Abstract
Smoking is the second reason for developing lung cancer. Our goal was to determine how the amount of 210Po in the tobacco is distributed among the cigarette parts and what percentage reaches the respiratory system, calculating the effective dose. 210Po from tobacco, filter and ash samples were measured from seven Romanian cigarette brands by alpha spectrometry. The obtained average results were 13.97 ± 1.75 mBq/cigarette in the tobacco; 1.61 ± 0.25 mBq/cigarette in the filter and 3.33 ± 0.29 mBq/cigarette in the ash. The dose originating from active smoking was estimated to have an average of 8.36 ± 0.91 μSv/year, while the passive smoking dose reaching the respiratory system was 5.92 ± 0.49 μSv.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cigarettes are produced using as main ingredient various types of tobacco leaves. Many studies show that tobacco leaves contain radioactive 210Po, an element harmful to human health [1–3, 5, 6, 8–13, 16–19, 24] 210Po in tobacco comes from the decay of 222Rn through airborne fallout, which is then caught by the fine hairs of the surface tobacco leaves (trichomes). Additionally, 210Po enters the tobacco plant through the uptake of 226Ra containing phosphate fertilizers [1] absorbed by the roots [2, 3]. Unlike other plants, which are always washed before consumption, tobacco leaves are directly dried in order to obtain quality cigarettes, so these will contain a great amount of 210Po.
All polonium (atomic number 84) isotopes are radioactive (total of 33 with masses ranging from 188 to 220 [4, 5]. Polonium is a very rare natural element, being present in uranium ores in traces (100 μg per ton of ore) [6, 7], most of which have short half-lives. 210Po is an alpha-emitting (5.297 MeV) and low abundance (1.06 × 10−5) gamma-emitting (0.802 MeV) radionuclide, with a half time of 138 days. It is a member of the natural uranium-238 series, due to which it present in trace amounts in most plants and foodstuff as well as in human tissues.
Medical researches indicate that tobacco smoking has serious consequences on human health, some of these being lung cancer, respiratory infections and heart diseases [8, 9]. There are about 100 hazardous compounds in cigarette smoke, including 210Po [10]. The 210Po concentration in tobacco has a mean of 13 ± 2 Bq/kg [11], concentrations ranging from 2.8–37 Bq/kg varying with the cigarette brand due to the different varieties of tobacco used and different manufacturing procedures [3, 12]. Other studies mention 210Po as one of the most powerful carcinogens in tobacco smoke [13] and exposure of the lungs to this element alone can cause lung cancer in rats and hamsters [14, 15]. Cigarette smoke is a complex aerosol consisting of a vapour and a particulate phase. The particles range from 0.1–1 μm and undergo a very fast coagulation [8, 16–18] at volatilising temperatures from 600 to 800 °C [19]. The tobacco leaves being consumed as cigarettes will contain 210Po which will be deposited on the surface of the teeth, lungs and respiratory tract [20]. On average, 50 % of the 210Po found in cigarette tobacco is transferred to the smoke, 35 % remains in the butt, the rest being found in the ash [21].
Smoking is common among the Romanian population, placing the country in a leading position regarding the per capita average cigarette consumption (tobaccoatlas.org). According to 24/7 Wall St. [22], Romania was an the 8-th place in the top 10 most smoking countries in 2012, with an overall adult smoking percenage of 34 % and yearly, a per capita cigarette consumption of 1404 [23].
Materials and methods
Active smokers
Seven popular cigarette brands have been chosen for this analysis, six of them having a cellulose filter and one having an active carbon filter. Also, five were worldwide known brands and two locally produced ones.
0.5 g of each cigarette tobacco, filter (before and after smoking) and ash have been added 0.5 ml of 100 mBq/ml 209Po tracer. Samples have been put then to acidic digestion, using 3 × 20 ml 65 % HNO3, 3 × 20 ml 35 % HCl and H2O2, until the reaction between the reagent and the sample takes place. Samples were then leached with 3 × 20 ml distilled water. The obtained solutions were poured in 100 ml balloons, which were filled with distilled water. Half of these were put into 50 ml Berzelius glasses. The 210Po content of these solutions was spontaneously deposited on high nickel content stainless steel discs in an oven at 80 °C for 3 h. Interferents (Fe3+) were eliminated using 0.5 g of ascorbic acid.
Measurements were carried out using a PIPS detector, having a resolution of 25–30 keV at an area of 900 mm2 and the minimal detectable activity of 0.75 mBq. This detector type has a compact size, an excellent stability and a low sensibility towards gamma rays. The acquisition system is an Aspec 927 multichannel analyser and the spectra acquisition program is Maestro32.
Passive smokers
The passive smoking measurements were carried out in two popular pubs in Cluj-Napoca, Romania, using two pumps: an Alpha Pump with the capacity of 0.5 l/min and 60 μm paper filters, and a Leland Legacy SKC pump with the capacity of 7 l/min and 25 μm paper filters. Measurements were made for 3 and 5 h.
The filter papers have been measured using the same method mentioned in the previous section.
Results and discussion
Active smokers
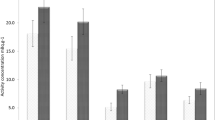
After measuring the activity concentration for each cigarette tobacco, we got the result summarized in Table 1.
The measured average mass of the tobaccos was 0.64 ± 0.06 g, having an average activity concentration of 21.76 ± 2.88 mBq/g, resulting an average activity concentration per cigarette of 13.97 ± 1.75 mBq/cigarette.
The 210Po concentration of the cigarette filters before smoking have been measured, but the activities were under the detection limit. The activity concentrations for cigarette filters after smoking are shown in Table 2.
The average mass of the cigarette filters has been measured to be 0.18 ± 0.02 g, having an average activity concentration of 8.67 ± 2.02 mBq/g, with an average cigarette filter activity of 1.61 ± 0.25 mBq/cigarette filter.
The activity concentration of cigarette ashes is summarized in Table 3.
The average mass of the cigarette ash has been 0.12 ± 0.01 g. The average activity concentration has been 26.16 ± 2.83 mBq/g and, respectively, 3.33 ± 0.29 mBq/cigarette ash.
After having these results, the activity concentration reaching the respiratory system was calculated using Eq. (1):
where Α RS signifies the activity concentration reaching the lung, Α T the activity concentration of the tobacco, Α F the activity concentration of the filter and Α A the activity concentration of the ash. The obtained activity concentrations for the respiratory system and the percentage representing it from the tobacco concentrations are summarised in Table 4, showing that approximately 63.41 % of the tobacco 210Po concentration reaches the respiratory system.
The effective dose reaching the respiratory system was calculated using the following formula: [24]
where K h is the inhalational dose factor, for 210Po being 3.3 × 10−6 (Sv/Bq) [25], Α RS is the activity concentration reaching the respiratory system (Bq/cigarette), F is the fraction of an ingested element absorbed directly into body fluids (equalling 0.2 in our case) [25], G is the consumption of cigarettes a year (for Romanians being 1404 cigarettes/year [23]) and τ is the consumption period, taken as a year.
The calculated effective doses are summarised in Table 5, the average effective dose of 210Po reaching the respiratory system being 8.36 ± 0.91 μSv/year.
Passive smokers
The measured activity concentrations using the pumps are summarised in Table 6.
As measurements show, the 25 μm filter has captured aerosols in the same range as the bigger 60 μm filter. The air activity concentration (A a) was measured using the following formula:
where A m is the measured activity of 210Po for the exposure time (3, respectively 5 h) and PC is the pump capacity of each measuring device. The air activity varies between 60.02 and 72.78 μBq/l, having an average of 69.18 μBq/l.
The effective dose originating from passive smoking was calculated on the basis of the formula used at the active dose measurement:
where L tv is the lung tidal volume, meaning the amount of air inspired by an average person during normal relaxed breathing; its value being 0.5 l [26], r is the average human respiratory rate, having an average of 16 breaths/min [26], t is the average, weekly residence time, taken as 5 h (estimation, considering that inside smoking is allowed in most closed spaces) and τ is the number of weeks in a year, taken as 54. Using this formula we got an average passive effective dose of 5.92 ± 0.49 μSv/year. Although this value was measured, the estimation of the filtered 210Po is hard to make. There is no absolute certainty that the only 210Po radionuclides were deposited on the applied filters and which other nuclides passed through. Presumably, the used filters do not filter the whole amount of 210Po, so that the filtered amount can only be less than the present amount. This is why the effective dose originating from passive smoking is underestimated.
Conclusions
The research was started under the assumption, that not all activity concentration originating from the tobacco reaches the respiratory system. The average activity concentration for tobaccos was 13.97 ± 1.75 mBq, which can be divided into four parts: the part which first reaches the filter, where a small amount of the 210Po is captured, then reaching the respiratory system and the ash originating from the burning of the tobacco and the smoke.
Measurements show that the filter is able to capture an average 1.61 ± 0.25 mBq (11.5 %), so only an average of 9.03 ± 0.91 mBq (63.41 %) activity concentration of the tobacco reaches the respiratory system. The ash has an average measured activity concentration of 3.33 ± 0.29 mBq (34.9 %), the smoke having the activity concentration of 6.9 × 10−6 mBq/m3 air, representing less than 1 % of the tobaccos activity concentration. For a precise measurement of the passive dose, the retention efficiency of the filters should be determined.
Locally produced cigarettes have a significantly lower activity concentration in the tobacco, which is probably caused by the different manufacturing methods and the characteristics of the area where the tobacco plant has been grown. Also, the cigarette having an active carbon filter was able to retain twice as much 210Po than the other filters made out of cellulose. Future investigations will be made both regarding the differences in the growing areas of the tobaccos and regarding the filter composition in comparison with the activity concentrations.
References
Santos MS, Azeredo AMGF, Melo DR, Juliao LMQC (1994) Determination of alpha-emitters in Brazilian tobacco. J Radioanal Nucl Chem 182(1):57–62
Desideri D, Meli MA, Feduzi L, Roselli C (2007) 210Po and 210Pb inhalation by cigarette smoking. Italy Health Phys 92:58–63
Skwarzec B, Struminska DI, Borylo A, Ulatowski J (2001) Polonium 210Po in cigarettes produced in Poland. J Environ Sci Health A 36:465–474
Stannard JN (1988) Radioactivity and health: a history. US Dep for Energy, Washington, DC
Murtiwardhani YEH, Indriyani VD, Noor JAE (2012) Estimation of Polonium-210 concentration in smokers and nonsmokers’ teeth in East Java, Indonesia. Asian Trans Sci Technol 2(5):1–4
Roessler G (2007) Why 231 210Po?, Health Phys News, The Official Newsletter of the Health Physics Societ,. XXXV(2) For Specialists in Radiation Safety, p. 8
Seiner BN, Morley SM, Beacham TA, Haney MM, Gregory S, Metz L (2014) Effects on digestion, chemical separation and deposition on Po-210 quantitative analysis. J Radioanal Nucl Chem. doi:10.1007/s10967-014-3255-2
Khater AEM, Al-Sewaidan HAI (2006) 210Po in cigarette tobacco. Int. J. Low Radiation 3(2–3):224–233
Iwaoka K, Yonehara H (2012) Nuclear radioactive nuclides in cigarettes and dose estimation for smokers. J Radioanal Nucl Chem 293(3):973–977
Talhout R, Schultz T, Florek E, van Benthem J, Wester P, Opperhuizer A (2011) Hazardous compounds in tobacco smoke. Int J Environ Res Public Health 8:613–828
Presson BRR, Holm E (2011) 210Po and lead-210 in the terrestrial environment: a historical review. J Environ Radioact 102:420–429
Kelecom A, Gouvea RCS, Santos PL (2002) Levels of 210Po and 210Pb in cigars. J Radioanal Nucl Chem 253(1):129–133
Seiler RL, Wiemels JL (2012) Occurrence of 210Po and biological effects of low-level exposure: the Need for Reaserch. Environ Health Perspect 120:1230–1237
Little JB, O’Toole WF (1974) Respiratory tract tumours in hamsters induced by benzol(a)pyrene and 210Po α-radiation. Cancer Res 34:3026–3029
Yuile CL, Berke HL, Hull T (1967) Lung cancer following 210Po inhalation in rats. Radiat Res 31:760–774
Khater AEM (2004) 210Po budget in cigarettes. J Environ Radioact 24:33–41
Dhar P (2004) Measuring tobacco smoke exposure: quantifying nicotine/continine concentration in biological samples by colorimetry, cromathography and immunoass methods. J Pharm Biomed Anal 35:155–168
Smith CJ, Fisher TH (2001) Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis 158:257–267
Radford E, Hunt VR (1964) 210Po: a volatile radioelement in cigarette. Science 143:247–249
Kovács T, Somlai J, Nagy K, Szeiler G (2007) 210Po and 210Pb concentration of cigarettes traded in Hungary and their estimated dose contribution due to smoking. Radiat Meas 42(10):1737–1741
Parfenov YD (1974) 210Po in the environment and in the human organism. Atmos Energy Rev 12:75–143
The countries with the heaviest smokers, 24/7 Wall St. http://247wallst.com/special-report/2012/11/01/the-countries-with-the-heaviest-smokers/. Accessed 10 Jan 2015
Worldwide annual cigarette consumption, Tobaccoatlas.org, (http://www.tobaccoatlas.org/products/cigarette_consumption/annual_cigarette_consumption/. Accessed 10 Jan 2015)
UNSCEAR (1993) Report: Source and effects of ionizing radiation, United Nations, New York
ICRP (2012) Compendium of dose coefficients based on ICRP Publication 60. ICRP publication 119. Ann ICRP 41(Suppl.)
Frank’s Hospital Workshop, Medical Equipment, Spirometry. http://www.frankshospitalworkshop.com/equipment/spirometry_equipment.html. Accessed 10 Jan 2015
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Begy, RC., Simon, H. & Kelemen, S. 210Po inhalation due to smoking: a dose estimation. J Radioanal Nucl Chem 306, 257–261 (2015). https://doi.org/10.1007/s10967-015-4073-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4073-x