Abstract
A rapid, simple and non-destructive method was developed for the determination of thorium, uranium by gamma ray spectrometry (high purity Ge detector) in thorium–uranium mixture. Using the calibration curves, thorium and uranium were analyzed in synthetic samples with RSD ~2 %. Inter element effect suggested that 583 and 185.7 keV gamma lines are more suitable for the determination of Th and U, respectively in a mixture of Th–U. The newly developed method was validated by routinely employed method using synthetic samples. An ICP-AES based method was developed for the determination of uranium and thorium for comparison purpose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a country like India, due to the unavailability of high quality uranium resources, the nuclear power production depends on the utilization of thorium resources in a three-stage nuclear energy programme using thorium fuel cycle [1]. The thorium fuel cycle uses the naturally abundant isotope of thorium, 232Th, as the fertile material. In the reactor, 232Th is transmuted into the fissile artificial uranium isotope 233U which is the nuclear fuel. Unlike natural uranium, natural thorium contains only trace amounts of fissile material (such as 231Th), which are insufficient to initiate a nuclear chain reaction. Additional fissile material or another neutron source is necessary to initiate the fuel cycle. In a thorium-fueled reactor, 232Th absorbs neutrons eventually to produce 233U. This parallels the process in uranium breeder reactors whereby fertile 238U absorbs neutrons to form fissile 239Pu. Depending on the design of the reactor and fuel cycle, the generated 233U either fissions in situ or is chemically separated from the used nuclear fuel and formed into new nuclear fuel. The thorium fuel cycle claims several potential advantages over a uranium fuel cycle, including thorium’s greater abundance, superior physical and nuclear properties, better resistance to nuclear weapons proliferation [2, 3] and reduced plutonium and actinide production [3]. Therefore, it is required to develop simple, rapid and non-destructive technique to determine thorium and uranium in a thorium–uranium mixture at each stages of fuel fabrication.
The investigation of flooding processes in former underground mines, where uranium was leached in situ with dilute sulfuric acid, requires the activity determination of several radionuclides of the 238U, 235U and 232Th decay chains in waters of various origin. Commonly used methods for determination of uranium and thorium include direct γ-ray measurements [4], γ-ray measurements after chemical separation [5, 6], α-particle spectrometry [7–9], radon emanation, liquid scintillation counting [10, 11], α- or β- particle counting methods with proportional counter [6], inductively coupled plasma atomic emission spectroscopy [12, 13] and mass spectrometry. The measurement of 238U activity in samples by the high resolution γ-ray spectrometry is generally based on the quantification of the more intense γ-ray peaks either at 63.3 keV (3.8 %) or at ~92.6 keV (doublet: ~4.8 %) of 234Th [14–16]. Recently, a relatively weaker intense but a cleaner high energy peak at 1001.0 keV (~0.84 %) of 234mPa is also preferably used for measuring 238U activity [17, 18], especially in uranium ore, processed uranium materials and also environmental samples.
In the present study, an attempt was made to develop a methodology for the determination of thorium and uranium in a thorium–uranium mixture. The method was found to be simple, rapid and non-destructive. No significant interference was observed from uranium on thorium determination and vice versa. The method was validated using synthetic samples and the analytical results were compared with that of the routinely employed method. ICP-AES being multi-elemental, sensitive and routinely employed analytical technique [19–23] due to desired analytical performance has been employed here for comparison purpose.
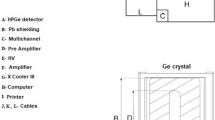
Generally thorium is determined by complexometric titration using ethelene diamine tetra acetic acid (EDTA). Though the method is very accurate and precise (RSD ~2 %), it does not tolerate interference of any other complexing anions such as fluorides, carbonates which are normally used for the dissolution of refractory thorium oxide (ThO2) [24]. In a three stage nuclear work programme in a country like India, bulk of thorium with different proportions of natural uranium is used as reactor fuel materials. Therefore, in the present investigation, gamma spectrometric determination of thorium, uranium and thorium–uranium mixture was studied using high purity germanium (HPGe) detector.
Experimental
The working standard of thorium in the concentration range 100–200 mg/g and the working standard of uranium in the concentration range 60–170 mg/g and the mixture were counted at a fixed geometry using a high purity germanium detector coupled to a 4 k—multi channel analyzer. The resolution of the detector 2 keV for the 1332 keV gamma line of 60Co. Depending upon the concentration, counting time ranged from 5000 to 10,000 s. The area under peaks corresponding to the energies 63.3, 185.7, 238.0, 583.0 and 1001.0 keV were calculated after subtracting the linear Compton baseline. The count rates in the peak regions were plotted against the thorium and uranium concentration respectively to study the correlation. The concentrations of the working standards of thorium and uranium and their gamma energy counts, relative to these, have been used for the estimation of respective concentrations in the mixtures.
ICP-AES based analyses were carried out using Spectro-Arcos spectrometer with both DC Arc and ICP as excitation source and charged coupled device (CCD) as detector systems. The instrumental specifications and optimized experimental parameters were given elsewhere [23]. The samples were analyzed after proper peak search, auto attenuation and multi point calibration. Quartz double distilled water and supra pure nitric acid were used for dilution. The method was validated with working reference materials obtained from inter laboratory comparison experiment involving different laboratories of Department of Atomic Energy (DAE), India.
Results and discussion
Determination of thorium
In the present paper attempt was made to determine Th using gamma energies emitted by the daughter 212Pb at 238 keV gamma line (γ abundance ~43.6 %) and daughter 208Tl at 583 keV gamma line (γ abundance 30.9 %). Calibration curves were established for Th using these lines and were presented in Fig. 1. The sensitivity of Th using 238 keV γ line (0.05 cps/mg/g) was found to be better than that of 583 keV line (0.016 cps/mg/g). High intercept of 238 keV line make the detection limit of this line poorer (0.41 mg/g) than that of 583 keV line (0.04 mg/g). A series of synthetic samples was prepared with concentration ranging from 0.01 to 500 mg/g. It was observed that using both the lines Th can be determined successfully with a RSD ~2 %. Table 1 summarizes the analytical results for the determination of Th using the γ lines of its daughters specified above. The methodology for the determination of thorium by using 911 keV gamma line of 228Ac and 1079 keV gamma line of 212Bi as the high energy gamma lines are less influenced by interference from other gamma energies [25].
Equation (1) and (2) depicted the nuclear reactions which lead to the formation of 212Pb and 208Tl responsible for the emission of the γ lines that were used for the determination of thorium.
In the present case, a systematic study was carried out for the determination of natural uranium by gamma energies emitted by daughter 234Th at 63.3 keV (γ abundance 4.8 %) and by daughter 234mPa at 1001 keV (γ abundance 0.84 %) as well as by 235U gamma energy (γ abundance 57.2 %). Figure 2 presented the calibration curves for the determination of uranium using the above mentioned lines while Table 2 summarizes the analytical results for the determination of uranium in different synthetic samples. At 63.3 keV, correlation between count rate and concentration of uranium was found to be non-linear due to the interference from other gamma energies or prominence of self attenuation at low energy. Count rates of uranium are very low at 1001 keV, but still the correlation between count rate and the concentration of uranium is as good as that of 1001 keV, since 1001 keV energy is less attenuated by other gamma energies. Apart from this, 185.7 keV energy gamma line showed better analytical performance with detection limit 0.58 mg/g and the sensitivity 0.011 cps/mg/g, than the other. The suitability of various gamma lines have been evaluated in literature for the determination of 238U in environmental samples [26]. 234Th and 234mPa generally formed from 238U by α decay.
Inter element effect
The effect of the determination of thorium on uranium and vice versa is extremely important for a real sample of U–Th mixture. Five synthetic samples (identified as S1–S5) with various concentrations of uranium and thorium in U–Th mixture were taken for this purpose. From the calibration curves (obtained from pure U and Th) the area under the mentioned peaks were calculated for both U and Th in synthetic sample. If there is any inter element interference present in the synthetic samples, the measured area under the mentioned peaks (in terms of counts per second) will be different than the calculated area (in terms of counts per second). Tables 3 and 4 summarizes the analytical results obtained from actual counting and its comparison with the calculated values for thorium and uranium, respectively. No significant interference was observed (except 1001 keV) for all the mentioned gamma lines for the determination of both thorium and uranium. It was also noticed that for thorium determination 583 keV gamma line appeared to be better than the other with observed count rate within ±2 % of expected count rate. In case of uranium, 185.7 keV gamma line appeared to be more suitable than that of other with observed count rate within ±2 % of the expected count rate.
ICP-AES methods
To compare the results obtained by gamma spectrometric method, an ICP-AES based methodology was developed for the determination of uranium and thorium after establishing proper calibration curves. Table 5 and 6 summarizes the analytical performance of the analytical lines of uranium and thorium, respectively. Four analytical lines of uranium were chosen for the present study namely 385.958, 409.041, 367.007 and 279.394 nm. The detection limits of uranium using these lines was found to follow the trend: 385.958 < 409.041 < 367.007 < 279.394 nm. 385.958 nm line was found to be the most sensitive analytical lines while 409.041 nm was the least one. Comparable sensitivity was observed for the rest two lines chosen for the present case. The linear dynamic range for 385.958 and 409.041 nm line was found to be up to 1000 mg/L while the same for 367.007 and 279.394 nm line was up to 700 and 850 mg/L, respectively. The analytical performance revealed that 385.958 nm line of uranium is the best line for its determination.
Similar study was carried out for thorium by establishing calibration curves for the identified analytical lines: 401.913, 283.730, 274.716 and 283.231 nm. The overall detection limits of thorium were found to be more than one order of magnitude better than that of uranium (due to the high background intensity of uranium). The detection limits of all the lines were comparable while the sensitivity followed the trend: 401.913 > 283.730 > 283.231 > 274.716 nm. Based on the analytical performance Th 401.913 nm line was found to be the best for its determination.
Validation of the methodology using synthetic samples
Synthetic samples were prepared and uranium and thorium were analyzed using gamma spectrometric method developed in this study. The analytical results were compared with the routinely employed method i.e. Davies & Gray method for uranium [27] and titrimetric determination for thorium [28]. Tables 7 and 8 summarizes the analytical results. It was observed that the analytical results of gamma spectrometric method are comparable with the routinely employed method (for uranium Davies & Gray method was employed while for Th complexometric titration was used) with RSD <5 %. Using 385.958 nm analytical line of uranium and 401.913 nm line of thorium, the analyses were done for the synthetic samples by ICP-AES. The analytical results obtained by gamma spectrometric study compared well with the ICP-AES technique also. The main advantage of the present method over conventional methods lies in its simplicity and non-destructive nature. X-ray absorption florescence (XRF), being a routinely employed method was also employed for the comparative study and the analytical results obtained by newly developed method found to be in good agreement with other analytical techniques.
Conclusions
A simple, rapid and non-destructive, gamma spectrometric method was developed for the determination of thorium and uranium content in a thorium–uranium mixture using γ energy at 583 keV for thorium and 185.7 keV for uranium. The detection limit for thorium using 583 keV γ line was found to be 0.04 mg/g while that for uranium using 185.7 keV γ line was 0.58 mg/g. Both these lines were found to be interference free in Th–U matrix. The newly method was validated using synthetic samples and was compared with the routinely employed methods for the determination of thorium and uranium. An ICP-AES based method was developed to compare the analytical results obtained by gamma spectrometry and was found to be satisfactory. Since, 212Pb obtained from both 232U and 232Th, was not considered for the estimation to avoid interference.
References
Kademani BS, Kumar V, Sagar A, Kumar A, Mohan L, Sutwase G, Scientometric (2006) Dimensions of thorium research in lndia. DESlDOC Bull Inf Technol 26:9–25
Kang J, Von Hippel FN (2001) 232U and the proliferation-resistance of 233U in spent fuel. Sci Glob Secur 7(3):237–269
Hargraves R, Moir R (2011) Liquid fuel nuclear reactors. Am Phys Soc Forum Phys Soc 40(2):10–13
Johnston A, Martin P (1997) Rapid analysis of 226Ra in waters by gamma-ray spectrometry. Appl Radiat Isot 48(5):631–638
Holtzmann RB, Jiang H (1989) Simultaneous determination of 224Ra, 226Ra and 228Ra in large volumes of well waters. J Health Phys 57(1):167–168
Kahn B, Rosson R (1990) Analysis of 228Ra and 226Ra in public water supplies by a gamma-ray spectrometer. J Health Phys 59(1):125–131
Bojanowski R, Holm E, Whitehead NE (1987) Determination of 227Ac by α-particle spectrometry. J Radioanal Nucl Chem 115(1):23–37
Hancock GJ, Martin P (1991) Determination of Ra in environmental samples by α-particle spectrometry. Appl Radiat Isot 42(1):63–69
Martin P, Hancock GJ, Paulka S, Akber RA (1995) Determination of 227Ac by α-particle spectrometry. Appl Radiat Isot 46(10):1065–1070
Chalupnik J, Lebecka M (1993) Determination of 226Ra, 228Ra and 224Ra in water and aqueous solutions by liquid scintillation counting. In: Noakes JE, SchoÈnhofer F, Polach HA (Eds.) Liquid scintillation spectrometry. Radiocarbon, p 397
Salonen L, Hukkanen H (1997) Advantages of low-background liquid scintillation alpha spectrometry and pulse shape analysis in measuring 222Rn, uranium and 226Ra in groundwater samples. J Radioanal Nucl Chem 226(1–2):67–74
Sengupta A, Adya VC, Godbole SV (2013) Spectral interference study of uranium on other analytes by using CCD based ICP-AES. J Radioanal Nucl Chem 298:1117–1125
Sengupta A, Adya VC, Godbole SV (2012) Development of a methodology for the determination of americium and thorium by ICP-AES and their inter-element effect. J Radioanal Nucl Chem 292(3):1259–1264
Dowdall M, Selnaes ØG, Gwynn JP, Davids C (2004) A comparison of alpha and gamma spectrometry for environmental natural radioactivity surveys. J Radioanal Nucl Chem 261(3):513–515
Kaste JM, Bostick BC, Heimsath AM (2006) Determining 234Th and 238U in rocks, soils, and sediments via the doublet gamma at 92.5 keV. Analyst 131(6):757–763
Saidou Bochud F, Laedermann JP, Kwato Njock MG, Froidevaux P (2008) A comparison of alpha and gamma spectrometry for environmental natural radioactivity surveys. Appl Radiat Isot 66(2):215–222
Yucel H, Cetiner MA, Demirel H (1998) Use of the 1001 keV peak of 234mPa daughter of 238U in measurement of uranium concentration by HP-Ge gamma-ray spectrometry. Nucl Instrum Methods A 413(1):74–82
Papachristodoulou CA, Assimakopoulos PA, Patronis NE, Ionnadis KG (2003) Use of HP-Ge gamma-ray spectrometry to assess the isotopic composition of uranium in soils. J Environ Radioact 64(2–3):195–203
Sengupta A, Thulasidas SK, Natarajan V (2015) Trace level determination of precious metals in aqueous medium, U, Th and Zr based nuclear materials by ICP-AES and EDXRF—a comparative study. J Radioanal Nucl Chem 303:2421–2429
Sengupta A, Kulkarni MJ, Godbole SV (2011) Analytical application of DHOA for the determination of trace metallic constituents in U based fuel materials by ICP-AES. J Radioanal Nucl Chem 289(3):961–965
Sengupta A, Rajeswari B, Kadam RM, Acharya R (2011) Determination of trace elements in carbon steel by inductively coupled plasma atomic emission spectrometry. At Spectrosc 32(5):200–205
Sengupta A, Rajeswari B, Kadam RM, Kshirsagar RJ (2011) Characterization of serpentine: a potential nuclear shielding material. J Radioanal Nucl Chem 292(2):903–908
Sengupta A, Thulasidas SK, Natarajan V (2014) Study on the spectral interference of thorium on critical elements and rare earths by CCD-based ICP-AES. At Spectrosc 35(5):213–222
Rao RM, Mirashi NN, Agarwal SK (2003) Proceeding of the annual 40th convention of chemists, Jhansi, p 61–62
Shriwastawa BB, Kumar A, Raghunath B, Nair MR, Abani MC, Ramchandra R, Majumdar S, Ghosh JK (2001) Rapid non-destructive quantitative estimation of urania/thoria in mixed thorium uranium di-oxide pellets by high-resolution gamma-ray spectrometry. Appl Radiat Isot 54(6):941–945
Garcia-Talavera M (2003) Evaluation of the suitability of various γ lines for the γ spectrometric determination of 238U in environmental samples. Appl Radiat Isot 59(2–3):165–173
Davis W, Gray W (1964) A rapid and specific titrimetric method for the precise determination of uranium using iron(II) sulphate as reductant. Talanta 11:1203–1211
Fritz JJ, Ford JJ (1953) Titrimetric determination of thorium. Anal Chem 25:1640–1642
Acknowledgments
The authors wish to acknowledge the support of Dr. A. Goswami, Head, Radiochemistry Division and Dr. S. K. Agarwal, Head, Fuel Chemistry Division and Associate Director, RC & I Group, Bhabha Atomic Research Centre, Mumbai, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sengupta, A., Sankhe, R.H. & Natarajan, V. Rapid and non-destructive determination of uranium and thorium by gamma spectrometry and a comparison with ICP-AES. J Radioanal Nucl Chem 306, 401–406 (2015). https://doi.org/10.1007/s10967-015-4088-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4088-3