Abstract
In this study, the activity concentration of polonium 210 in cigarette for Tunisian consumers was investigated by alpha spectrometry. After chemical digestion of tobacco, 210Po was extracted, auto-deposited on disc and measured. The activity of 210Pb was assessed after radioactive equilibrium was achieved. The activity levels of 210Po ranged between 7.8 ± 0.3 and 17 ± 0.5 mBq per cigarette with an average of 12.9 ± 0.4 mBq per cigarette. Effective doses per year due to cigarette smoking were calculated assuming that 22% of the 210Pb and 210Po in tobacco were retained in the lungs of the smokers. It is concluded that for a smoker in Tunisia, the average effective dose is about 90.6 ± 3.3 μSv per year for a cigarette consumption of one pack of cigarettes per day. This value is somewhat lower than 106.4 ± 5.3 μSv per year estimated as the mean global effective dose from smoking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nicotiana tabacum, commonly known as tobacco, is a leafy plant, which is used for cigarettes, cigars, pipes and narghiles. Tobacco and tobacco products contain several toxic compounds and, consequently, smoking these products can cause serious health damage (Sakoda et al. 2012; Funck-Brentano et al. 2006; Goodwin et al. 2010). Tobacco smoke is considered one of the potential causes of lung cancer (Zaga et al. 2011). Among the toxic compounds are natural radioactive isotopes of uranium and thorium (234U, 238U, 228Th, 230Th, 232Th) as well as their daughter products (e.g., 226Ra, 210Po, 210Pb) (Goodwin et al. 2010). Polonium-210 is the daughter of 210Pb. These radioisotopes are present among the hazardous chemicals in cigarettes and represent a source of ionizing radiation in the smoke which is inhaled into the respiratory system (Kilthau 1996; Martell 1982; Jenkins et al. 1984; Burns 1991; Skwarzec et al. 2001a, b; Khater 2004).
The origin of polonium and lead in tobacco plants can be absorption from the soil (supported Po), direct deposition of fallout on the tobacco leaf (unsupported Po) and 222Ra which is deposited on tobacco leafs. Polonium concentration can be enhanced by use of phosphate fertilizers (Desideri et al. 2007; Lopes dos Santos et al. 1970). Phosphate rocks, with high concentrations of uranium and the daughter nuclide 210Po, are extensively mined to produce phosphate fertilizers (Mussealo-Rauhammaa et al. 1985; Hussein 1994; Alam et al. 1997).
The distribution of radionuclides in area tissues, which are exposed to air as for example leaves, follows an acropetal gradient suggesting that Po-210 and Pb-210 are more concentrated in older leaves of tobacco (Athalye 1972). A smoker inhales such natural radionuclides into the respiratory system by smoking a cigarette (Zaga and Mistry 2011). Polonium-210 is the radioactive isotope most often investigated in the literature, and its half-life is 138.376 ± 0.002 days (Singh and Nikelani 1976; Martell 1974). The alpha particle emitted after the decay of 210Po has a high energy (5.305 MeV), it represents a local source of radiation and can damage the lung cells (Martell 1982).
As compared to other radionuclides, 210Pb and its daughter 210Po contribute the largest radiation dose to the lungs (Peres and Hiromoto 2002; Skwarzec et al. 2001a, b). Martell estimated that the cumulative alpha dose is about 16 Sv in bifurcations of smokers who died with lung cancer (Martell 1982).
The objective of the present work was to measure the 210Po activity concentrations in selected brands of the most frequently purchased cigarettes in Tunisia. In this study, the activity concentrations of 210Pb in cigarettes were assessed; the effective dose received by a smoker per year was calculated and compared with those reported by other studies.
Materials and methods
Sample preparation
All reagents used in the present study for chemical procedures were of analytical grade. Chemical recoveries were assessed using 209Po as a radiotracer. Eleven brands of cigarettes mostly smoked in Tunisia were selected for analysis; 6 of them were local products (coded as L1-L6), while the others were imported (I1-I7) from other countries. Three packs of cigarettes of each brand were bought from the local market; the 60 cigarettes of each brand were dried at 60 °C overnight, ground and mixed well. A suitable quantity of a dry cigarette sample (i.e., 2.5 g) was transferred to beakers, treated with a mixture of acid concentrated solution [HNO3, HCl], spiked with a known 209Po activity (i.e., 37.4 mBq), and settled overnight. Pure sodium chloride was added to the sample which was then heated for 3 h at 70 °C near dryness. Then the residue was treated two times with concentrated HCl and H2O2 30% and evaporated. The dry residue was dissolved in 30 mL of a solution of 0.3 M HCl. For the reduction of Fe3+, a solution of NH2OH.HCl was added to the mixture and polonium was auto-deposited on a stainless steel disc (diameter: 20 mm). It is noted that Pb and Bi could be co-deposited in a disc made of metal other than silver. To avoid any overestimation of 210Po, the measurements were performed immediately after auto-deposition (Ehinger et al. 1986).
Measurements
For the measurements, a 7400 model alpha spectrometer (Canberra) was used including six vacuum chambers and a silicon detector (Canberra alpha PIPS detector); the active surface area was 450mm2. A TRUMP 8 k-W3 multichannel analyzer connected the spectrometer with a computer. The distance between the source and the detector was 5 mm. The detection efficiency was of the order of 24% and the Canberra Genie-2000 software tool was applied for the acquisition of spectra. Depending on the sample, the counting time varied between 2 and 4 days. Consequently, the lowest limit of detection (LLD) calculated according to Currie (1968) was approximately 0.3 mBq L−1for 2 days counting. For the local calibration, a multi alpha reference material was used for quality control (standard source with certificate with radionuclides (238U, 235U, 239Pu, 241Am)). Blank samples were used to check the purity of the chemicals.
Assessment of effective dose
Yearly effective dose was assessed from210Po and 210Pb in cigarette smoke according to Eqs. 1 and 2 (Sakoda et al. 2012):
where E is the effective dose (µSv/year), A is the activity of the radionuclide inhaled by smokers per year (Bq year−1), and D is the effective dose per activity due to inhalation of 210Po and 210Pb (µSv Bq−1). The effective dose conversion factor D was taken from Publication 119 of the International Commission of Radiological Protection (ICRP 2012) as 3.3 µSv Bq−1 for 210Po and 1.1 µSv Bq−1 for 210Pb.
where F is the experimentally determined radionuclide activity per cigarette (Bq cigarette−1), C is the number of cigarettes smoked per day (assumed to be 20 per day), and T is the smoking time per year (365 days per year).
Results and discussion
210P concentrations
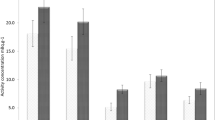
The results of the different concentrations of 210Po in cigarettes (local and imported brands) in this study are summarized in Table 1. The data indicate that there are large differences of activities between the local and imported products. These differences depend essentially on the tobacco varieties and on differences in manufacturing procedures (Skwarzec et al. 2001a, b; Carvalho 1995). The average activity concentrations of 210Po in cigarettes locally produced in Tunisia were between 11.7 ± 0.5 and 21.1 ± 0.8 mBq g−1 with a mean value of 16.3 ± 0.6 mBq g−1. The differences observed are probably due to the quality of tobacco in the cigarettes: Athalye et al. assumed that the distribution of radionuclides in the tobacco plant follows an acropetal gradient and, consequently, radionuclides are more concentrated in older than in younger leaves (Athalye et al. 1972). For the imported brands, the average concentrations were between 18.8 ± 0.8 and 25.3 ± 0.8 mBq g−1 with a mean value of 22.9 ± 0.7 mBq g−1. These high activities of polonium 210 in tobacco can be explained by the use of phosphate fertilizers during cultivation (Tso et al. 1968). The average of 210Po activity concentration value in cigarettes sold in Tunisia, 19.6 mBq per gram, is not significantly different from the average of 210Po concentrations reported in Finland, Cuba, Philippines, and Greece (Mussealo-Rauhammaa and Jaakkola 1985; Brigido Flores et al. 2015; Iwaoka et al. 2012; Savidou et al. 2006). On the other hand, Persson reported that the average activity of 210Po was 13 ± 2 mBq g−1 in tobacco harvested in various countries and at different times (Persson and Holm 2011).
It takes about 24–30 months between the harvest of tobacco leaves and the final production of cigarettes. This represents six to seven half-lives of 210Po. Therefore, secular equilibrium with 210Pb is considered as completed (Sakoda et al. 2012), meaning that the activity concentration of both radionuclides should be in equilibrium (Tahir and Alaamer 2008; Papastefanou 2009; Nagamatsu et al. 2011). This was verified by several studies (Desideri et al. 2007; Godoy et al. 1992; Schayer et al. 2009). Consequently it was assumed in the present study that, when the measurements were conducted, secular equilibrium between 210Pb and 210Po was well accomplished and the activity concentrations of 210Pb could be estimated from the activity concentration of 210Po (Table 1).
Assessment of effective dose
In most studies, the transfer factor (F) assumed for 210Pb and 210Po from cigarettes to mainstream smoke varies between 10 and 35% (Schayer et al. 2009; Radford and Hunt 1964; Hill 1965; Ferri and Baratta 1966; Ferri and Christiansen 1967; Sakanoue et al. 1987; Carvalho and Olivira 2006). Peres and Hiromoto (2002), assuming that 10% of 210Pb and 20% of 210Po in the tobacco are inhaled. Schayer et al. (2009) indicated that an average of 13% of 210Po and 8% of 210Pb are transferred to the mainstream smoke and inhaled by a smoker. For calculation of the yearly effective dose, they used 3.7 µSv Bq−1 and 1.2 µSv Bq−1 as the conversion factors for 210Po and 210Pb (DCAL 2006), respectively. Jankovic-Mandic et al. (2016) used the arithmetic mean values as suggested by Iwaoka and Yonehara (2012), which were 12% for 210Pb and 18% for 210Po. M. Horvath et al. (2017) reported, after having performed a smoking experiment with a smoking machine, that the net intake of 210Po from tobacco is 15 ± 10% (the range was between 5 and 25%). Kubalek et al. (2016) found in a smoking experiment with volunteer smokers that approximately 22% of 210Po and 210Pb are retained in the lungs of smokers. In the present study, this value was used to estimate the effective yearly dose from smoking of the investigated local and imported cigarettes (Table 2). The values calculated for the annual effective dose due to 210Po in cigarette smoke varied between 41.5 ± 1.8 and 89.8 ± 2.8 µSv year−1 with a mean value 68 ± 2.4 µSv year−1, while for 210Pb annual effective dose varied between 13.9 ± 0.6 and 30.0 ± 0.9 µSv year−1 with an average 22.6 ± 0.8 µSv year−1.
It is known that, for a smoker, the effective dose depends on several parameters such as the conversion factor and the percentage of concentration of radionuclides retained in the lungs. Effective doses reported in different studies are often difficult to compare, because often different approaches and parameters were used to calculate them. For this reason, the doses estimated in the majority of studies were re-calculated in the present study on the basis of their reported activity concentrations of 210Po and 210Pb using the same parameters as used in the present study (i.e., using a 22% net intake and the conversion factors recommended by ICRP (2012) for 210Po and 210Pb of 3.3 nSv/Bq and 1.1 nSv/Bq, respectively). The new estimated effective doses due to inhalation of 210Pb and 210Po and the combined doses in different countries of the world are summarized in Table 3.
The data shown in Table 3 demonstrate that some countries such as Syria, Canada, Japan, and Norway have low total effective doses per year of 13.2, 55.7, 56.5, and 60.7 µSv year−1, respectively, while Iran, Germany, Hungary, France, Serbia, and Vietnam have higher doses of 131.5, 135.6, 153.3, 164, 184.6, and 185.4 µSv year−1, respectively. The mean value of the total effective dose due to inhalation of 210Po and 210Pb for a smoker in Tunisia is 90.6 ± 3.3 µSv year−1. The mean value of the total effective dose from these radionuclides in the world is estimated to be 106.4 ± 5.3 µSv year−1. Persson and Holm assumed that for a heavy smoker (30 cigarettes per day) and with the conversion factor of 1.1 µSv Bq−1, the committed annual dose equivalent is in the order of 100 µSv year−1 (Persson and Holm 2011). Thus, for a smoker who consumes 20 cigarettes per day, the annual effective dose will be about 200 µSv if a conversion factor of 3.3 µSv Bq−1 for 210Po is used (ICRP 2012). This value, without the contribution of 210Pb, is rather high compared to the values calculated in the present study.
Conclusion
The annual effective doses due to inhalation of 210Po and 210Pb estimated for Tunisian tobacco smokers as obtained in the present study were 68 ± 2.4 µSv and 22.76 ± 0.8 µSv year−1, respectively, which is lower than the corresponding global mean values. It is known that tobacco smoking is a global pandemic affecting over 1.1 billion people and causing nearly 8 million deaths annually (WHO 2019) by inhaling various chemical and radioactive materials. The radiological risk associated with cigarette smoking was assessed in the present study with focus on 210Po and 210Pb. This assessment must be supplemented by accurate estimation of the activity concentrations of thorium isotopes and their progeny. Consequently, further work will be focused on the investigation of the intake of thorium isotopes and progeny in cigarettes that are also retained for quite a long time in the lungs of smokers.
References
Alam MN, Chowdhury MI, Kamel M, Ghose S, Hamida B, Chakaborty D (1997) Radioactivity in chemical fertilizers used in Bangladesh. Appl Radiat Isotopes 48:1165–1168
Athalye VV, Mistry KB (1972) Uptake and distribution of polonium-210 and lead-210 in tobacco plants. Radiat Bot 12(6):421–425
Batarekh K, Teherani DK (1987) Determination of polonium-210 in cigarettes from Syria. J Radioanal Nucl Chem 117:75–80
Black SC, Brethauer EW (1968) Polonium-210 in tobacco. Radiat Data Rep 9:145–152
Brigido Flores O, Montalvan Estrada A, Fabelo Bonet O& Barreras Caballero A (2015) Polonium-210 concentration of cigarettes traded in Cuba and their estimated dose contribution due to smoking. In: Proceedings of XV Workshop on Nuclear Physics IX International Symposium on Nuclear and Related Techniques WONP-NURT.
Burns D (1991) Cigarette and cigarette smoking. Clin Chest Med 12:631–642
Carvalho FP (1995) 210Po and 210Pb intake by the Portuguese population: the concentration of seafood in the dietary intake of 210Po and 210Pb. Health Phys 69:469–480
Carvalho FP, Olivira JM (2006) Po-210 in cigarette smoke and radiation exposure of lungs. Czech J Phys 56:697–703
Christobher S, Periyasamy M, Athif P, Syed Mohamed HE, Sadiq Bukhari A, Shahul Hameed P (2019) Activity concentration of polonium-210 and lead-210 in tobacco products and annual committed effective dose to tobacco users in Tiruchirappalli District (Tamil Nadu India). J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06879-x
Desideri D, Meli A, Feduzi L, Roseli C (2007) 210Po and 210Pb inhalation by cigarette smoking in Italy. Health Phys 92:58–63
Ehinger SC, Pacer RA, Romines FL (1986) Separation of the radioelements 210Pb–210Bi–210Po by spontaneous deposition onto noble metals and verification by Cherenkov and liquid scintillation counting. J Radioanal Nucl Chem 98:39–48
Ferri ES, Baratta EJ (1966) Polonium 210 in tobacco, cigarette smoke, and selected human organs. Public Health Rep 81:121–127
Ferri ES, Christiansen H (1967) Lead-210 in tobacco and cigarette smoke. Public Health Rep 82:828–832
Funck-Brentano C, Raphael M, Lafontaine M, Arnould JP, Verstuyft C, Lebota M, Costagliola D, Roussel R (2006) Effects of type of smoking (pipe, cigars or cigarettes) on biological indices of tobacco exposure and toxicity. Lung Cancer 54:11–18
Godoy JM, Gouvea VA, Mello DR, Azeredo AMG (1992) 226Ra/ 210Pb/ 210Po equilibrium in tobacco leaves. Radiat Prot Dosim 45:299–300
Goodwin RD, Keyes KM, Hasin D et al (2010) Respond. Am J Public Health 100(2):196–197
Hill CR (1965) Polonium-210 in man. Nature 208:423–428
Horvath M, Shahrokhi A, Bator P, Toth-Bodrogi E, Kovacs T (2017) Determination of Po-210 content in cigarette smoke using a smoking machine: a case study of Iranian cigarettes. J Environ Radioact 174:66–70
Hussein EM (1994) Radioactivity of phosphate ore, superphosphate and phosphogypsum in Abu-Zaabal phosphate plant, Egypt. Health Phys 67:280–282
International Commission of Radiological Protection (ICRP) (2012) Compendium of dose coefficients based on ICRP 60. ICRP Publication 119 Ann ICRP (41)
Iwaoka K, Enriquez EB, Yajima K, Hosoda M, Tokonami S, Yonehara H, Garcia TY, Kanda R (2019) 210Po as a source of natural radioactivity in cigarettes distributed in the Philippines. Perspect Sci 12:100400
Iwaoka K, Yonehara H (2012) Nuclear radioactive nuclides in cigarettes and dose estimation for smokers. J Radioanal Nucl Chem 293(3):973–977
Jandl J, Petr I (1988) Ionizuyicí zarení v zyivotním prostredí, SNTL, Praha, pp 63–74
Jankovic-Mandic L, Ðoloc M, Markovic D, Todorovic D, Onjia A, Dragovic S (2016) Natural radionuclides in cigarette tobacco from Serbian market and effective dose estimate from smoke inhalation. Radiat Prot Dosim 168(1):111–115
Jenkins RA, Gueri MR (1984) Analytical chemical methods for the detection of environmental tobacco-smoke constituents. Eur J Respir Dis 65:33–46
Karali T, Olmez S, Yener G (1996) Study of spontaneous deposition of 210Po on various metals and application for activity assessment in cigarette smoke. Appl Radiat Isotopes 47:09–411
Khater AE, Al-Sewaidan HA (2006) Polonium-210 in cigarettes tobacco. Int J Low Radiat 3(2/3):224–233
Khater AEM (2004) Po-210 budget in cigarettes. J Environ Radioact 71:33–41
Kilthau GF (1996) Cancer risk in relation to the radioactivity in tobacco. Radiol Technol 67(3):217–222
Kovacs T, Somlai J, Nagy K, Szeiler G (2007) Po-210 and Pb-210 concentration of cigarettes traded in Hungary and their estimated dose contribution due to smoking. Radiat Meas 42:1737–1741
Kubalek D, Sersa G, Strok M, Benedik L, Jeran Z (2016) Radioactivity of cigarettes and the importance of Po(210) and thorium isotopes for radiation dose assessment due to smoking. J Environ Radioact 155–156:97–104
Lopes dos Santos P, Weinberg EM, Penna-Franca E (1970) Determinacao de 210Po emcigarrose tabaco. Revista de Biologia y Medicina Nuclear 2:73–77
Martell EA (1974) Radioactivity of tobacco trichomes and insoluble cigarette smoke particles. Nature 249:215–217
Martell EA (1982) Radioactivity in cigarette smoke. N Engl J Med 307:309–310
Mussealo-Rauhammaa H, Jaakkola T (1985) Plutonium-239, 240 and Po-210 content of tobacco and cigarette smoke. Health Phys 49:296–301
Nagamatsu T, Sakod A, Kataoka T, Ono T, Yamaoka K (2011) An assessment of radioactivity levels of 210Pb and 40K in tobacco and radiation exposure from smoking. Acta Med Okayama 65(2):91–95
Papastefanou C (2009) Radioactivity of tobacco leaves and radiation dose induced from smoking. Int J Environ Res Public Health 6:558–567
Parfenov YD (1974) Polonium-210 in the environment and in the human organism. Atom Energy Rev 12:75–143
Peres AC, Hiromoto G (2002) Evaluation of 210Pb and 210Po in cigarette tobacco produced in Brazil. J Environ Radioact 62:115–119
Persson ERR, Holm E (2011) 210Po and lead-210 in the terrestrial environment: a historical review. J Environ Radioact 102:420–429
Radford EP Jr, Hunt VR (1964) Po-210: a volatile radioelement in cigarettes. Science 143:247–249
Robert-Csaba B, Hedvig S, Szabolcs K (2015) 210Po inhalation due to smoking: a dose estimation. J Radioanal Nucl Chem 306(1):257–261
Sakanoue M, Yamamot M, Kamura K (1987) Determination of environmental actinide nuclide and Pb-210 (Po-210) by low energy photon spectrometry with ALFA-spectrometry. J Radioanal Nucl Chem 115(1):71–82
Sakoda A, Fukao K, Kawabe A, Kataoka T, Hanamoto K, Yamaoka K (2012) Radioactivity of 210Pb in Japanese cigarettes and radiation dose from smoking inhalation. Radiat Prot Dosim 150:109–113
Savidou A, Kehagia K, Eleftheriadis K (2006) Concentration levels of 210Pb and 210Po in dry tobacco leaves in Greece. Environ Radioact 85:94–102
Schayer S, Nowak B, Wang Y, Qu Q, Cohen B (2009) 210Po and 210Pb activity in Chinese cigarettes. Health Phys 96(5):543–549
Skwarzec B, Struminska DI, Ulatowski J, Gołebiowski M (2001a) Determination and distribution of polonium 210Po in tobacco plants from Poland. J Radioanal Nucl Chem 250:319–322
Skwarzec B, Ulatowski J, Struminska DI, Borylo A (2001b) Inhalation of 210Po and 210Pb from cigarette smoking in Poland. J Environ Radioact 57:221–230
Tahir SN, Alaamer AS (2008) Determination of natural radioactivity in rock salt and radiation doses due to its ingestion. J Radiol Prot 28(2):233–236
Taroni M, Zaga V, Bartolomei P, Gattavecchia E, Pacifici R, Zuccaro P, Esposito M (2014) Pb-210 and Po-210 concentrations in Italian cigarettes and effective dose evaluation. Health Phys 107:195–199
Tran TN, Le CH, Chau VT (2014) 210Po and 210Pb activity concentrations in cigarettes produced in Vietnam and their estimated dose contribution due to smoking. In: Proceedings of the 12th Asia Pacific physics conference, JPS conference proceedings, vol 1, 019002
Tso TC, Steffens G, Ferri ES, Baratta EJ (1968) Agronomic factors affecting Polonium-210 and Lead-210 levels in tobacco. I Soil Fertilizer Agron J 60:647
World Health Organization, WHO 2019. https://www.who.int/news-room/fact-sheets/detail/tobacco. Accessed May 2020
Zaga V, Lygidakisb C, Chaouachi K, Gattavecchia E (2011) Polonium and lung cancer. J Oncol 2011:1–11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boujelbane, F., Samaali, M., Rahali, S. et al. The activities of 210Po and 210Pb in cigarette smoked in Tunisia. Radiat Environ Biophys 59, 565–570 (2020). https://doi.org/10.1007/s00411-020-00853-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-020-00853-y