Abstract
Based on the preparation of chloromethylated polysulfone(CMPS), two types of sulfonated polysulfone, naphthalenesulfonic acid type sulfonated polysulfone(PS-NS) and pyrenesulfonic acid type sulfonated polysulfone(PS-TS) were successfully obtained via nucleophilic substitution with 2-naphthol-6,8-disulfonic acid dipotassium(NS) and 8-hydroxy-1,3,6-pyrene trisulfonic acid sodium(TS) as reagents, respectively. The PS-NS and PS-TS Proton exchange membranes (PEMs) were obtained by solution casting method after characterizing their structure by FT-IR and 1H-NMR. The impact of phenyl side chain on performance including water uptake(WU), swelling ratio(SR) and proton conductivity(PC) of PEMs were explored. Atomic force microscopy(AFM) shows that the phase separation structures of PS-NS and PS-TS PEMs are distinct due to the positioning of the hydrophilic sulfonic acid groups far away from the hydrophobic main chain. So the PS-NS and PS-TS PEMs keep excellent size stability, oxidative stability and methanol permeability at high WU. At the same bonding amount(BA) of sulfonic acid, due to less hydrophilic chain segment and a larger number of sulfonic acid groups in the hydrophilic chain segment, which is conducive to the aggregation of ion cluster. The PS-TS present better size stability and anti-oxidative at high WU. The SR of PS-TS-3 PEM was only 20.3 and 48.1%. Meanwhile, the methanol permeability was only 7.54 × 10−7cm2·s−1 at room temperature, much lower than commercialization Nafion115 PEM (16.8 × 10−7cm2/s at room temperature). The research results are of great significance for the design and preparation of high-performance membrane materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proton Exchange Membrane Fuel Cells (PEMFCs) have gained attention as a promising alternative for energy conversion in the past few decades which have been employed in a large number of fields (e.g. stationary power, portable power sources) due to the zero pollution emissions and high energy efficiency [1, 2]. As the core components of PEMFCs, PEMs play a crucial role by serving as the electrolyte, facilitating the transfer of hydrogen ions from the anode to the cathode, and acting as a separator between gases/fuels and oxidant [3]. Nafion series PEMs, a perfluorosulfonic acid membrane have been the most widely used PEMs due to their excellent mechanical properties, high proton conductivity and thermal stability. However, high cost, high methanol permeability in direct methanol PEMFCs and low proton conductivity at high temperature (> 80℃) have limited the further application of PEMFCs [3,4,5]. To overcoming the shortcoming of Nafion PEMs, many methods including modified Nafion materials [6], acid–base blends [7], inorganic–organic composites [8] have been used. Also, much attention has been focus on research and development of alternative PEMs materials [9, 10].
Sulfonated aromatic polymer materials including sulfonated polysulfone [11], sulfonated poly (arylene ether) [12], sulfonated poly(arylene ether ketone) [13] and sulfonated polybenzimidazole [14] were considered to be one of the most promising non-fluorine film materials due to the excellent mechanical stability, lower methanol permeability and excellent membrane-forming properties [11, 12]. Most of these sulfonated aromatic polymer PEMs are main chain type, where the sulfonic acid groups are directly attached onto the backbones of aromatic polymers [13, 14]. However, these main chain PEMs suffer from high water swelling ratio(SR) and poor in hydrolytic stability with increasing degree of sulfonation [15]. Obviously, it is difficult to obtain high performance PEMs by preparing main chain type aromatic polymers. The side chain sulfonated PEMs can overcome these drawbacks by locating the hydrophilic groups far from the hydrophobic main chain resulting in significant micro-phase separation between hydrophilic and hydrophobic domains. Because the absorbed water is confined to the hydrophilic region, which has little effect on the main chain of aromatic polymers. Finally, the side chain PEM can maintain good dimensional stability and oxidative stability at high water uptake(WU). Consequently, much attention has been paid on the research and development of side chain type aromatic polymer PEMs [16, 17]. Bagher et al. prepared side chain and main chain type sulfonated polybenzimidazole PEMs, respectively. The effect of side chain structure on the properties of PEMs was studied. The results show that the side chain PEMs exhibited better size stability due to the better phase separation structure [18]. Zeng et al. prepared a series of long flexible alkylsulfonated side chains poly (phthalazinone ether ketone). The effect of side chain lengths on properties of PEMs were investigated systematically. The PC of PEM were up to 107mS/cm at 30 ℃ and 184mS/cm at 80 ℃, higher than that of Nafion115 [19]. Thuyavan et al. prepared a series of poly(aryl ether sulfone)s containing eight alkyl sulfide sulfonated side chains. Due to the clear hydrophilic/hydrophobic phase separation structure. The PC of PEM was up to 82 mS/cm at 30 ℃. which exceeds the PC of Nafion 117 (79 mS/cm) [20].
In this study, PS was selected as polymer backbones due to its excellent thermal stability, good hydrolytic stability against hot water and outstanding resistance to inorganic acid and bases. Two kinds of side chain type sulfonated polysulfone, PS-NS and PS-TS with different hydrophilicsegments were successfully prepared. By locating hydrophilic sulfonic acid group far away from hydrophobic main chain of PS, the PS-NS and PS-TS PEMs exhibited remarkable dimensional stability, oxidative stability and methanol permeability at high WU due to the obvious characteristic of micro-phase separation structures. Meanwhile, the effect of hydrophilic segment structure on performance including water sorption, size stability and proton conductivity of PEMs at the same bonding amount of sulfonic group were explored. The results hold significant scientific and practical value for the preparation of high-performance sulfonated aromatic polymer PEMs.
Experimental
Materials
Polysulfone (PS, Ningbo Deqi Special Plastics Co., Ltd); 2-naphthol-6,8-potassium disulfonate (NSK, Hubei Jusheng Technology Co., Ltd)was of industry grade; Trisodium 8-hydroxy-1,3,6-pyrene trisulfonate (TS, Shanghai Jizhi Biochemical Technology Co., Ltd)was of industry grade; Anhydrous tin tetrachloride(Tianjin Yuanli Chemical Co., Ltd)was of analytical grade; Dimethyl sulfoxide (DMSO,Tianjin Bodi Chemical Co., Ltd)was of analytical grade. All solvents shall be dried with anhydrous magnesium sulfate before use.
Preparation and characterization of modified polysulfone
Dry trichloromethane (30.0 ml) and PS (1.0 g) were added into a three-neck bottom flask (250.0 ml) equipped with a stirrer and a reflux condenser. The anhydrous stannic chloride(0.26 ml) and 1,4-Bis(chloromethixy)butane 5.6 ml were added after PS was dissolved. The reaction solution was stirred at 50 ℃ for several hours.Then the resultant solution was washed with hydrochloric acid to remove stannic chloride. The resultant mixture was precipitated in 120.0 mL ethanol. After being filtered and washed with ethanol three times, CMPS was thereby obtained.
PS-NS and PS-TS were prepared by similar experimental methods, the preparation process of PS-TS was illustrated as an example. CMPS(1.0 g) was dissolved in 50.0 ml DMSO. After dissolution, Na2CO3, KI and TS(3.8 g) were added. The mixture was obtained by controlling the reaction time at 110 ℃. The resulting solution was cooled to room temperature, and PS-TS was obtained after precipitating in 200.0 mL ethanol, followed by centrifugal separation and ethanol washing for three times. PS-TS was dried at 50 ℃ under vacuum until the weight of the polymer reached a constant value.
By controlling the reaction time, PS-NS and PS-TS with different content of sulfonic acid groups were obtained, which were recorded as PS-NS-1, PS-NS-2, PS-NS-3, PS-TS-1, PS-TS-2 and PS-TS-3. The chemical reaction process of PS-NS and PS-TS was shown in Fig. 1.
The structures of PS-NS and PS-TS were characterized by FT-IR and 1H-NMR spectra. The data of 1H-NMR spectra can be used to calculate the degree of substitution of sulfonic acid group(DS) = 3A2/A1 (where A2 is the integral area of the side chain methylene proton; A1 is the integral area of the polysulfone main chain isopropyl proton) [20]. The chlorine contents of CMPS, PS-NS and PS-TS were determined by Volhard oxygen–nitrogen combustion and back-titration method, and the bonding amount(BA, mmol/g) of sulfonic acid group was calculated [13, 14].
Preparation of PS-NS and PS-TS PEMs
The PS-NS and PS-TS PEM materials were dissolved in CHCl3. Then the resulting solution was casted onto a clean Petri dish and dried at 50 ºC for 24 h in a vacuum oven to remove the solvent, followed by being soaked in de-ionized water to fabricate a transparent film with 50–60 um thickness. The film was converted into PEM by immersing in 2 M hydrochloride for 48 h, followed by washing with de-ionized water for several times until the pH value of washing solution reached a constant value. The as-prepared PS-NS and PS-TS PEM was kept in de-ionized water.
Characterization
The chemical structures of PS-NS and PS-TS were confirmed via FT-IR (1700 infrared spectrometer, Perkin-Elmer Company, USA) and 1H-NMR spectrometry (DRX300 nuclear magnetic resonance spectrometer, Bruker Company, Switzerland); The FTIR spectra were collected at 64 scans in the 4000–400 cm−1.The degree of chloroacylation was measured using combustion-Volhard titration method (Oxygen bomb calorimeter WGR-1Changsha instrument company, China) [21].
Ion exchange capacity
IEC value directly depends on the content of sulfonic acid group incorporated onto the polymer and was an indication of the actual ion bits for proton conduction. The IEC value was measured by acid–base titration method. The dry PEMs (m) were immersed in 1 M NaCl solution for 48 h to replace the H+ with Na+, the released H+ were titrated by 0.1 M NaOH solution using phenolphthalein as an indicator, the IEC value was calculated by the following equation:
where \(C_{NaOH}\) and \(V_{NaOH}\) were the concentration and the volume of NaOH, respectively. m was the weight of dry membrane.
Water uptake and swelling ratio
The PEM samples were dried at 100 ºC under vacuum for 24 h until the weight value reached a constant value. The samples were immersed in de-ionized water at different temperature from 25–85 ºC for 48 h, then the PEMs were taken out, wiped with tissue paper, and their weight was measured. The values of water uptake (WU) and swelling ratio(SR) were calculated by following formula:
where W1 and W2 were the weigh of dried and wet membranes, L1 and L2 were the length of dried and wet membranes, respectively.
Proton conductivity
The resistance (R) of PEM was estimated using electrical impedance spectroscopy method using electrochemical workstation (CHH660 electrochemical workstation, Chenhua instrument company, China). The PEM was clamped between two platinum electrodes and immersed into de-ionized water (PH = 7) to keep the relative humidity (RH) at 100% during the measurement and electrochemical interface over the frequency range of 10 ~ 106 Hz at different temperatures. The proton conductivity \(\sigma\) (s·cm−1) of PEMs were calculated by formula (4).
where R is the resistance of the PEMs, L is the thickness of PEMs and A is the effective area of the membrane.
Morphology test
The morphology structure of PEM was studied by AFM (Veeco dilnnova SPMA atomic force microscope). The experiments were carried out in Acoustic Alternating Current Mode at room temperature. Point probe pulse/non-contact/long cantilever (PPP-NCL) tips were used in the experiments. The set-point used for imaging varied between 0.5 and 1.0 V. All phase images were shown as recorded without any additional image processing. TEM images were determined by JEOL2010. Before testing, the PEMs were stained with silver by ion exchange of sulfonic acid groups, immersed in 0.5 M AgNO3 aqueous solution for 48 h, rinsed with water, and dried at room temperature for 12 h. The H+ in the sulfonated polysulfone PEMs was replaced by Ag + to form a good inverted phase in transmission photographs.
Oxidative stability and methanol permeability
PEM samples were immersed into Fenton’s reagent (3% H2O2 and 2 × 10–6 mg·L−1 FeSO4) at 80 ℃ for different time. Oxidative stability was evaluated by the retained weight (RW) and dissolved time of PEMs.
Methanol permeability was measured by methanol diffusion coefficient(DK). The higher the diffusion coefficient (DK)of alcohol, the higher the permeability of methanol. A two-chamber glass diffusion cell equipped with two compartments which was separated by a vertical PEM was used to measure the methanol permeability of PEMs at 25 ℃. One side of the glass diffusion cell was filled with 1.0 M methanol and the other side was pure water. Each side of diffusion cell was stirred by magnetic stirring continuously throughout the process of measurement and the methanol concentration of two compartments was measured by gas chromatograph (P230) liquid chromatography, Elite Analytical Instruments Co. Ltd, China). The DK was calculated by formula (5).
where CA, CB were the methanol concentration in feed and permeate, respectively; VB was the volume of diffusion cell; A, L and t were the effective area, the thickness of PEMs and the time of diffusion, respectively.
Mechanical properties
The mechanical properties of PEMs were measured using a universal testing machine by CMT6502. at 27 °C with a 5 mm·min−1 speed. The properties of PEM were measured at 25℃ with a thickness of 0.1 mm and area was 1 × 5cm2.
Results and discussions
FT-IR
Taking the preparation of PS-TS as an example, the infrared spectra of PS, CMPS and PS-TS were showed in Fig. 2.
In the infrared spectrum of PS, the characteristic absorption peaks of PS were appeared. The absorption peaks of carbon atoms on the benzene ring of the main chain were at 1490 and 1585 cm−1, and the absorption peaks of main chain -SO2- were at 1080, 1295 and 1325 cm−1, respectively. The vibration absorption peaks of phenoxy-oxygen bond were 1245 and 1015 cm−1. In the infrared spectrum of CMPS, in addition to all the absorption peaks of PS, two new absorption peaks appeared at 1440 and 670 cm−1, which were the stretching vibration absorption peak and the bending vibration absorption peak of C–Cl bond. In the infrared spectrum of PS-TS, the absorption peak of sulfonic acid group was 1050 cm−1 and the stretching vibration absorption peakwas 1499 cm−1. The above changes in the infrared spectrum indicate that we had successfully prepared PS-TS.
1H-NMR spectrum
The 1H-NMR spectrum of CMPS, PS-NS and PS-TS were obtained by dissolving the polymer with deuterated chloroform as solvent, as shown in Fig. 3.
As shown in above figure, the 1H-NMR spectrum of CMPS was shown in Fig. (A). There were many protons in the main chain of polysulfone, which were concentrated between 6.831 and 7.883 ppm. A new chemical shift of chloromethyl protons in the side chain of polysulfone appears at 4.541 ppm. The 1H-NMR spectrum of PS-NS was shown in Fig. (B). Due to the introduction of the side chain naphthalene disulfonic acid group, the 1H-NMR spectrum has changed greatly. The peaks in the range of b-j coincide with each other, and a group of large absorption peaks appear in the range of 6.782–8.016 ppm. The proton shift at the side chain methylene k was 4.557 ppm, and the chemical shift at the m, n and l of the side chain naphthalene ring was at 7.802 ppm, 7.312 ppm and 7.963 ppm, respectively. The 1H-NMR spectrum of PS-TS was shown in Fig. (C). The chemical shifts of the main chain protons coincide with each other and are concentrated at 6.783 ~ 7.847 ppm. Due to the introduction of the side chain pyrene sulfonic acid group, the nuclear magnetic hydrogen spectrum of CMPS has changed a lot, and the proton chemical shift of the side chain methylene appears at 3.581 ppm. The chemical shifts of protons at s, o, p, q, m and n on pyrene sulfonic acid occured at 7.201 ppm, 7.852 ppm, 6.927 ppm, 7.352 ppm, 8.952 ppm and 7.832 ppm respectively. It was further confirmed that we have successfully prepared the modified polymers PS-NS and PS-TS through this spectrum.
Properties of PEM
Two series of PSF-NS and PS-TS were prepared by controlling the nucleophilic substitution reaction time and corresponding PEMs were obtained by solution casting method. According to the bond amount of sulfonic acid groups, sulfonated polysulfones PEMs were labled as PS-NS-1, PS-NS-2, PS-NS-3, PS-TS-1, PS-BDS-2, and PS-TS-3, respectively. The basic properties of PS-BS and PS-BDS PEMs were listed in Table 1.
PS-NS and PS-TS PEMs with different BA of sulfonic acid groups were prepared by controlling the reaction time of nucleophilic substitution reaction on the basis of preparing CMPS. According to the sulfonic acid group content, PS-NS and PS-TS PEMs were recorded as PS-NS-1, PS-NS-2, PS-NS-3, PS-TS-1, PS-TS-2 and PS-TS-3 respectively. With the change of sulfonic acid group content, the basic properties including IEC, RW and DK of PS-NS and PS-TS PEMs have undergone great changes in performance. As seen from Fig. 1, although PS-NS and PS-TS PEMs have the same main chain structure of PS, their side chain structure was different, resulting in the different properties under the same conditions. In the following sections the effect of side chain structure on performance of PS-NS and PS-TS PEMs would be studied in detail.
Morphology properties
PS-NS and PS-TS PEM could form an obvious micro-phase separation structure by locating the hydrophilic sulfonic groups far away from hydrophobic backbone, resulting in the formation of connected water channels for ion conduction. The higher the degree of phase separation, the wider the proton transport channel, the more favorable the enhancement of proton conductivity. AFM and TEM was often used to study the microstructure of PEM. Figs. 4 and 5 showed the AFM and TEM images of PS-NS and PS-TS PEM.
Figure A, B, C, D represent the AFM diagram of PS-NS-2, PS-NS-3, PS-TS-2 and PS-TS-3 respectively. They all show clear phase separation morphology due to the large polarity difference between the densely hydrophilic flexible side chains and the rigid polymer backbone.The dark region represents the hydrophilic sulfonic acid areas and the brighter areas refer to the hydrophobic moiety which is composed of the main chain of PS. The black areas were connected to each other to form ion transport channels.The degree of phase separation of these PEMs increases with the increase of the degree of sulfonation. For PS-NS and PS-TS PEM at the same BA, PS-TS PEM has many rigid benzene rings, which was not conducive to the rotation of side chain sulfonic acid groups. Hindering the formation of hydrated ion clusters, and ultimately leading to the reduction of PC [23, 24]. So the order of PC of PEM was always PS-NS > PS-TS.
The dark region represents the hydrophilic sulfonic acid groups areas and the brighter areas refer to the hydrophobic main chain moiety. PS-NS and PS-TS all exhibited obvious micro-phase separation structure by locating the hydrophilic sulfonic acid groups areas far away from main chain of polymers.The phase separation structure is beneficial to improve ion transfer efficiency and ionic conductivity of PEMs, which keep the PEMs maintain excellent size stability under high water absorption. With the increase of sulfonic acid group content, we can obviously see the increase of black hydrophilic region.For PS-NS and PS-TS at the same BA of sulfonic acid group,due to the small number of sulfonic acid groups at the end of the side chain and the larger number of hydrophilic chain segments, PS-NS has more black regions in the TEM images,which were conducive to improving the water absorption and conductivity of PS-NS PEM.
Relationship between PEM performance and temperature
Water uptaking
PEM absorbs water, hydrophilic groups and water molecules form hydrated ion clusters, which gather and connect to form proton transport channels. The higher the water content, the more favorable the proton conduction, but too high WU leads to too high SW of PEM, which reduces the dimensional stability of PEM and greatly reduces the practical use of fuel cells. Therefore, the WU of PEM should be controlled while ensuring high PC. Figure 6 shows the relationship between WU and temperature of PS-TS and PS-NS PEM.
The WU of the PEMs increases with the increase of temperature, which was related to the increase of the diffusion rate of water molecules. The increase of temperature promotes the movement of sulfonic groups and water molecules, which was conducive to their mutual combination to form hydrated ion clusters, leading to the increase of WU. At the same sulfonic acid group content, the WU of PS-NS was significantly larger than that of PS-TS PEM, which was related to the molecular structure of PEMs. At the same BA of sulfonic acid group, PS-NS PEM had more hydrophilic segments, which could form more hydrophilic points, and the surrounding space for absorbing water molecules is larger, resulting in an increase in WU. The WU of PS-NS-3 PEM with BA of 1.51 mmol/g reached 23.3% at 25 ℃ and 42.7% at 85 ℃, higher than that of some side chain sulfonated aromatic polymer proton exchange membranes, even higher than that of Nafion115 membrane under the same conditions [22, 23, 25, 26].
Swelling ratio
As one of the important performance of PEM, the dimensional stability directly determines the service life of PEM, and SW determines the dimensional stability of PEM. The lower the SW, the better the dimensional stability of the PEM. In this study, the dimensional stability of PEM is measured by the SW. The smaller the SW, the greater the dimensional stability of PEM. Figure 7 shows the relationship between SW and temperature.
With the increase of temperature, the SR of the PEMs increase, which was consistent with the change of WU. This was because the increase of temperature increases the movement ability of water molecules and sulfonic acid groups, which was easier to combine with each other, and ultimately leads to the increase of SW. Meanwhile, it could be seen that when the temperature was higher than 65 ℃, the water absorption swelling rate increases suddenly, which may be due to the existence of a limit value when the size of the PEM is stable at 65 ℃, and when it reached this limit value, the SR increases. At the same temperature, the size relationship of dimensional stability is PSF-TS > PSF-NS, which is related to their molecular structure. Under the same BA of sulfonic acid group, PS-TS PEM had fewer hydrophilic chain segments, higher water absorption efficiency and more obvious phase separation structure. The absorbed water is limited in the hydrophilic region, which reduces the influence of water on the main chain of hydrophobic polymer and keeps it in good dimensional stability. The SR of PS-TS-3 PEM at 25 ℃ and 85 ℃ was only 20.3% and 48.1%, lower than that of some side chain sulofonated aromatic polymer PEMs and Nafion115 membrane under the same conditions [22, 26, 24, 27, 28].
Proton conductivity
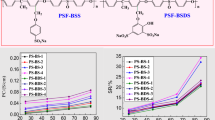
As one of the most important performance indicators of PEM, PC directly determined the performance of PEM. PEM can be used for fuel cells only when it meets the minimum requirements of fuel cell for PC. Figure 8 shows the relationship between the PC of PEM and temperature and Fig. 9 gives the relation of proton conductivity with humidity.
It can be seen from the Fig. 7 that the PC of the two PEMs increases with the increase of temperature. This is because the movement of the sulfonic acid group and water molecules increases with the increase of temperature, which makes it easier to combine with each other to form hydrated ion clusters, and the diffusion ability of water molecules also increases. The transmission of hydrogen protons is mainly determined by the diffusion of water molecules and the transport of hydrated ion clusters. The two effects jointly lead to the increase of PC of PEMs. Their PC is higher than 0.01S/cm (the minimum requirement for PC of membrane in fuel cells) [26, 27, 29], which can be applied to the practical application of fuel cells. Under the same BA of sulfonic acid group, the size relationship of PC is PSF-NS > PSF-TS, which is consistent with their WU. More water molecules and sulfonic acid groups combine with each other to form hydrated ion clusters, then the hydrated ion clusters combine with each other to form proton transport channels, resulting in an increase in proton conductivity. The PC of PS-NS is 0.083S/cm at 25 ℃ and 0.141S/cm at 85 ℃, higher than some side chain sulfonated polymer PEMs [30, 31], which is close to that of commercial Nafion115 (0.09S/cm and 0.157S/cm at 25 ℃ and 85 ℃, respectively) [22, 29, 31, 32].
The proton conductivity was closely related to the relative humidity(RH) and IEC of the PEM. Figure 8 shows the variation curve of proton conductivity of PS-NS-3 and PS-TS-3 PEMs with relative humidity. Sulfonic acid groups bind water molecules to form hydrated ion clusters, and hydrated ion clusters conduct proton conduction. The more hydrated ion clusters, the stronger the proton conduction ability. Therefore, with the increase of RH at the same temperature, the proton conduction ability was enhanced. When the humidity was less than 50%, the increase rate of proton conductivity of the PS-NS-3 and PS-TS-3 PEMs was relatively large, while when the humidity is higher than 50%, the increase rate of proton conductivity was significantly reduced. This is because proton conductivity was mainly determined by RH and BA of sulfonic acid group. RH was the determinant of proton conductivity at low humidity. Meanwhile, bonding amount of sulfonic acid group content was the determinant of proton conductivity at high humidity.
Oxidative stability and methanol permeability
The fuel cells will produce free radicals such as HOO• and HO• during their operation, which will attack the PEM, resulting in the rupture and even dissolution of the PEM, greatly reducing the service life of the PEM. When methanol is used as fuel, poor alcohol resistance will cause waste of fuel gas. Therefore, oxidative stability and methanol permeability are important indicators of PEM.
Table 1 shows that the mass retention rate of the two PEMs is above 98% after being immersed in Fenton reagent for 1 h, which is higher than that of commercial Nafion membrane. At the same time, we also found that PS-NS and PS-TS PEM have a certain degree of weight loss and did not be dissolved after soaking in Fenton’s reagent for 7 h. We also observed with our eyes that the surface of PEMs was smooth and transparent, did not break track, which fully suggested the three PEMs had excellent oxidative stability. PS-NS and PS-TS PEM maintain excellent methanol permeability and the methanol permeability is much lower than some side chain sulfonated aromatic polymers PEMs [33, 34], even lower than Nafion115 membrane under the same conditions [16, 19, 20]. Meanwhile, we can see that the methanol permeability of PS-NS and PS-TS PEM increases with the increase of the capacity and water absorption, which indicates that the methanol permeability strongly depends on the water absorption of the PEM, but the alcohol resistance of the two PEMs is much higher than that of the Nafion115 membrane. In addition, under the same sulfonic acid group content, the antioxidant stability and alcohol resistance are both PSF-TS > PSF-NS, and their order remains consistent, which is consistent with their dimensional stability. Good dimensional stability increases the density of the PEM, which can effectively prevent the passage of free radicals and methanol, so as to maintain good antioxidant stability and alcohol resistance [22, 29, 35, 36].
Mechanical properties
As one of the important indicators of the proton exchange membrane, mechanical property determines the stability of the PS-NS and PS-TS, excellent mechanical properties can keep the PEM to maintain a long service life. Mechanical properties were determined by the structure of the PS-NS and PS-TS. The mechanical properties of PS-NS and PS-TS were given in Table 2.
As seen from the Table 2, with the increase of BA of sulfonic acid group, the tensile strength and Young’s modulus of the PS-NS and PS-TS decrease. The introduction of side chain increases the flexibility of PS. At the same time, sulfonic acid group has a great plasticizing effect on PS, which reduces the strength of PS and increases the toughness, resulting in a decrease in tensile strength and Young’s modulus, on the contrary, the elongation at break increases. Compared to PS-NS, more rigid benzene rings and fewer hydrophilic chain segments were bonded on PS at the same IEC, PS-TS exhibits stronger rigidity, the tensile strength and Young’s modulus of PS-TS PEM were greater than PS-NS at the same condition. On the contrary, the toughness of PS-NS was stronger, and the elongation at break was stronger than PS-TS.
Conclusion
In this work, two types of sulfonated polysulfone, PS-NS and PS-TS with phenyl side chain were obtained. The PEM maintained obvious micro-phase separation structure by locating the sulfonic group far away from main chain of polysulfone. The side chain structure has an important effect on the properties of the two proton exchange membranes. The WU of PS-NS-3 PEM with BA of 1.51 mmol/g reached 23.3% at 25 ℃ and 42.7% at 85 ℃, higher than that of Nafion115 PEM under the same conditions. Due to fewer hydrophilic chain segments and higher water absorption efficiency, PS-TS PEM has better dimensional stability, antioxidant stability and alcohol resistance. The SR of PS-TS-3 PEM was only 20.3% and 48.1%. Meanwhile, the methanol permeability was only 7.54 × 10−7cm2·s-1 at room temperature, much lower than commercialization Nafion115 PEM. This study has important reference significance for the design and preparation of high-performance proton exchange membranes.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article at https://doi.org/10.1007/s10965-023-03856-7.
Change history
19 September 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10965-024-04141-x
References
Yang X, Kim JH, Kim YJ (2022) Enhanced proton conductivity of poly(ether sulfone) multi-block copolymers grafted with densely pendant sulfoalkoxyl side chains for proton exchange membranes. J Power Sources 242:124604
Vinothkannan M, Kannan R, Kim RA, Gnana Kumar GG, Nahm SK, Yoo JD (2016) Facile enhancement in proton conductivity of sulfonated poly(ether ether ketone) using functionalized graphene oxide—synthesis, characterization, and application towards proton exchange membrane fuel cells. Colloid Polym Sci 294:1197
Yusoff YN, Shaari N (2021) An overview on the development of nanofiber-based aspolymer electrolyte membrane and electrocatalyst in fuel cell application. Int J Energy Res 45:18441
Naffati N, Fernandes M, Bermudez VZ (2022) Chitosan-based electrolytes containing carbon nanotube-titanium dioxide for energy conversion devices applications. Iran Polym J 31:1197
Daud SNSS, Norddin MNAM, Jaafar J, Sudirman R (2021) Development of sulfonated poly(ether ether ketone)/polyethersulfone-crosslinked quaternary ammonium poly(ether ether ketone) bipolar membrane electrolyte via hot-press approach for hydrogen/oxygen fuel cell. Int J Energy Res 45:9210
Liu QT, Li YQ, Yang SK (2023) Transfer-free in-situ synthesis of high-performance polybenzimidazole grafted graphene oxide-based proton exchange membrane for high-temperature proton exchange membrane fuel cells. J Power Sources 559:232666
Wang ZW, Zheng H, Chen Q, Zhang SM, Yang F, Kang J, Chen JY, Cao Y, Xiang M (2019) Preparation and characterization of PVA proton exchange membranes containing phosphonic acid groups for direct methanol fuel cell applications. J Polym Res 26:200
Jang J, Ahn MK, Lee SB (2021) Conductive and Stable Crosslinked Anion Exchange Membranes Based on Poly(arylene ether sulfone). Macromol Res 29:157
Liu X, Li Y, Li M, Xie N, Zhang J, Qin Y, Yin Y, Guiver MD (2021) Durability enhancement of proton exchange membrane fuel cells by ferrocyanide or ferricyanide additives. J Membr Sci 629:119282
Orcid AK, Orcid KV, Hegde GA (2020) Transport and Morphology of a Proton Exchange Membrane Based on a Doubly Functionalized Perfluorosulfonic Imide Side Chain Perflourinated. Polym Chem Mater 32:38
Zhang X, Xia Y, Gong X, Geng P, Gao Z, Wang Y (2009) Preparation and characterization of sulfonated polysulfone/titanium dioxide composite membranes for proton exchange membrane fuel cells International. J Hydrogen Energ 34:3467
Miyatake KJ, Chikashige Y, Watanabe M (2003) Novel Sulfonated Poly(arylene ether): A proton conductive polymer electrolyte designed for fuel cells. Macromolecules 36:9691
Abate L, Asarisi V, Blanco I, Cicala G, Recca G (2010) The influence of sulfonation degree on the thermal behaviour of sulfonated poly(arylene ethersulfone)s. Polym Degrad Stab 95:1568–1574
Kim DS, Liu B, Guiver MD (2006) Influence of silica content in sulfonated poly(arylene ether ether ketoneketone) (SPAEEKK) hybrid membranes on properties for fuel cell application. Polymer 47:7871
Mahalingam A, Deivanayagam P, Pushparaj H (2022) Synthesis and fabrication of BST/SPVdF-co-HFP composites for proton exchange membrane fuel cell applications. J Polym Res 29:513
Wu Z, Shen C, Gao S (2021) Crosslinked Proton Exchange Membranes with a Wider Working Temperature Based on Phosphonic Acid Functionalized Siloxane and PPO. Macromol Res 29:199
Kumar P, Dutta K, Das S (2014) Membrane prepared by incorporation of crosslinked sulfonated polystyrene in the blend of PVdF-co-HFP/Nafion: A preliminary evaluation for application in DMFC. Appl Energy 123:66
Bagher MK, Khadijeh H, Parisa S (2022) A comprehensive review on the proton conductivity of proton exchange membranes (PEMs) under anhydrous conditions: Proton conductivity upper bound. Int J Hydrogen Energ 46(69):34413
Zeng GB, Yan LM, Xue BH, Zhao HB, Zhang JJ (2021) Synthesis of the sulfonated poly(ether ether ketone ketone) proton exchange membrane with butane-1,4-diylbis(oxy) spacers and sulfopropyloxy pendants through Friedel-Crafts polyacylation. Int J Hydrogen Energy 46:2770
Thuyavan LY, Juhana J, Fauzi AI, Sean PG, Arthanareeswaran G, Radzi FMH (2021) Functionalized boron nitride embedded sulfonated poly (ether ether ketone) proton exchange membrane for direct methanol fuel cell applications. J Environ Chem Eng 9:105876
Kobayashi T, Rikukawa M, Sanui K (1998) Proton conducting polymers derived from poly(ether-etherketone) and poly(4-phenoxybenzoyl-1,4-phenylene). Solid State Ionic 106:219
Zhu YQ, Manthiram A (2011) Synthesis and characterization of polysulfone-containing sulfonated side chains for direct methanol fuel cells. J Power Sources 196:7481
Jin CH, Zhu XL, Zhang S, Li S (2018) Highly conductive flexible alkylsulfonated side chains poly(phthalazinone ether ketone)s for proton exchange membranes. Polymer 148:269
Cai SJ, Wang CY, Tao ZW, Qian JF, Zhao XY, Li J, Ren Q (2022) Proton exchange membranes containing densely alkyl sulfide sulfonated side chains for vanadiumredox flow battery. Int J Hydrogen Energy 47:9319
Li YT, Zhang XL, He GH, Zhang FX (2017) Sulfonated poly(phenylene sulfide) grafted polysulfone proton exchange membrane with improved stability. Int J Hydrogen Energy 4:22360
Qiao ZW, Gao BJ, Chen T (2015) Design and preparation of proton exchange membrane of sulfonated polysulfone with side chain-type and studies on its properties. Acta Polym Sin 05:571
Liu D, Dong B, Zhang HB, Xie YJ, Pang JH, Jiang ZH (2021) High methanol resistant polyelectrolyte membrane based on semi-crystalline Poly(ether ketone) with densely sulfonated side chain for direct methanol fuel cell. J Power Sources 482:228982
Ban T, Guo ML, Wang YJ, Zhang YY, Zhu XL (2023) High-performance aromatic proton exchange membranes bearing multiple flexible pendant sulfonate groups: Exploring side chain length and main chain polarity. J Membra Sci 668:121255
Gouda MH, Konsowa AH, Farag HA, Elessawy NA, Tamer TM, Eldin MS (2021) Development novel eco-friendly proton exchange membranes doped with nano sulfated zirconia for direct methanol fuel cells. J Polym Res 28:263
Xu JM, Zhang ZG, Yang K, Zhang HX, Wang Z (2019) Synthesis and properties of novel cross-linked composite sulfonated poly (aryl ether ketone sulfone) containing multiple sulfonic side chains for high-performance proton exchange membranes. Renew Energy 138:1104
Liu D, Dong B, Zhang HB (2021) High methanol resistant polyelectrolyte membrane based on semi-crystalline Poly(ether ketone) with densely sulfonated side chain for direct methanol fuel cell. J Power Sources 482:228982
Chen XL, Lü H, Lin QL, Zhang X, Chen DY, Zheng YY (2018) Partially fluorinated poly(arylene ether)s bearing long alkyl sulfonate side chains for stable and highly conductive proton exchange membranes. J Membra Sci 549:12
Mohammadi M, Mehdipour-Ataei S (2020) Durable sulfonated partially fluorinated polysulfones as membrane for PEM fuel cell. Renew Energ 158:421
Liu LL, Lu Y, Ning C, Li N, Chen SW, Hu ZX (2022) Comb-shaped sulfonated poly(aryl ether sulfone) proton exchange membrane for fuel cellapplications. Int J Hydrogen Energy 47:16249
Li X, Wang S, Zhang H, Lin C, Xie X, Hu C, Tian R (2021) Sulfonated poly(arylene ether sulfone)s membranes with distinct microphase-separated morphology for PEMFCs. Int J Hydrogen Energy 46(68):33978
Liu D, Xie Y, Zhong J, Yang F, Pang J, Jiang Z (2022) High methanol resistance semicrystalline sulfonated poly(ether ketone) proton exchange membrane for direct methanol fuel cell. J Membr Sci 650:120413
Acknowledgements
This work was supported by Natural Science Project of Shaanxi Province (2023-JC-YB-142), the Natural Science Special Project of Shaanxi Province(No.18JK0069) and Natural Science Research Program of Shaanxi Institute of Technology(Gfy23-19)
Funding
Natural Science Project of Shaanxi Province,2023-JC-YB-142,Zongwen Qiao,Natural Science Special Project of Shaanxi Province,18JK0069,Zongwen Qiao,Natural Science Research Program of Shaanxi Institute of Technology,Gfy23-19,Zongwen Qiao
Author information
Authors and Affiliations
Contributions
Qiao Zongwen: Data curation, writing—original draft, methodology, and investigation. Deng Jiaqi: writing- review & editing. Chen Tao: Supervision,writing-review & editing.
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s10965-024-04141-x
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiao, Z., Deng, J. & Chen, T. RETRACTED ARTICLE: Sulfonated polysulfone containing phenyl side chain as proton exchange membrane. J Polym Res 31, 8 (2024). https://doi.org/10.1007/s10965-023-03856-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03856-7