Abstract
Lithium titanate is a proposed tritium breeding blanket material in D-T based fusion reactor under International Thermonuclear Experimental Reactor programme. For optimization of sol–gel preparation method and chemical quality control, compositional characterization of Li2TiO3 was carried out by particle induced gamma-ray emission using 8 MeV proton beam at BARC–TIFR pelletron facility. For the first time, a non-destructive method has been standardized for simultaneous determination of Li, Ti and O in this ceramic sample, which is otherwise difficult by various wet-chemical as well as radio-analytical methods. Thick targets of samples, synthetic samples and standards prepared in graphite matrix were used for the experiment. Rutherford backscattering spectrometry method was used for beam current monitoring using a thin Au foil. The gamma-rays at 478, 983 and 6129 keV from 7Li(p, p′γ)7Li, 48Ti(p, p′γ)48Ti and 16O(p, p′γ)16O nuclear reactions, respectively, were measured using high resolution gamma-ray spectrometry and corresponding peak areas were used for concentration calculations by relative method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In D-T based fusion reactor under International Thermonuclear Experimental Reactor (ITER) programme, lithium based ceramics like lithium titanate (Li2TiO3), lithium aluminate (LiAlO2), lithium silicate (Li4SiO4) and lithium zirconate (Li2ZrO3) are proposed tritium breeding blanket materials [1]. For a material to be used as a tritium breeder in this reactor, it should have good tritium release characteristics at low temperature, high physical, chemical and mechanical stability along with low neutron absorption characteristics. Tritium is produced from 6Li(n,α)3H reaction during reactor operation. Presently, studies are in progress for the above mentioned ceramics synthesized by different routes using natural Li. Among these ceramics, Li2TiO3 is a preferred choice, as it meets the above mentioned desired properties. It has been reported that Li2TiO3 can be prepared by three different routes, namely, sol–gel [2, 3], solid-state reaction [4, 5] and in situ hydrolysis methods [6].
In the present work, Li2TiO3 spheres were prepared by sol–gel route using three different starting materials namely LiCl, LiNO3 and (1:1) mixture of LiCl and LiNO3 and their corresponding sintering temperatures were 800, 1000 and 1000 and 1250 °C. The details of sol–gel synthesis procedure are given in Reference 2. Compositional characterization of Li2TiO3, thus prepared, is essential for optimization of synthesis procedure and also for its chemical quality control (CQC). Since Li2TiO3 is a ceramic sample, it is difficult to analyze by wet chemical destructive methods like AAS, ICP-AES and ICP-MS. Though laser induced breakdown spectroscopy (LIBS), in principle, can determine Li and Ti in solid samples, this technique is not practised for chemical characterization of Li2TiO3 as it is strongly dependent on matrix matching composition of standard. Non-destructive radio-analytical methods like neutron activation analysis (NAA), particle induced X-ray emission (PIXE) and X-ray fluorescence (XRF) are not suitable for low Z elements like Li and O determination. Determination of oxygen is difficult by many conventional and nuclear techniques. Techniques like charge particle activation analysis (CPAA), fast neutron activation analysis (FNAA) and inert gas fusion in conjunction with IR are used for oxygen determination at trace concentration levels (about 30 to ≥100 mg kg−1) [7–9]. Prompt gamma-ray NAA (PGNAA) is not suitable for oxygen determination because of very low neutron absorption cross section of 16O. Thus, it is necessary to standardize a simple, sensitive and non-destructive method which can quantify Li, Ti and O simultaneously. One of the ion beam analysis (IBA) techniques namely particle induced gamma-ray emission (PIGE) using proton beam is capable of determining many low Z elements including Li, Ti and O [10–12].
PIGE involves measurement of characteristic prompt gamma-rays emitted during nuclear processes like inelastic scattering (p, p′γ), and nuclear reactions namely (p, αγ), (p, nγ) and (p, γ). It has been observed that PIGE using low energy (2–5 MeV) proton beam [11–15] is capable of determining concentration of low Z elements like Li, Be, B, F, Na, Al, Mg and Si. In our previous work [10, 11] we have quantified F in borosilicate glass [13], Li in Li doped Nd2Ti2O7 [14] and Li2TiO3 [15] by PIGE method using 4 MeV proton beam. In our earlier work on Li2TiO3, Ti was determined by INAA and both Ti and O concentrations could not be determined by PIGE [15]. Using 4 MeV proton beam, it was difficult to determine Ti (Z = 22) by measuring 983 keV gamma-ray due to its lower analytical sensitivity. Whereas determination of oxygen using 495 and 871 keV gamma-rays from 16O(p, γ)17F and 17O(p, p′γ)17O nuclear reactions was difficult due to lower thick target gamma-ray yield (of the order of 103) at 4 MeV proton beam [11]. Additionally at this lower proton beam energy, oxygen concentration could not be determined using 6129 keV gamma ray obtained from 16O(p, p′γ)16O, since this reaction needed proton energy greater than 6.1 MeV to occur [12, 16]. Thus, in general using low energy proton beam, it is difficult to determine elements like C, N, O, P, S, Cl, K, Ca and even higher Z element due to their lower sensitivity or in some cases like C they need higher proton energy [11, 16]. In view of this, literature reports for these elements by PIGE are rare [16]. PIGE method using higher proton energy ≥7 MeV is capable of determining all the above elements including C, N and O [16] and even higher Z elements beyond Zn. Thus using proton energy higher than 7 MeV, it is possible to quantify Li, O and Ti simultaneously in Li2TiO3. The prompt gamma-rays 478 and 429 keV from 7Li(p, p′γ)7Li and 7Li(p, nγ)7Be; 889, 983, 1312 and 1437 keV from 48Ti(p, p′γ)48Ti and 6129 keV from 16O(p, p′γ)16O can be used for the quantification of respective elements [16].
In the present work, PIGE method using 8 MeV proton beam has been standardized for simultaneous determination of Li, Ti and O concentrations. The method has been validated using synthetic samples and applied for compositional characterization of four samples of Li2TiO3 synthesized by sol–gel route.
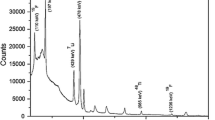
Experimental
Pellets of sample and standards were prepared in graphite matrix (total mass 650 mg). Standards for lithium, titanium and oxygen were prepared by homogenously mixing Li2CO3 (~50 mg) and TiO2 (~50 mg) in graphite matrix. Four samples of Li2TiO3 (100 mg each), two synthetic samples using Li2SO4·2H2O and TiO2 and two graphite blank samples were prepared in similar way. The target pellets were mounted on an aluminum ladder and kept at 45° with respect to beam direction inside a reaction chamber under vacuum conditions (10−6 torr). The targets were irradiated with 8 MeV proton beam (beam current ~10–15 nA) using BARC–TIFR Pelletron facility. Prompt gamma-rays at 478, 983 and 6129 keV corresponding to 7Li, 48Ti and 16O were measured using a 30 % relative efficiency HPGe detector coupled to an 8 k multi-channel analyzer (MCA). The detector was placed at 90° angle with respect to the beam direction. The PIGE set up at BARC–TIFR Pelletron facility is shown in Fig. 1. A typical PIGE spectrum of a Li2TiO3 is shown Fig. 2. The gamma-ray spectrum clearly indicates the characteristic peaks of Li, Ti and O which were used to obtain the concentrations of the respective elements. The gamma-ray peaks of aluminium may be due to the scattered proton falling on the aluminium ladder used. Additionally, gamma-ray spectrum contains characteristic peak of 12C (4430 keV) and first and second escape peaks of C and O. In the present work, lithium sulphate was used as a stoichiometric compound for Li and used for validation of PIGE method and high purity graphite (C) was used as the matrix for preparing samples and standards. The blank graphite pellet did not show any gamma ray peak at the peaks of interest of Li, Ti and O. Also there was no indication of other micro- or trace elements in the gamma ray spectrum that would affect the experiment. Rutherford backscattering spectrometry (RBS) method was used for beam current monitoring using a thin Au foil (1.6 mg cm−2), which was fixed in a holder placed in front of the target ladder. A silicon surface barrier detector, kept at an angle of 135° with respect to the beam direction, was used for measuring backscattered protons from the gold foil. Figure 3 shows a typical RBS spectrum of Au foil.
Calculations
Prompt gamma-rays 478, 983 and 6129 keV corresponding to 7Li, 48Ti and 16O, respectively, were used for calculating concentrations due to their higher thick target γ-ray yields at higher proton energy (≥7 MeV) [16]. Beam current normalized count rate was obtained by dividing the count rate of gamma-ray of interest (Li, Ti and O as the case may be) by RBS count rate of Au-foil. The concentrations of Li, Ti and O were calculated using Eq. 1.
where CPSi, Sample (counts per second) is the count rate due to the ith element in the sample, CPSRBS, Sample is the count rate due to Au in the RBS spectrum and S i is the current normalized (RBS count rate) sensitivity of elemental standard of interest. S i values of Li, Ti and O were obtained from count rate (CPS i ), concentration of the elemental standard C i,Std and RBS count rate as given in Eq. 2.
Results and discussion
The PIGE method was validated by determining Li, Ti and O concentrations in two synthetic samples (Table 1). The obtained concentration values of Li, Ti and O were found to be in good agreement (±1–2 %) with the calculated concentration values (Table 1). The results of four sol–gel synthesized samples are given in Table 2. The concentrations obtained for Li, Ti and O in these four samples are in the range of 11.8–12.7 wt%, 43.3–43.8 wt% and 43.7–44.3 wt%, respectively. The calculated values of Li, O and Ti in stoichiometric Li2TiO3 are 12.67, 43.66 and 43.67wt %, respectively. The combined propagated uncertainties at ±1 s confidence limits in the results (Tables 1, 2) were obtained from counting statistics of sample, standard and current normalizing element (Au) of RBS and the respective masses of sample and standard. The uncertainty values for Li, Ti and O concentrations were within ±3, ±3 and ±8 %, respectively. Higher uncertainty for O is due to low γ-ray detection efficiency at 6129 keV γ-ray leading to higher counting statistics error.
When LiCl was used as starting material for Li, the product Li2TiO3 obtained was crack-free but with loss of Li in the final product [2, 14], whereas Li loss was negligible when LiNO3 was used as starting material for Li2TiO3 but the product obtained was not crack-free. Thus, attempts were made to prepare the said compound using 1:1 mixture of LiCl and LiNO3 at sintering temperatures of 1000 and 1250 °C [2] to get crack-free stoichiometric compound. The composition of Li2TiO3 thus formed from LiCl and LiNO3 mixture (1:1) sintered at 1000 °C temperature, compared to sintered at 1250 °C, was found to be in good agreement with stoichiometric Li2TiO3 (Table 2).
The 3σ detection limits of PIGE at 8 MeV proton beam for Li, Ti and O, in one representative sample of Li2TiO3, were calculated using sample background and respective elemental sensitivities. The detection limit (L D in mg kg−1) was evaluated using Eq. 3.
where σ b is square root of background counts (√C b ) under γ-ray peak of interest of sample, LT is the live time of counting in seconds and S is the sensitivity of element (Li, Ti or O) in terms of counts per second (CPS) per unit concentration (mg kg−1). The detection limits for Li, Ti and O are 4.0, 8.0 and 136 mg kg−1 (Table 2) in the sample. Though detection limit for oxygen by PIGE is slightly poorer compared to other techniques [7–9] but its concentration could be determined simultaneously with Li and Ti without any interference. Thus, a non-destructive method for lithium, titanium and oxygen concentration determination could be standardized which is an important application of PIGE using this medium (8 MeV) energy proton beam.
Conclusions
A relative PIGE methodology using 8 MeV proton beam was optimized for simultaneous determination of Li, Ti and O in Li2TiO3 samples, which was the main objective of this work. The PIGE method optimized has several advantageous: (i) non-destructive analysis, which is most important for a ceramic sample, (ii) simultaneous determination of these three elements (Li, Ti and O) and (iii) there were no gamma-ray interferences. The method thus standardized is a promising non-destructive approach for compositional characterization of lithium based ceramic tritium breeders under chemical quality control exercise.
References
Roux N, Avon J, Floreancig A, Mougin J, Rasneur B, Ravel S (1996) J Nucl Mater 233–236:1431–1435
Vittal Rao TV, Bamankar YR, Mukerjee SK, Aggarwal SK (2012) J Nucl Mater 426:102–108
Tsuyoshi H, Fumiaki O (2011) Fusion Eng Des 86:2172–2175
Tang T, Zhang Z, Meng J-B, Luo D-L (2009) Fusion Eng Des 84:2124–2130
Mandal D, Sathiyamoorthy D, Govardhana Rao V (2012) Fusion Eng Des 87:7–12
Li Y, Cang X, Wang X, Li L, Kong L (2012) Mater Lett 89:25–27
Chowdhury DP, Arunachalam J, Verma Rakesh, Sujit Pal, Gangadharan S (1992) J Radioanal Nucl Chem 158:463–470
Ehmann WD, Ni BF (1992) J Radioanal Nucl Chem 160:169–179
Ryo I, Hideaki S (1991) Mater Trans 12:1164–1169
Calzolai G, Chiari M, Lucarelli F, Nava S, Taccetti F, Becagli S, Frosini D, Traversi R, Udisti R (2014) Nucl Instr Meth B318:125–129
Kiss ÃZ, Koltay E, Nyako B, Somorjai E, Antilla AJ, Räisäien J (1985) J Radioanal Nucl Chem 89:123–141
Savidou A, Aslanoglou X, Paradellis T, Pilakouta M (1999) Nucl Instr and Meth B152:12–18
Chhillar S, Acharya R, Sodaye S, Sudarshan K, Santra S, Mishra RK, Kaushik CP, Choudhury RK, Pujari PK (2012) J Radioanal Nucl Chem 294:115–119
Chhillar S, Acharya R, Vittal Rao TV, Bamankar YR, Mukerjee SK, Pujari PK, Aggarwal SK (2013) J Radioanal Nucl Chem 297:1597–1603
Chhillar S, Acharya R, Pai RV, Sodaye S, Mukerjee SK, Pujari PK (2012) J Radioanal Nucl Chem 293:437–441
Räisänen J, Witting T, Keinonen J (1987) Nucl Instr Meth B28:199–204
Acknowledgments
Authors thank Dr. A. Goswami, Head, RCD and Dr. S. K. Aggarwal, Associate Director, RC&I Group and Head, FCD, BARC, Mumbai for their support and encouragement. Authors thank Mr. T.V.Vittal Rao and Mr. Y.R. Bamankar, FCD, Dr. Suresh Kumar and Dr. A. Gupta, NPD, BARC and Prof. V. Nanal, TIFR for their help. Authors thank crew members of BARC–TIFR pelletron facility, Mumbai for their help and support during the experiment. One of the authors (Sumit Chhillar) thanks HBNI-DAE for the PhD fellowship to pursue this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chhillar, S., Acharya, R., Tripathi, R. et al. Compositional characterization of lithium titanate ceramic samples by determining Li, Ti and O concentrations simultaneously using PIGE at 8 MeV proton beam. J Radioanal Nucl Chem 305, 463–467 (2015). https://doi.org/10.1007/s10967-015-4037-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4037-1