Abstract
In this work, a facial approach was proposed to improve the thermal and electrical conductivity of poly(vinylidene fluoride) (PVDF) by constructing a segregated graphene nanoplatelets (GNP) network structure. PVDF/GNP composites with segregated filler network were fabricated by coating GNP on the surface of PVDF powders using ball milling treatment, followed by compression molding technique. PVDF powders of different particle size (e.g., 100 and 400 μm) were employed to elucidate the influence of matrix particle size on the properties of PVDF/GNP composites. Results showed that the formation of segregated GNP network contributed to a concurrent enhancement of both thermal and electrical conductivity of corresponding composites. A thermal conductivity as high as 2.23 W/mK coupling with an electrical conductivity of about 0.2 S/m was achieved for segregated PVDF100/GNP 6 wt% composite, which demonstrates potential applications in the areas that require both high electric and heat dissipations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is growing interest of metal-replacement using polymer composites in the areas of automotive, aerospace, electronics among others due to their easy processability, tailorable properties, lightweight and excellent resistance to corrosive conditions [1,2,3,4,5,6]. The intrinsic thermal conductivity of pure polymers is quite low (0.1 ~ 0.5 W/mK) and a certain amount (≥ 30 vol%) of thermal conductive fillers are always required to enhance the thermal conductivity to meet practical application requirements [7,8,9]. A consensus has been acknowledged that both the formation of three-dimensional thermal conductive pathways and the increase of filler packing density are crucial to the overall enhancement of thermal conductivity for filler-containing polymers composites [10]. It also becomes inevitable to deteriorate both the processability and mechanical properties of polymers due to the formation of filler agglomerates. As a result, it becomes critical to construct thermally conductive pathways within polymer matrices at lower filler concentrations.
A variety of approaches have been proposed to enhance the thermal conductivity of polymer composites [11, 12]. For example, surface modification of fillers is considered as an effective way to reduce the interfacial thermal resistance between host matrix and the added fillers, thereby leading to an increase of thermal conductivity for subsequent composites. Wie et al. [13] reported the thermal conductivity of surface modified boron nitride (BN) loaded epoxy composites reached 11.8 W/mK at a filler concentration of 75 wt%. The enhanced interaction between polymer and BN was considered as the contributing factor that improves the thermal conductivity. A core–shell SiC@GNP structure consisting of silicone carbide (SiC) and graphene (GNP) was proposed by Wang et al. [14], and the thermal conductivity of epoxy composite with 20 wt% core–shell SiC@GNP reached 1.84 W/mK, which is more than 8 times higher than pure epoxy.
Recently, researchers proposed that constructing novel nanostructures is a more appropriate method which can reduce surface thermal resistance and enhance thermal conductivity of polymer composites [15,16,17,18,19,20,21,22]. The closer distance between fillers can reduce phonon scattering at interfaces between adjacent fillers. Zhang et al. [23] reported that the thermal conductivity of polypropylene/alumina (PP/Al2O3) composites reached 1.07 W/mK at 27.5 vol% Al2O3 by constructing continuous segregated filler network structure. The selective localization of Al2O3 to form continuous thermal conductive pathways is believed to be the contributing factor. Li et al. [24] prepared poly(3-hydroxylbutyrate)/BN composites using powder mixing method and the obtained composites exhibited higher thermal conductivity when compared with the samples prepared by solution mixing. The formation of segregated BN network is thought to be crucial for the enhancement of thermal conductivity by reducing thermal boundary resistance between adjacent fillers. Moreover, Wang et al. [25] found that PVDF composites with segregated (BN + CNT) hybrid fillers exhibited a thermal conductivity of 1.8 W/mK at a total filler concentration of 25 vol%, which was 169% higher than that of PVDF/BN/CNT counterparts with random filler distribution state. The above finding was ascribed to the effective utilization of fillers by constructing segregated structure and the reduction of interfacial thermal resistance between adjacent BN particles via the bridging effect of using high aspect ratio CNT.
In the present study, a series of poly(vinylidene fluoride) (PVDF) composites were prepared with graphene nanoplatelets (GNP) being adopted as the thermal conductive fillers. Ball milling treatment method was employed to prepare GNP@PVDF hybrids which were further adopted to prepare PVDF-based composites with segregated filler structure. The effect of constructing segregated GNP network on the properties such as morphology, thermal and electrical conductivity, rheological behavior and thermal stability of corresponding composites were studied in detail.
Experimental section
Materials
Poly(vinylidene fluoride) (PVDF) powders of different particle size (e.g., 100 and 400 μm) under the trademark of FR906 were provided by Shanghai Huayi 3F Co., Ltd (China). Graphene nanoplatelets (GNPs) with an average size of 7 ~ 12 µm were purchased from Shenzhen Tulingjinhua Technology Co., Ltd (China). All materials were used as-received without further treatment.
Sample preparation
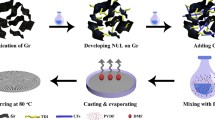
PVDF composites with segregated GNP network were prepared as per the following procedure: firstly, powder form PVDF and a predetermined amount of GNP (0, 1, 2, 4 and 6 wt%) were treated using an omnidirectional planetary ball mill. The weighed PVDF and GNP powders were placed in an agate tank with the presence of 5 mm agate balls (the mass ratio of grinding ball to powder is 100:1). The duration of ball milling process lasted 1 h and the rotational speed was set at 500 rpm. Then, the obtained GNP@PVDF hybrids were hot pressed at 200 °C and 10 MPa for 10 min. Samples prepared with 100 µm PVDF particles were termed at s-PVDF100/GNP-x, where s- indicated samples with segregated GNP network and x referred to the weight fraction of GNP in respective samples. The same nomenclature rules applied to samples prepared using 400 µm PVDF particles. The schematic diagram illustrating the preparation of s-PVDF/GNP composites is displayed in Scheme 1.
In addition, melt blending of PVDF particles with GNP was conducted to prepare PVDF/GNP composites with discretely distributed GNP, which were termed as m-PVDF/GNP. The concentration of GNP in melt blended PVDF/GNP composites was the same to that of s-PVDF/GNP counterparts.
Characterization
The morphology of PVDF (particle size was about 400 µm) before and after coating GNP was observed using scanning electron microscopy (SEM, JSM-7800F, JEOL, Japan). The morphology of GNP was observed using transmission electron microscopy (TEM, JEM-2100, JEOL, Japan) at 200.0 kV. The segregated structure was observed and confirmed using a polarized optical microscope (POM, DM2700P, Leica, Germany). The thermal conductivity of all samples was measured by a thermal constant analyzer (TPS2200, Hot-Disk, Sweden). Electrical conductivity of was tested by a Keithley multimeter system (DAQ6510, Keithley, USA). The thermal stability of all samples was measured by a simultaneous thermogravimetric analyzer (TGA/DSC3 + , Mettler Toledo, Switzerland) at a heating rate of 10 °C/min in N2 atmosphere.
Results and discussion
Figure 1a, b demonstrated the morphology of PVDF particles (about 400 μm) and GNP, respectively. After ball milling, the surface of PVDF became rougher due to the coating of GNP, as displayed in Fig. 1c, d. The morphology of s-PVDF/GNP composites prepared with different PVDF particle sizes was investigated by optical microscope. Figure 1e, f showed that GNP particles which were selectively localized at the interfaces were observed for composites prepared with both PVDF particle sizes. Under such circumstances, the formed segregated structure facilitates the construction of intact thermal conductive pathways, which is crucial to improving the thermal conductivity for subsequent composites.
The thermal conductivity of PVDF/GNP composites prepared by different methods was shown in Fig. 2. Figure 2 showed that pure PVDF exhibited inferior thermal conductivity (0.24 W/mK) at room temperature. There is obvious increase of thermal conductivity with an incremental loading content of GNP. The thermal conductivity of PVDF/GNP 6 wt% composites that prepared by melting mixing reached 1.46 W/mK, which is 6.1 times higher than that of neat PVDF. The above improvement was related to the overlapping of GNP within the host matrix, which is beneficial to the formation of intact thermal conductive pathways and therefore improve the thermal conductivity of corresponding composites.
As expected, the thermal conductivity of PVDF/GNP composites with segregated filler network exhibited much higher values when compared with the melt blended PVDF/GNP counterparts. For example, the thermal conductivity of s-PVDF400/GNP 6 wt% composites reached 1.76 W/mK which is 20.5% higher than melt blended PVDF/GNP counterparts. The above observation is undoubtedly related to the formation of thermal conductive network arising from the formation of segregated GNP network. However, samples prepared using melt blending method exhibited random filler distribution, and intact thermal conductive network pathways were hardly formed at lower GNP concentrations. In this scenario, the thermal conductivity of m-PVDF/GNP composites was lower than that of s-PVDF/GNP counterparts. In addition, samples with segregated filler network that prepared from different particle sizes of PVDF showed different increasing trend of thermal conductivity, especially when the content of GNP is above 2 wt% (as given in Fig. 2). Figure 1c, d showed that the GNP could be coated on the surface of PVDF particles with the aid of ball milling treatment, and the PVDF particles with 100 μm are believed to exhibit higher specific surface area which is beneficial to the attachment of GNP during powder mixing. This led to the formation of denser heat conductive pathways in s-PVDF100/GNP composite. According to literature [2], both the formation of thermal conductive pathways and increasing filler packing density were favorable for improving thermal conductivity of filler-containing polymer composites. Thus, a denser and more perfect thermal conductive network is likely constructed in terms of samples prepared with small size PVDF particles, thereby leading to a significant improvement of thermal conductivity for s-PVDF100/GNP composites when compared with s-PVDF400/GNP counterparts with increasing filler concentrations.
The electrical conductivity of PVDF/GNP composites as a function of filler content was given in Fig. 3. The neat PVDF is electrically insulative [9, 26] with an electrical conductivity of about 1.5 × 10–14 S/m. In spite of the preparation method, the electrical conductivity of PVDF/GNP composites increased monotonically with increasing GNP concentration. For example, the electrical conductivity of melt blended PVDF/GNP 6 wt% composites reached 0.01 S/m, which is nearly 12 orders of magnitude higher than that of pure PVDF.
It is known [27,28,29] that insulative polymers will become electrically conductive once the added fillers attain an intact physical network within the host matrix. Figure 3 clearly exhibited that the electrical conductivity of s-PVDF/GNP composites is invariably higher than the melt blended counterparts. In addition, the electrical conductivity of s-PVDF400/GNP composites is higher than that of s-PVDF100/GNP composites when the content of GNP is lower than 4 wt%. For example, the electrical conductivity of s-PVDF100/GNP 1 wt% is 5.1 × 10–12 S/m, which is about 4 orders of magnitude lower than that of s-PVDF400/GNP 1 wt% counterpart. This indicated that the formation of more intact electrically conductive network is favored when larger size PVDF particles were used to prepare s-PVDF/GNP composites. According to the percolation theory [30], the electrical conductivity of polymer composites would experience a distinct increase when the added fillers attain a three-dimensional network structure. More intact conductive pathways are expected to be formed with further increasing GNP content. As a result, the difference of electrical conductivity between s-PVDF100/GNP and s-PVDF400/GNP composites is minimized when the concentration of GNP is no less than 4 wt%.
The thermal stability of pure PVDF and PVDF/GNP 6 wt% composites that prepared by different methods was evaluated using TGA and corresponding results for respective samples were displayed in Fig. 4 and Table 1. Results indicated that the main pyrolysis process occurred in the temperature range of 400 ~ 500 °C, which is believed related to the degradation of chain backbone of PVDF [31, 32]. The thermal decomposition temperatures (i.e., T5 and T30 are the temperatures at which 5 and 30 wt% mass loss were recorded), heat-resistance index (HRI) [33, 34] and peak decomposition temperatures (Tp, the maximum mass loss temperature that determined from DTG curves) of all samples were listed in Table 1. Results showed that the T5, HRI and Tp of pure PVDF are 424.6, 218.8 and 467.0 °C. Figure 4 and Table 1 revealed that the thermal stability of PVDF improved with the incorporation of GNP. Additionally, the melt blended PVDF/GNP composites exhibited the highest T5, HRI and Tp, suggesting that the randomly distributed GNP particles were favorable for the enhancement of thermal stability of PVDF. This could be explained by the following aspects: (1) the presence of planar GNP particles would, to some extent, barrier the volatilization of decomposed fragments due to the “tortuous effect”, which improved the thermal stability of polymer composites [35, 36]; (2) the GNP particles acted as radical scavengers which might suppress the chain transfer reaction and thus prevent the degradation of polymer chains [37].
Figure 4 also showed that the thermal stability of PVDF/GNP composites followed a sequence of m-PVDF/GNP 6 wt% > s-PVDF100/GNP-6 > s-PVDF400/GNP-6. The elevated thermal stability of melt blended PVDF/GNP composites is ascribed to the random distribution of GNP within the host matrix, as described previously. However, it should be noticed that either the “tortuous effect” or “scavenge effect for free radicals” would be somewhat compromised in terms of PVDF/GNP samples with segregated filler structure, which leads to a reduced thermal stability for s-PVDF/GNP composites. Moreover, smaller size PVDF particles were more likely to be fully covered with GNP particles during the ball milling process. Under such circumstances, s-PVDF100/GNP-6 composites exhibited relatively higher thermal stability as compared with that of s-PVDF400/GNP-6 counterparts.
Rheological analysis is considered as a reliable way to characterize the relationship between the internal microstructure and properties of polymer composites. Figure 5 demonstrated the storage modulus (G’) and loss modulus (G”) of PVDF/GNP composites with different filler content and preparation method. It is clear that the values of G’ and G” increased with increasing GNP content, especially in the low frequency region. The presence of GNP particles restricted free movement of polymer chains, which resulted in an enhancement of subsequent composites. It is worth mentioning that both s-PVDF100/GNP and s-PVDF400/GNP composites exhibited lower G’ when compared with that of melt blended PVDF/GNP counterparts, which is likely associated with the state of distribution of GNP in corresponding samples. It is believed that GNP particles were randomly distributed within melt-blended PVDF composites, which could limit free mobility of polymer chains. However, the selective localization of GNP between adjacent PVDF particles had milder chain confinement effect on the polymer chains. As a result, a relatively lower reinforcement effect was observed for s-PVDF/GNP composites.
Conclusion
In this study, we proposed a feasible method to fabricate thermally and electrically conductive poly(vinylidene fluoride)/graphene (PVDF/GNP) composites with segregated filler network structure. PVDF particles of different size (i.e., 100 and 400 μm) were employed to prepare GNP@PVDF hybrids using ball milling treatment, followed by hot pressing. Regardless of the size of PVDF particles, composites prepared with segregated filler structure exhibited much higher thermal and electrical conductivity than those prepared by melt mixing. However, the size of PVDF particles seemed to play a crucial role in building thermally and electrically conductive pathways. The thermal conductivity of samples prepared with smaller PVDF particles was higher when compared with the other composites, which was related to the construction of a much denser and more perfect thermal conductive network. For example, the thermal conductivity of s-PVDF100/GNP 6 wt% composite reached 2.23 W/mK, which is nearly 829% and 52.7% higher than pure PVDF and m-PVDF/GNP 6 wt% counterparts, respectively.
References
He S, Zhang J, Xiao X, Hong X (2018) Effects of ultrasound vibration on the structure and properties of polypropylene/graphene nanoplatelets composites. Polym Eng Sci 58(3):377–386
Zhou S, Chen Y, Zou H, Liang M (2013) Thermally conductive composites obtained by flake graphite filling immiscible Polyamide 6/Polycarbonate blends. Thermochim Acta 566:84–91
Bai Y, Shi Y, Zhou S, Zou H, Liang M (2021) A Concurrent Enhancement of Both In-Plane and Through-Plane Thermal Conductivity of Injection Molded Polycarbonate/Boron Nitride/Alumina Composites by Constructing a Dense Filler Packing Structure. Macromol Mater Eng 306(9):2100267
Thomas S, George SC, Thomas S (2018) Evaluation of mechanical, thermal, electrical, and transport properties of MWCNT-filled NR/NBR blend composites. Polym Eng Sci 58(6):961–972
Lyu M, Choi T (2015) Research trends in polymer materials for use in lightweight vehicles. Int J Precis Eng Man 16(1):213–220
Guo Y, Ruan K, Shi X, Yang X, Gu J (2020) Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos Sci Technol 193(6402):108134
Han Z, Fina A (2011) Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog Polym Sci 36(7):914–944
Yan H, Mahanta NK, Majerus LJ, Abramson AR, Cakmak M (2014) Thermal conductivities of electrospun polyimide-mesophase pitch nanofibers and mats. Polym Eng Sci 54(4):977–983
Yang Y, Gao J, Lei T, Yang J, Wang J, Liu J (2020) Thermal conductivity and mechanical properties of polyimide composites with mixed fillers of BN flakes and SiC@SiO2 whiskers. Polym Eng Sci 60(5):1044–1053
Zhou S, Yu L, Song X, Chang J, Zou H, Liang M (2014) Preparation of highly thermally conducting polyamide 6/graphite composites via low-temperature in situ expansion. J Appl Polym Sci 131(1):39596
Wang Z, Wang X, Wang S, He J, Zhang T, Wang J, Wu G (2021) Simultaneously enhanced thermal conductivity and dielectric breakdown strength in sandwich AlN/epoxy composites. Nanomaterials 11:1898
Wang Z, Zhang T, Wang J, Yang G, Li M, Wu G (2022) The investigation of the effect of filler sizes in 3D-BN skeletons on thermal conductivity of epoxy-based composites. Nanomaterials 12:446
Wie J, Kim M, Kim J (2020) Enhanced thermal conductivity of a polysilazane-coated A-BN/epoxy composite following surface treatment with silane coupling agents. Appl Surf Sci 529(1):147091
Wang R, Xie C, Gou B, Xu H, Luo S, Zhou J, Zeng L (2020) Epoxy nanocomposites with high thermal conductivity and low loss factor: Realize 3D thermal conductivity network at low content through core-shell structure and micro-nano technology. Polym Test 89:106574–106585
Choi S, Zhang Z, Yu W, Lockwood FE, Grulke EA (2001) Anomalous thermal conductivity enhancement in nanotube suspensions. Appl Phys Lett 79(14):2252–2254
Sun K, Stroscio MA, Dutta M (2009) Thermal conductivity of carbon nanotubes. J Appl Phys 105(7):527–531
Xie H, Wang J, Xi T, Liu Y, Ai F, Wu Q (2002) Thermal conductivity enhancement of suspensions containing nanosized alumina particles. J Appl Phys 91(7):4568–4572
Zhang X, Gu H, Fujii M (2006) Effective thermal conductivity and thermal diffusivity of nanofluids containing spherical and cylindrical nanoparticles. J Appl Phys 100(4):044325
Zhang P, Ding X, Wang Y, Gong Y, Zheng K, Chen L, Tian X, Zhang X (2019) Segregated double network enabled effective electromagnetic shielding composites with extraordinary electrical insulation and thermal conductivity - ScienceDirect. Compos Part A: Appl S 117:56–64
Jiang Y, Liu Y, Min P, Sui G (2017) BN@PPS core-shell structure particles and their 3D segregated architecture composites with high thermal conductivities. Compos Sci Technol 144:63–69
Zhang Y, Ruan K, Gu J (2021) Flexible Sandwich-Structured Electromagnetic Interference Shielding Nanocomposite Films with Excellent Thermal Conductivities. Small 17:2101951
Guo Y, Qiu H, Ruan K, Zhang Y, Gu J (2022) Hierarchically Multifunctional Polyimide Composite Films with Strongly Enhanced Thermal Conductivity. Nano-Micr Lett 14:26
Zhang X, Xia X, You H, Wada T, Chammingkwan P, Thakur A, Taniike T (2020) Design of continuous segregated polypropylene/Al2O3 nanocomposites and impact of controlled Al2O3 distribution on thermal conductivity. Compos Part A-Appl S 131:105825
Li Z, Kong J, Ju D, Cao Z, Han L, Dong L (2017) Thermal conductivity enhancement of poly(3-hydroxylbutyrate) composites by constructing segregated structure with the aid of poly(ethylene oxide). Compos Sci Technol 149(8):185–191
Wang Z, Huang Y, Zhang G, Wang H, Xu J, Lei J, Zhu L, Gong F, Li Z (2018) Enhanced Thermal Conductivity of Segregated Poly(Vinylidene Fluoride) Composites via Forming Hybrid Conductive Network of Boron Nitride and Carbon Nanotubes. Ind Eng Chem Res 57:10391–10397
Zhao Z, Zheng W, Yu W, Long B (2009) Electrical conductivity of poly(vinylidene fluoride)/carbon nanotube composites with a spherical substructure. Carbon 47:2118–2120
Jin J, Lin Y, Song M, Gui C, Leesirisan S (2013) Enhancing the electrical conductivity of polymer composites. Eur Polym J 49(5):1066–1072
Miyasaka K, Watanabe K, Jojima E, Aida H, Sumita M, Ishikawa K (1982) Electrical conductivity of carbon-polymer composites as a function of carbon content. J Mater Sci 17(6):1610–1616
Fang C, Zhang J, Chen X, Weng G J, A Monte Carlo model with equipotential approximation and tunneling resistance for the electrical conductivity of carbon nanotube polymer composites. Carbon 146:125–138.
Al-Saleh MH, Gelves GA, Sundararaj U (2011) Copper nanowire/polystyrene nanocomposites: Lower percolation threshold and higher EMI shielding. Compos Part A-Appl S 42(1):92–97
Behera K, Chiu F (2013) Evident improvements in the rigidity, toughness, and electrical conductivity of PVDF/HDPE blend with selectively localized carbon nanotube. Polym Test 90:106736–106739
Campos JSC, Ribeiro AA, Cardoso CX (2007) Preparation and characterization of PVDF/CaCO3 composites. Mat Sci Eng B 136(2–3):123–128
Yang X, Fan S, Li Y, Guo Y, Li Y, Ruan K, Zhang S, Zhang J, Kong J, Gu J (2020) Synchronously improved electromagnetic interference shielding and thermal conductivity for epoxy nanocomposites by constructing 3D copper nanowires/thermally annealed graphene aerogel framework. Compos Part A-Appl S 128:105670
Gu J, Lv Z, Wu Y, Guo Y, Tian L, Qiu H, Li W, Zhang Q (2017) Dielectric thermally conductive boron nitride/polyimide composites with outstanding thermal stabilities via in-situ polymerization-electrospinning-hot press method. Compos Part A-Appl S 94:209–216
Jang JW, Min BG, Yeum JH, Jeong YG (2013) Structures and Physical Properties of Graphene/PVDF Nanocomposite Films Prepared by Solution-mixing and Melt-compression. Fiber Polym 14(8):1332–1338
Zhang Q, Wang Y, Bailey C, Yuen RKK, Parkin J, Yang W, Valles C (2018) Quantifying effects of graphene nanoplatelets on slowing down combustion of epoxy composites. Compos Part B-Eng 146:76–87
Yu J, Huang X, Wu C, Jiang P (2011) Permittivity, thermal conductivity and thermal stability of poly(vinylidene fluoride)/graphene nanocomposites. IEEE T Dielect El In 18(2):478–484
Acknowledgements
This work was funded by the National Natural Science Foundation of China (52103040), China Postdoctoral Science Foundation (2020M673217), and the State Key Laboratory of Polymer Materials Engineering (sklpme2020-3-06).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, Y., Bai, Y., Shi, Y. et al. Composite nanoarchitectonics of poly(vinylidene fluoride)/graphene for thermal and electrical conductivity enhancement via constructing segregated network structure. J Polym Res 29, 213 (2022). https://doi.org/10.1007/s10965-022-03052-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03052-z