Abstract
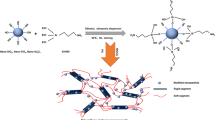
It is a challenge to manufacture multifunctional, two-dimensional, nanosized additive for polymer by a simple and universal method. Herein, a phytic acid-doped polypyrrole shell was successfully grown on the surface of exfoliated g-C3N4 nanosheets (CNPPy) by an in-situ polymerization method. CNPPy was directly incorporated into thermoplastic polyurethane (TPU) to prepare multifunctional TPU nanocomposites with near-infrared (NIR) induced self-healing properties. Rheological analysis showed that the combination of carbon nitride and polypyrrole formed a stable network in the TPU matrix. Compared to pure TPU, the incorporation of 3 wt% CNPPy into TPU exhibited 43.4% lower peak heat release rate and 53.4% lower peak smoke release rate. In addition, the mechanical performance of the CNPPy-TPU nanocomposite remain basically unchanged. Meanwhile, the tensile strength of a cut composite was restored to 31.3% in 45 s via NIR. The strategy of in-situ polymerization of conjugated polymers on the surface of two-dimensional nanosheets enables polyurethane composites with both improved rheological and self-healing properties and broadens the application field of two-dimensional nanosheets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research on flame retardant polymer is primarily focused on the fire scenario and the development of new kinds of flame retardant additives [1]. Additive flame retardants, as one of the most effective ways, has been widely employed to protect a polymer against fire [2]. Polymer flame retardants are divided into halogen-containing (bromine/chlorine compounds [3, 4] and halogenated phosphates [5], etc.), inorganic (metal hydroxides/metal oxides [6], silicon-containing additives [7] and ammonium polyphosphate [8], etc.), organic phosphorus/nitrogen (aliphatic phosphate [9], aromatic phosphonates [10] and nitrogen-containing flame retardants [11], etc.) and nanosized materials (zero/one/two-dimensional nanomaterials [12]) flame retardants categories. Among them, two-dimensional nanomaterials, nanoplatelets with layered structures, have been widely applied to induce fire resistance and smoke suppression of polymeric materials [13, 14]. This flame retardant effect is primarily attributed to the so-called “tortuous path” effect of nanosheets, which can act as a barrier to heat and mass transfer, and delay the release of pyrolysis gases of polymers during thermal degradation [15, 16]. However, it is believed that the challenge in applying two-dimensional nanomaterials as flame retardant additives is the high cost of peeling. Meanwhile, due to the improved polymer performance, it will be a long-term challenge to select suitable two-dimensional nano materials for specific polymers and prepare two-dimensional nano additives that are functionally controllable by simple and universal methods, so as to overcome the multi-functionality of polymers.
TPU is a widely used plastic [17, 18]. Designing flame retardant additives for TPU not only requires flame retardant and smoke suppression performance but also certain self-healing performance [19]. To solve this challenge, we have designed flame retardants via in-situ growth of polypyrrole on the surface of two-dimensional carbon nitride nanosheets (g-C3N4). Two-dimensional graphitic carbon nitride, polyheptazine, has been employed in the flame retardant field due to its condensed-phase flame retardancy mechanism that forms a physical barrier in the polymer matrix. Polypyrrole (PPy) is an important heterocyclic conjugated polymer material. Many studies have shown that the incorporation of PPy can improve the thermal stability and flame retardancy of polymers [20,21,22]. In the PPy molecule, the introduction of P through the surface functionalization of phytic acid has proven to be a feasible method to enhance the flame retardant effects [17, 23]. Meanwhile, PPy and g-C3N4 nanosheet can form stable organic-organic heterojunctions through π-π stacking interactions [24,25,26], so that the conductive polymer can grow on the surface of the g-C3N4 nanosheet. It has been reported that PPy can be employed in the field of biomedicine [27] and self-healing [28] due to its excellent photothermal effects. It is speculated that in-situ growth of PPy coatings on exfoliated g-C3N4 nanosheets can improve the dispersion and interfacial interaction of g-C3N4 in the TPU matrix, aiding its flame retardancy, rheological and self-healing properties.
To achieve a multifunctional additive for TPU with both improved rheological and self-healing ability, we designed a synergetically-enhanced, multifunctional flame retardant using exfoliated carbon nitride as the core and phytic acid-doped PPy as the shell. Then, a multifunctional additive was incorporated into TPU, and the fire hazards, rheological behavior, mechanical and self-healing performances were investigated.
Experimental
Materials
Urea (99%) was obtained from J&K Scientific Ltd. Phytic acid (PA) solution (70%), pyrrole solution, (NH4)2S2O8 (APS), methanol, ethanol, and N,N-dimethylformamide (DMF) were provided by Macklin Ltd (China). All chemicals were used as received. TPU (S80A, BASF SE) with a density of 1.21 g/cm3 was used as received.
Synthesis of few-layered carbon nitride (ECN)
In a typical synthesis, 20 g urea was calcined at 550 ℃ in air for 4 h (heating rates: 8.7 K/min) to synthesize bulky carbon nitride (CN). Then, 0.7 g bulky CN powder was heated to 500 °C (heating rates: 5 K/min) for 4 h in a tube furnace under a water atmosphere using an Ar-carrying gas to synthesize ECN nanosheets (yield: 55%).
Synthesis of CNPPy
0.6 g ECN powder was dispersed in 600 mL of deionized water for 0.5 h to form a uniform suspension. Then, 0.369 g of pyrrole solution and 15.0 g of PA acid were added into the suspension under ultrasonication for 15 min. Next, 0.72 g of APS was dissolved into 50 mL of deionized water and the formed solution was slowly added into the above suspension dropwise. Pyrrole polymerization proceeded for 4 h, and the black product was collected by filtration, and washed with deionized water, methanol, and ethanol three times. The product of CNPPy was subsequently dried overnight at 90 ℃. The product of PA-doped polypyrrole particles (PPy) was prepared in the absence of ECN. The preparation procedure of multifunctional CNPPy was illustrated in Scheme 1.
Fabrication of pure TPU and its nanocomposites
Pure TPU and its nanocomposites were fabricated by a solution blending method. Typically, 3.0 g of CNPPy was dispersed in 200 mL of DMF with ultrasonication and stirring for 0.5 h. Then, the dispersion was heated to 50 ℃, and 97.0 g of TPU pellets was dissolved into the dispersion via stirring for 3 h. Then, the mixture was slowly poured into low temperature deionized water to sufficiently remove the solvent, which was further dried at a 100 ℃ oven for 12 h to remove the remaining solvent. The sample containing 3.0 wt% CNPPy was hot-pressed at 185 ℃ to obtain sheets of suitable and named as CNPPy-TPU. Other samples with 3.0 wt% ECN and 3.0 wt% PPy were defined as ECN-TPU and PPy-TPU, respectively. The control sample of pure TPU was also prepared by the process above.
Characterization
X-ray diffraction (XRD) patterns were obtained using an X-ray diffractometer (Empyrean, Malvern Panalytical, Netherlands) equipped with CuKα radiation. Fourier transform infrared (FT-IR) spectra were obtained on a Fourier transform infrared spectrometer (EQUINOX 55, Bruker) and scanned from 4000 to 400 cm−1. Transmission electron microscopy (TEM) were performed using a FEI Talos 200S FEI microscope integrated with an energy dispersive X-ray spectrometer (EDS). Thermogravimetric analysis (TGA) was performed on a NETZSCH TG209F1 libra thermogravimetric analyzer. Thermal analysis was carried out from room temperature to 800 °C at a heating rate of 10° C min−1 in N2 or air atmosphere. Cone calorimetry tests (CCT) were carried out by a FTT0007 cone calorimeter under radiation heat flux of 35 kW/m3. The sample dimension was 100 × 100 × 1 mm3 or 100 × 100 × 3 mm3. Small-scale combustion (MCC) performance was investigated by a Govmark MCC-2 microcalorimeter (New York, NY, USA). Tensile testing of three groups of composites (dumbbell specimens) were examined by a tensile testing machine (CMT4304GD, Zhuhai SUST Electrical Equipment Co., Ltd, China) in accordance with the GB/T 1040.2–2006 standard at room temperature. The assay was performed under a linear deformation loading rate of 30 mm/min until mechanical failure occurred. A laser system (LASER DIODE DRIVER-808, DS3-11,312–705) was used to generate near-infrared light at 808 nm, which was used to measure the near-infrared light response. A spectrophotometer (PE Lambda950) was used to record the ultraviolet–visible-near-Infrared (UV–vis-NIR) absorption spectrum of all nanocomposites. The rheological behavior was measured on a rheometer (ARES, TA Co., New Castle, USA) with a disk-like sample size of about 25 mm in diameter. Frequency sweeping was selected from 0.1 to 1000 rad s−1 with a strain of 0.1%, and the testing was carried out at 195 °C under nitrogen.
Results and discussion
Fourier transform infrared spectroscopy (FT-IR) and X-Ray diffraction (XRD) were employed to confirm the structures of ECN, CNPPy, and PPy. For the typical FT-IR spectrum of exfoliated carbon nitride (Fig. 1a), the peaks at 1245, 1330, 1415, 1573, and 1639 cm−1 correspond to the typical stretching mode of CN heterocycles. The broad peak at 3000–3500 cm−1 corresponds to the stretching vibration of N–H and C-H [14]. The physically adsorbed H2O molecules are also attributed to this broad peak [24]. Furthermore, the characteristic breathing vibration of the triazine unit at 810 cm−1 was observed. It was noted that the peak at 1562 cm−1 shown by the blue curve is attributable to the C–C and C = C tensile vibrations of the pyrrole ring [17], confirming the successful synthesis of PPy. The peaks at 1105 and 931 cm−1 are attributed to the C-H and N–H vibrations in pyrrole [29, 30]. The N–H bond vibration of a typical pyrrole of 931 cm−1 was also observed in CNPPy, indicating the successful loading of polypyrrole on carbon nitride. In addition, the two peaks of PPy at 1205 and 1055 cm−1 are attributed to the P = O and P-O bonds [31, 32], which prove the successful doping of PA on polypyrrole. For the XRD spectrum of ECN (Fig. 1b), two peaks were identified. The peak at 12.90° corresponds to the in-plane structure filling motif of tri-s-triazine units (100), and the peak at 27.76° corresponds to the interlayer stacking of aromatic planes (002) [33]. Compared with ECN, the XRD peak position and morphology of CNPPy were unchanged, indicating that the in-situ polymerization of PPy had no effect on the lattice structure of ECN.
The morphology and structure of ECN were investigated by TEM. ECN showed a smooth surface without other nanoparticles attached (Fig. 2a). ECN exhibited a thin nanosheet structure, which showed obvious differences when compared with unexfoliated g-C3N4 that had shown a stacked sheet structure in the literature [14]. ECN nanosheets tend to be fragmented with a smaller area than g-C3N4 because of thermal steam reforming [34]. PPy showed a dark round structure in the TEM (Fig. 2c). The morphology of CNPPy was studied as shown in Fig. 2b. The PPy nanoparticles accumulated in the darker area were observed on the ECN surface, indicating that the PPy was successfully loaded on the surface of the ECN nanosheets. In addition, the EDS spectra of CNPPy showed the presence of C, N, O, and P elements, indicating the successful doping of PA on PPy. The digital photos of ECN and CNPPy are shown in Fig. 2d. ECN is typically light yellow, while CNPPy was obviously darkened to almost black due to the in-situ polymerization of PPy on the ECN surface. The distribution of the CNPPy nanosheets in the polyurethane host was further dissected by TEM, as depicted in Fig. 2f, g and h. As shown in Fig. 2g, the CNPPy can be uniformly dispersed in the matrix, presenting a better dispersion as compared with ECN (see Fig. 2f). From Fig. 2h, some particles with about 200 nm were dispersed in the TPU. This demonstrated that the combination of g-C3N4 nanosheets and PPy can further exfoliate ECN.
Thermal properties of ECN, CNPPy, and PPy were characterized by thermogravimetric analysis (TGA) under nitrogen and air, as presented in Fig. 3 and Table 1. The temperatures at 5% and 50% weight loss were defined as T-5 and T-50, respectively, and the residues at 750 °C were investigated in Table 1. The first mass loss of PPy below 100 °C was attributed to the trapped moisture (Fig. 3a). However, the second mass loss from 230 °C could be attributed to the loss of PA and PPy chain degradation, as the char residues at 750 °C reached 60.1% in nitrogen. ECN exhibited a similar one-step degradation process whether under nitrogen or air. The char residues of CNPPy were 21.1% under nitrogen, which due to the high thermal stability of ECN, protecting PPy to a certain extent. PPy was almost completely degraded, leaving only 1.9% in air. However, the residues of CNPPy in the air were 4.1% (Fig. 3b), indicating that PA-doped PPy exhibiting a small degree of crosslinking to form char with ECN during thermal degradation.
In addition, the thermal stabilities of pure TPU and its nanocomposites in nitrogen and air were investigated, as shown in Fig. 3c, d, and Table 2. The T-5 of CNPPy-TPU was 313 °C in nitrogen, which is higher than the other three samples, indicating that CNPPy had good thermal stability in the polymer matrix. The T-5 of PPy-TPU was only 305 °C, which was due to the relatively poor thermal stability of the PA-doped PPy. The char residues of CNPPy-TPU at 500 ℃ and 750 ℃ were 11.9% and 8.6%, respectively, which were higher than those of the other three samples, indicating that CNPPy improved the flame retardance of TPU. The higher char residues of CNPPy-TPU at 500 °C were attributed to the high thermal stability of ECN. The char residues of CNPPy-TPU at 750 °C were the highest and likely attributed to the synergistic char formation of ECN and PPy during degradation. It is notable that the char residues of pure TPU and ECN-TPU were about 2% lower than that of CNPPy-TPU and PPy-TPU at 500 °C in air, which may be due to the presence of PA-doped PPy changing the decomposition process of the soft segment of TPU to a certain extent, leading to the formation of a small quality of unstable char.
The mechanism of thermal degradation can be further elucidated by investigating the rheological properties of polymers [35]. Figure 4 shows the dependence of storage modulus (G') and complex viscosity (η*) on the shear frequency (ω) of TPU and its nanocomposites at 195 ℃ and 0.1% strain, respectively. For the binary ECN-TPU and PPy-TPU, the storage modulus trend with ω is similar to that of pure TPU. However, for ternary CNPPy-TPU, a much higher modulus in the low frequency region was clearly observed, which may be due to the formation of a network structure. The dependence of G' on ω weakens with an obvious plateau at low frequencies. It demonstrated that a transition from liquid to solid was accompanied by the formation of a mechanically stable network structure [36]. For CNPPy-TPU and PPy-TPU (as shown in Fig. 5), there was no intersection point between the storage modulus and loss modulus curves. PPy-TPU melts in the whole shear frequency range as exhibited by G' < G'', while CNPPy-TPU melts in the shear frequency range as exhibited by G' > G'', showing a solid-like behavior [37, 38]. The complex viscosity results of TPU and its nanocomposites also exhibited a similar conclusion (Fig. 4b). Therefore, the combination of carbon nitride and polypyrrole can form a more stable network in the TPU matrix, which is a prerequisite to improve the flame retardancy (reduce the peak heat release rate) of the polymer.
Fire behaviors of TPU and its nanocomposites were investigated by CCT and MCC, and the results were shown in Figs. 6, 7 and 8 and Tables 3 and 4. Pure TPU possessed a pHRR value of 666.1 kW/m2, 872.3 kW/m2 and 347.7 W/g in CCT (1 mm-thick), CCT (3 mm-thick) and MCC test, indicating its high inflammability in fire. After the incorporation of 3 wt% ECN and PPy separately, the pHRR values of TPU nanocomposites decreased to varying degrees. When TPU was blended with 3wt% CNPPy, the pHRR values of corresponding nanocomposites experienced a 29.9%, 43.4% and 26.6% reduction in CCT (1 mm-thick), CCT (3 mm-thick) and MCC test compared with pure TPU, respectively, revealing further reduced in the peak heat release rate by the combined effects of carbon nitride and polypyrrole. It was worth noting that the law of the total heat release curve under different thicknesses was not consistent. The total heat release of CNPPy-TPU in the 1 mm-thick samples was the lowest among comparable samples, but it was not consistent in the 3 mm-thick samples. This may be attributed to the different products of thermal oxidative degradation and thermal degradation of TPU nanocomposites. The peak smoke production rate (pSPR) values of pure TPU (1 mm-thick) and pure TPU (3 mm-thick) were 0.101 m2/s and 0.176 m2/s during burning. The introduction of 3 wt% ECN, CNPPy, and PPy decreased the pSPR of TPU nanocomposites (1 mm-thick) to 0.068, 0.072, and 0.071 m2/s, respectively. The introduction of 3 wt% ECN, CNPPy, and PPy decreased the pSPR of TPU nanocomposites (3 mm-thick) to 0.129, 0.082, and 0.129 m2/s, respectively. When compared to pure TPU, the TSR (total smoke release) values (1 mm-thick) of ECN-TPU, CNPPy-TPU and PPy-TPU were reduced by 17.0%, 24.2%, and 13.2%, respectively, as shown in Fig. 6d. However, the TSP in the 3 mm-thick CCT test of the nanocomposites did not decrease compared to the pure TPU. The above results demonstrated that CNPPy possessed better smoke suppression performance than others, which would reduce the fire risk from smoke to a certain extent.
Figure 9 shows the tensile strength and elongation at break of the TPU nanocomposites. For pure TPU, the elongation at break and tensile strength were 2861.4% and 40.5 MPa, respectively. The elongation at break of ECN-TPU was almost equal as that of pure TPU. Unfortunately, all TPU nanocomposites had a lower tensile strength and shorter elongation at break than pure TPU. The tensile strengths of ECN-TPU and PPy-TPU were 33.4 MPa and 25.2 MPa, respectively, and reduced by 17.7% and 37.8% as compared with that of pure TPU. With 3 wt% CNPPy, the tensile strength and elongation at break of the TPU nanocomposites were 37.1 MPa and 2672.1%, respectively. Obviously, the effect of polypyrrole on the mechanical properties of TPU was effectively improved after the incorporation of ECN, compared with the corresponding values of PPy-TPU and ECN-TPU (Fig. 9b). This demonstrated that the formation of a stable network (the combination of carbon nitride and polypyrrole) in the TPU matrix could improve the tensile strength.
The polymer nanocomposites possess excellent photothermal conversion performance, so that it can reach the appropriate temperature in a short time and realize self-healing [28]. The process and mechanism of near-infrared (NIR) self-healing are shown in Fig. 10a. First, the sample was cut into a dumbbell shape, and then the dumbbell shape was completely cut off. Next, two of the cut surfaces were put back together, placed under a near-infrared laser light, and then irradiated to self-healing. After irradiation, the treated sample was placed in air for 2 days. Finally, a tensile test was performed to evaluate the repair efficiency. The self-healing efficiency was defined as: \(healing\;efficiency\;\mathrm{ \%}=\frac{\sigma\;of\;healing\;sample}{\sigma\;of\;original\;sample}\times 100\mathrm{\%}\), where σ is the tensile strength of the samples.
(a) Schematic illustration of NIR light-induced self-healing. The (b) DSC and (c) UV–vis-NIR absorption spectra of TPU and its nanocomposites. (d) The temperature elevation versus illumination time (808 nm, 1 W, distance from the sample surface to laser: 2 cm) curves of TPU and its nanocomposites. The (e) stress–strain and (f) healing efficiency curves of TPU and its nanocomposites after exposure to NIR light
The DSC curves of the TPU nanocomposites are shown in Fig. 10b. The first heating scan of pure TPU showed two melting temperature peaks (Tm), 154.2 ℃ and 182.2 ℃, respectively. The peaks of Tm at lower and higher temperatures can be attributed to the melting of less and better ordered structures [39, 40], respectively. With the PPy and ECN incorporation into pure TPU, the first and second melting temperatures almost disappear, respectively. The melting peak of CNPPy-TPU was closer to that of ECN-TPU, which indicates that CNPPy still keeps the nanocomposite in a state similar to that of ECN-TPU. The results of UV–vis-NIR absorption spectra are given in Fig. 10c. Compared with pure TPU and ECN-TPU, CNPPy-TPU and PPy-TPU have obvious absorption at about 808 nm in the NIR region. Therefore, 808 nm was used to investigate the photothermal effect, so that it has excellent rapid heating performance. As shown in Fig. 10d, we analyzed the temperature increase of TPU and its nanocomposites after NIR light irradiation (808 nm, 1 W). The temperatures of pure TPU, ECN-TPU, CNPPy-TPU, and PPy-TPU could be elevated by 68.9 ℃, 51.4 ℃, 174.6 ℃, and 181.0 ℃, respectively. This demonstrated that the CNPPy-TPU has excellent photothermal effects compared with pure TPU. To further study the healing properties under NIR light, CNPPy-TPU was selected as the research object. Figure 10e, f present the stress–strain curves and mechanical healing efficiencies of CNPPy-TPU with different irradiation times. With increasing irradiation time, the healing efficiency of CNPPy-TPU gradually increased. The healing efficiency of CNPPy-TPU can reach 31.3% after 45 s. Unfortunately, when the irradiation time was 90 s, the healing efficiency of CNPPy-TPU drops to 19.7%. This shows that although prepared TPU nanocomposites can quickly heat up through NIR radiation to achieve self-healing, the effect is still limited. This is because overexposure will generate excessive heat, which may cause deformation of the TPU nanocomposites.
Conclusion
In this work, we designed and synthesized a multifunctional additive using exfoliated carbon nitride as the core and phytic acid-doped PPy as the shell. The microstructure of the TPU nanocomposites was characterized, and the distribution of phytic acid-doped polypyrrole on carbon nitride was further illustrated by TEM and EDS. Thermal analysis shows that the incorporation of PPy improves the thermal degradation residues of the prepared nano additive, and there was a synergistic effect between carbon nitride and PPy in the polymer matrix, which promoted the increased amounts of residues in the condensed phase. CNPPy has obtained the best dispersibility in TPU and can form an effective network structure in the polymer, but no such phenomenon was found when carbon nitride or phytic acid-doped polypyrrole were used alone. The formation of the network structure further reduces the peak heat release rate in the condensed phase and enables the TPU nanocomposites to obtain better flame retardant performance, as shown by the CCT test. Especially after the repair experiment, the introduction of CNPPy can make the composite material obtain self-healing effects under near-infrared light. The strategy of constructing a two-dimensional nanocomposite additive in this paper provides a simple and universal method for the preparation of multifunctional polymers.
References
Lu HD, Song L, Hu Y (2011) A review on flame retardant technology in china. Part ii: flame retardant polymeric nanocomposites and coatings. Polym Advan Technol 22:379–394
Chen L, Wang YZ (2010) A review on flame retardant technology in china. Part i: development of flame retardants. Polym Advan Technol 21:1–26
Oleszek S, Kumagai S, Grabda M, Shiota K, Yoshioka T, Takaoka M (2021) Mitigation of bromine-containing products during pyrolysis of polycarbonate-based tetrabromobisphenol a in the presence of copper (i) oxide. J Hazard Mater 409:124972
Zhang ZX, Dai XR, Luo P, Sinha TK, Kim JK, Li H (2019) Lightweight, elastomeric, and flame-retardant foams from expanded chlorinated polymers. Macromol Mater Eng 304:1900145
Allcock H, Taylor J (2000) Phosphorylation of phosphazenes and its effects on thermal properties and fire retardant behavior. Polym Eng Sci 40:1177–1189
Liu JC, He YP, Chang HB, Guo YB, Li H, Pan BL (2020) Simultaneously improving flame retardancy, water and acid resistance of ethylene vinyl acetate copolymer by introducing magnesium hydroxide/red phosphorus co-microcapsule and carbon nanotube. Polym Degrad Stabil 171:109051
Feng YZ, He CG, Wen YF, Ye YS, Zhou XP, Xie XL, Mai Y (2017) Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos A Appl Sci Manuf 103:74–83
Chen XL, Wang K, Gu YX, Jiao CM, Liang HJ, Li SX (2021) Influence of nickel citrate in flame retardant thermoplastic polyurethane elastomer composites based on ammonium polyphosphate. Express Polym Lett 15:445–458
Markwart JC, Battig A, Velencoso MM, Pollok D, Schartel B, Wurm FR (2019) Aromatic vs. Aliphatic hyperbranched polyphosphoesters as flame retardants in epoxy resins. Molecules 24:3901
Wendels S, Chavez T, Bonnet M, Salmeia K, Gaan S (2017) Recent developments in organophosphorus flame retardants containing p-c bond and their applications. Materials 10:784
Lu SL, Shen BT, Chen XD (2021) Construction of charring-functional polyheptanazine towards improvements in flame retardants of polyurethane. Molecules 26:340
Lu SL, Hong W, Chen XD (2019) Nanoreinforcements of two-dimensional nanomaterials for flame retardant polymeric composites: an overview. Adv Polym Tech 2019:1–25
Shi YQ, Yu B, Duan LJ, Gui Z, Wang BB, Hu Y, Yuen RKK (2017) Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J Hazard Mater 332:87–96
Lu SL, Zhou W, Yang MJ, Chen GJ, Hong W, Yu DS, Zheng ZK, Chen XD (2019) Preparation and flame-retardant mechanism of polyheptazine/pa6 nanocmposites. Polymer 182:121810
Zhou KQ, Liu CK, Gao R (2018) Polyaniline: a novel bridge to reduce the fire hazards of epoxy composites. Compos A Appl Sci Manuf 112:432–443
Shi YQ, Long Z, Yu B, Zhou KQ, Gui Z, Yuen RKK, Hu Y (2015) Tunable thermal, flame retardant and toxic effluent suppression properties of polystyrene based on alternating graphitic carbon nitride and multi-walled carbon nanotubes. J Mater Cbem A 3:1764–1773
Wang JL, Zhang DC, Zhang Y, Cai W, Yao CX, Hu Y, Hu WZ (2019) Construction of multifunctional boron nitride nanosheet towards reducing toxic volatiles (co and hcn) generation and fire hazard of thermoplastic polyurethane. J Hazard Mater 362:482–494
Jiao CM, Wang HZ, Chen XL (2019) An efficient flame-retardant and smoke-suppressant agent by coated hollow glass microspheres with ammonium molybdophosphate for thermoplastic polyurethane. J Therm Anal Calorim 137:1579–1589
Ha Y, Kim Y, Ahn S, Lee S, Lee J, Park M, Chung JW, Jung YC (2019) Robust and stretchable self-healing polyurethane based on polycarbonate diol with different soft-segment molecular weight for flexible devices. Eur Polym J 118:36–44
Kotal M, Srivastava SK, Paramanik B (2010) Enhancements in conductivity and thermal stabilities of polypyrrole/polyurethane nanoblends. J Phys Chem C 115:1496–1505
Attia NF (2017) Organic nanoparticles as promising flame retardant materials for thermoplastic polymers. J Therm Anal Calorim 127:2273–2282
Attia NF, El Ebissy AA, Hassan MA (2015) Novel synthesis and characterization of conductive and flame retardant textile fabrics. Polym Advan Technol 26:1551–1557
Dai HX, Wang N, Wang DL, Ma HY, Lin M (2016) An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of cd(ii) and pb(ii). Chem Eng J 299:150–155
Sui Y, Liu JH, Zhang YW, Tian XK, Chen W (2013) Dispersed conductive polymer nanoparticles on graphitic carbon nitride for enhanced solar-driven hydrogen evolution from pure water. Nanoscale 5:9150
Liu Y, Zhang H, Lu YF, Wu J, Xin BF (2016) A simple method to prepare g-c3n4/ag-polypyrrole composites with enhanced visible-light photocatalytic activity. Catal Commun 87:41–44
Li Q, Xu D, Guo JN, Ou X, Yan F (2017) Protonated g-c3n4@polypyrrole derived n-doped porous carbon for supercapacitors and oxygen electrocatalysis. Carbon 124:599–610
Zha ZB, Yue X, Ren QS, Dai ZF (2013) Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv Mater 25:777–782
Wu HH, Sheng DK, Liu XD, Zhou Y, Dong L, Ji FC, Xu SB, Yang YM (2020) Nir induced self-healing polyurethane/polypyrrole nanocomposites. Polymer 189:122181
Yao TJ, Lin Q, Zhang K, Zhao DF, Lv H, Zhang JH, Yang B (2007) Preparation of sio2@polystyrene@polypyrrole sandwich composites and hollow polypyrrole capsules with movable sio2 spheres inside. J Colloid Interf Sci 315:434–438
Cui LF, Shen J, Cheng FY, Tao ZL, Chen J (2011) Sno2 nanoparticles@polypyrrole nanowires composite as anode materials for rechargeable lithium-ion batteries. J Power Sources 196:2195–2201
Lei YH, Sheng N, Hyono A, Ueda M, Ohtsuka T (2013) Electrochemical synthesis of polypyrrole films on copper from phytic solution for corrosion protection. Corros Sci 76:302–309
Liu BJ, Robertson GP, Guiver MD, Shi Z, Navessin T, Holdcroft S (2006) Fluorinated poly(aryl ether) containing a 4-bromophenyl pendant group and its phosphonated derivative. Macromol Rapid Comm 27:1411–1417
Niu P, Zhang LL, Liu G, Cheng HM (2012) Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv Funct Mater 22:4763–4770
Yang PJ, Ou HH, Fang YX, Wang XC (2017) A facile steam reforming strategy to delaminate layered carbon nitride semiconductors for photoredox catalysis. Angew Chem Int Ed 56:3992–3996
Yang HF, Guan YY, Ye L, Wang S, Li SX, Wen X, Chen XC, Mijowska E, Tang T (2019) Synergistic effect of nanoscale carbon black and ammonium polyphosphate on improving thermal stability and flame retardancy of polypropylene: a reactive network for strengthening carbon layer. Compos Part B: Eng 174:107038
Yang HF, Gong J, Wen X, Xue J, Chen Q, Jiang ZW, Tian NN, Tang T (2015) Effect of carbon black on improving thermal stability, flame retardancy and electrical conductivity of polypropylene/carbon fiber composites. Compos Sci Technol 113:31–37
Mun SC, Kim M, Prakashan K, Jung HJ, Son Y, Park OO (2014) A new approach to determine rheological percolation of carbon nanotubes in microstructured polymer matrices. Carbon 67:64–71
Ji XY, Chen DY, Wang QW, Shen JB, Guo SY (2018) Synergistic effect of flame retardants and carbon nanotubes on flame retarding and electromagnetic shielding properties of thermoplastic polyurethane. Compos Sci Technol 163:49–55
Lu MG, Lee JY, Shim MJ, Kim SW (2002) Thermal degradation of film cast from aqueous polyurethane dispersions. J Appl Polym Sci 85:2552–2558
Rueda-Larraz L, d’Arlas BF, Tercjak A, Ribes A, Mondragon I, Eceiza A (2009) Synthesis and microstructure-mechanical property relationships of segmented polyurethanes based on a pcl-pthf-pcl block copolymer as soft segment. Eur Polym J 45:2096–2109
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFB0308600).
Author information
Authors and Affiliations
Contributions
Shaolin Lu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing–original draft. Haixian Shi: Resources, Investigation. Botao Shen: Resources, Data curation. Wei Hong: Writing–review & editing, Visualization, Supervision. Dingshan Yu: Writing–review & editing. Xudong Chen: Writing–review & editing, Supervision.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, S., Shi, H., Shen, B. et al. Polypyrrole-functionalized g-C3N4 for rheological, combustion and self-healing properties of thermoplastic polyurethane. J Polym Res 29, 263 (2022). https://doi.org/10.1007/s10965-022-03046-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03046-x