chem 307, 1093–1104 (2016). https://doi.org/10.1007/s10967-015-4252-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4252-9