Abstract
In this article, an efficient flame-retardant and smoke-suppressant agent HGM@AMP was prepared by coated hollow glass microspheres (HGM) with ammonium molybdophosphate (AMP) and applied in thermoplastic polyurethane (TPU). The fire safety characteristics including heat and smoke production of TPU composites were investigated using cone calorimeter test (CCT), smoke density test (SDT), thermogravimetric analysis/infrared spectrometry (TG-IR), etc. The CCT results revealed that HGM@AMP could significantly decrease the heat release rate (HRR), total smoke release, smoke factor and so on. For example, when the loading of HGM@AMP was 0.5 mass%, the peak HRR value of the sample was decreased to 512.4 kW m−2, reduced by 55.1% compared with the sample containing the same loading of HGM (1141.8 kW m−2). The SDT results indicated that HGM@AMP could obviously improve the luminous flux of TPU composites in the test with or without flame, decreasing the production of smoke. And the TG-IR results showed that HGM@AMP can improve the thermal stability of TPU composites at high temperature. In all, HGM@AMP would make a great influence in improving the fire safety of TPU.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermoplastic polyurethane (TPU), as one of the three general plastics, has become more and more popular due to its superior performance and environmental protection concept and has been widely used in many fields [1]. However, many fire safety problems, such as high heat, dense smoke generation and high toxic gases release during the combustion process, have also been brought into focus with the wide use of TPU, which has greatly limited its broad applications [2, 3]. TPU generates a large amount of heat and unsaturated structure, such as smoke precursors in the process of depolymerization. If the heat and unsaturated structures are bound in the condensed phase, it will greatly reduce the fire hazard.
Many investigations have been demonstrated that it is one of the most simple and effective ways to improve the fire safety performance of TPU by adding flame retardant in the past decades, and have achieved a better effect [4,5,6]. As is known, organic halogen flame retardants, especially bromine and chlorine compounds, are the most widely used flame retardants because of their high flame-retardant efficiency and low price [7,8,9]. However, for organic halogen flame retardants, the serious disadvantages are that a lot of smoke, poisonous and corrosive gases generate during the process of pyrolysis and combustion, which affects human health and environment. In addition, intumescent flame retardant with acid, carbon and gas sources has also been developed quickly in the past few years [10, 11]. It is proved that the flame-retardant effect is good, but the mechanical properties of the material are destroyed to a certain degree [12]. Thus, developing new flame retardant with halogen free, high performance, low smoke and toxicity becomes the trend in this field, achieving a good improvement in flame-retardant aspect.
Hollow glass microspheres (HGM), as an important component of fly ash, have attracted considerable interests in the past few decades because of its advantages including well-defined morphology, large surface area, strong filling ability, uniform size, low density, small and well-distributed internal stress in products [13]. So far, many researchers have explored its application. Among these applications, HGM was often used to reinforce polymer for its high compression strength and good fluidity [14,15,16]. However, there are few reports about the application of HGM from fly ash in flame-retardant fields. In our previous researches, it was found that HGM could significantly reduce the smoke production and the heat release of the TPU composites by catalyzing TPU carbonization and change the structure of char residue layer during the combustion process [17,18,19]. In addition, it is reported that ammonium AMP, as a molybdenum compound, has a good smoke suppression effect on polymer materials [20, 21]. It can decompose to form phosphoric acid, molybdate and NH3, which can act as the acid source and gas source in intumescent flame-retardant system. And TPU has carbon-forming performance during combustion, which can act as carbon source. Therefore, AMP may have a certain flame-retardant effect in TPU. Combing HGM and AMP, a new flame retardant maybe be obtained. To the best of our knowledge, the studies on HGM coated by AMP to improve the flame retardant of TPU are less.
In this paper, HGM@AMP was prepared by combining HGM with AMP. And the characterization of HGM@AMP was carried by SEM and XRD. Then, the TPU composites were prepared using HGM@AMP as flame retardants. The flame-retardant and smoke suppression characteristics of thermoplastic polyurethane (TPU) composites were evaluated using CCT and SDT. The thermal decomposition behavior of the composites was also studied by TG-IR.
Experimental
Materials
TPU (9380A) was produced by Bayer Germany, and the basic properties of TPU are as follows: hardness 82A (ISO868); density 1.110 g cm−3 (ISO1183); tensile strength 40 MPa (ISO527-1, -3); and elongation at break 500% (ISO527-1, -3). HGM composed of sodium borosilicate (grade 5020, production place: Australia, particle size 15–150 μm, medium particle 50 μm, density 0.2 g cm−3) was obtained from Guangzhou Qingcong trading Co., Ltd.
Synthesis of HGM@AMP
Ammonium hydrogen phosphate (10 g) and ammonium molybdate (10 g) are dissolved in ionized water (300 mL). Then, the pH of the solution was adjusted to 1 by HNO3 and stirred at 70 °C for 4 h. And the AMP was obtained. HGM was washed in 10% sodium hydroxide solution for 30 min and then filtrated, washed and dried. AMP was added, and the treated HGM in evaporating dish was kept stirring constantly. The solvent was distilled, and AMP was deposited on the surface of HGM. After drying, the product of HGM@AMP was obtained.
Sample preparation
TPU was dried in a vacuum oven at 80 °C for 24 h. Then, TPU mixed with HGM@AMP in different ratios (the formulations are given in Table 1), the quality of each group was 80 g, added to the internal mixer (SU-70B) with the rotation speed of 30 rpm for 10 min. The materials from the internal mixer were put into the tablet machine (the quality of materials for each sample is 45 g with the size 10 × 10 × 3 mm3) with the temperature of the splints was both 180 °C.
Measurements
Scanning electron microscope (SEM)
The surface morphologies of HGM and HGM@AMP were observed using a JEOL JSM 6700 LV scanning electron microscope (SEM) (JEOL, Japan) at an accelerating voltage of 5 and 8 kV.
X-ray diffraction (XRD)
The performance of XRD patterns was tested with the DX-1000 diffractometer using Cu-Ka tube and Ni filter (λ = 0.1542 nm), and the scanning rate is 0.02° per second in the 2θ range of 5–50°.
Cone calorimeter test (CCT)
The flame-retardant properties of the samples were measured by a cone calorimeter device (Fire Testing Technology, UK). The samples with the dimension of 100 × 100 × 3 mm3 were exposed to a radiant cone at a heat flux of 35 kW m−2.
Smoke density test (SDT)
The smoke production properties were measured by smoke density test (Qingdao University of Science and Technology; Naer Company in Dongguan). The samples with the dimension of 75 × 75 × 3 mm3 were exposed to a radiant cone at a heat flux of 25 kW m−2, 552 °C.
Thermogravimetric analysis/infrared spectrometry (TG-IR)
Thermogravimetric analysis/infrared spectrometry (TG-IR) of the pure TPU and flame-retardant TPU was performed using a DT-50 (Setaram, France) instrument that was interfaced to an IRAffinity-1 FTIR spectrometer. About 10.0 mg of sample was put in an alumina crucible and heated from 30 to 700 °C. The heating rate was set as 20 K min−1 (nitrogen atmosphere, flow rate of 60 mL min−1).
Results and discussion
Characterization of HGM@AMP
The SEM results of HGM (A and B) and HGM@AMP (C and D) are shown in Fig. 1. It described the surface characters of particles with 10 μm and 100 μm scale. Seen from the complete structure of particles (A and C), the surfaces of HGM and HGM@AMP were all spherical shape material. And it also could be seen from the broken HGM that HGM consists of an outer shell, a thin layer, which will result in good thermal insulation performance. Comparing A with C, the surfaces of HGM particles were quite smooth. However, the surfaces of HGM@AMP particles were rough, which may be that AMP had been coated on the surface of HGM. B and D also had given the SEM images of HGM and HGM@AMP. It can be seen that there was film layer on the surface of HGM@AMP, which further confirmed that AMP had been coated on the surface of HGM.
The XRD method was also used to explore the surface characteristics of HGM, AMP and HGM@AMP which are shown in Fig. 2. Combining AMP and HGM, it could be seen that there were new diffraction peaks appearing at about 16°, 23°, 29°, 33° and 45° for HGM@AMP, and the peak values were 101, 112, 200, 202 and 213, respectively, whereas no peak was found in HGM. The results implied the new diffraction peaks should be attributed to AMP formed on the surface of HGM.
Cone calorimeter test
Heat release rate (HRR)
Thermal performance is one of the important aspects to evaluate the flame retardant of polymer [22]. And the HRR from CCT is one of the representative thermal parameters to characterize the thermal performance [23,24,25,26]. The HRR curves of flame-retardant TPU composites are shown in Fig. 3. It could be seen that the HRR of sample with HGM@AMP (TPU-2) was much lower than the sample with HGM (TPU-1) or pure TPU (TPU-0), especially on the peak of HRR (pHRR). The pHRR value of TPU-0 was 1423.4 kW m−2, while the pHRR values of TPU-1 and TPU-2 were 1141.8 kW m−2 and 512.4 kW m−2. Obviously, the pHRR value of sample with 0.5 mass% HGM@AMP decreased by 64.0% and 55.1% compared with TPU-0 and TPU-1, respectively. Moreover, it could be seen that the ignition time of TPU-0 was 70 s, while the ignition times of TPU-1 and TPU-2 were about 65 s and 60 s, advanced on the basic of TPU-0. The reason may be that AMP can decompose at a low temperature to form phosphoric acid, molybdate, metaphosphate, polyphosphoric acid, and so on, which can catalyze the degradation of TPU to form flammable gases at low temperature, making the ignition time short. And it could also be seen that the double-peak phenomena appeared for the sample with HGM@AMP. However, there was only one peak for the sample with HGM. The formation of first peak could be illustrated that the HGM@AMP could migrate to the surface of the sample, and the phosphoric acid and metaphosphate from the decomposition of AMP could act with the polyols from the decomposition of TPU and form char residue layer on the surface of the sample, which acts as a barrier to prevent flammable gases into flame zone and heat to underlying materials. With the continuous exposure to high temperature, the protective carbon layer gradually degrades, leading to the second peak.
Total heat rate (THR)
The THR curves of the TPU composites are shown in Fig. 4. It was found that TPU-0 was highly flammable after ignition, exhibiting a THR as high as 97.5 MW m−2 at the end of the combustion. Compared with TPU-0, the samples with HGM and HGM@AMP burnt slowly and showed a lower THR. The THR decreased from 97.5 MW m−2 to 93.2 MW m−2 and 84.2 MW m−2, respectively. Compared with TPU-1, the THR value of TPU-2 with the same loading was further reduced. The significant reduction in THR indicated that more effective and protective char layers are formed on the surfaces of the sample, making the organic structures remain in the condensed phase, rather than being converted to flammable gas in the gas phase, which is in accordance with the HRR results.
Smoke production rate (SPR)
The smoke is one of the major causes of death in a real fire accident. In order to reduce the overall fire hazard, it is essential for flame retardant with low smoke production. SPR is an important parameter to evaluate the smoke performance of composites, and the SPR curves of flame-retardant TPU composites are shown in Fig. 5. It could be seen that the SPR values of TPU-1 and TPU-2 were significantly reduced compared with TPU-0. The peak SPR value of TPU-1 composite decreased to 0.058 m2 s−1 from 0.072 m2 s−1 (TPU-0). And for the sample with the same loading of HGM@AMP (TPU-2), the peak SPR value of TPU2 was reduced to 0.031 m2 s−1. It was no doubt that 0.5 mass% of HGM@AMP in TPU was the most effective match in the aspect of SPR and reduced by 46.6% on the basic of TPU-1. Moreover, for TPU-2, the time to the peak SPR value became longer compared with TPU-1, which meant the burning rate was slowed down. Smoke suppression by HGM@AMP may be caused by the following two reasons: on the one hand, HGM@AMP can migrate to the surface of the sample and then the phosphate acid from the decomposition of AMP can change the structure of char layer, which protects the inner matrix and reduces the amount of smoke-forming materials in the gas phase during combustion. Meanwhile, it is very hard for heat to enter into the underlying material, thus preventing further thermal decomposition and smoke production of the material. On the other hand, AMP, as a kind of molybdenum compound, can lead to cross-linking reaction and reduce the production of combustible materials; moreover, the AMP will decompose and form the matrix of aromatic smoke bonded in the composite firmly. In conclusion, the SPR confirms that HGM@AMP is a more effective additive to suppress smoke production in the combustion process of TPU composites than HGM.
Total smoke release (TSR)
The TSR curves of all flame-retardant TPU composites are shown in Fig. 6. It could be seen that the SPR value of the sample containing HGM@AMP (TPU-2) was much lower than those of the samples containing HGM and pure TPU. The TSR values of TPU-0 and TPU-1 were 760.6 m2 m−2 and 714.1 m2 m−2, respectively, while the TSR value of TPU-2 was 642.9 m2 m−2. It can be concluded that HGM@AMP has a better smoke suppression effect than HGM, which is consistent with the result of SPR.
Smoke factor (SF)
The SF is the production of HRR and TSP, which can be used to evaluate the combustion performances including the heat and smoke. Figure 7 presents the SF curves of flame-retardant TPU composites. The SF value of TPU-0 was about 849.9 MW/m2 at the end of combustion; however, it was about 629.8 MW m−2 when 0.5 mass% HGM was added and 362.4 MW m−2 when 0.5 mass% HGM@AMP was added, respectively. Obviously, the sample with 0.5 mass% HGM@AMP had the lowest SF value, which meant HGM@AMP had a better effect to prevent the hot and smoke during the combustion.
Mass
Figure 8 presents the mass loss curves of flame-retardant TPU composites. It could be seen from Fig. 8 that the residual mass of TPU-1 was not obviously increased compared with TPU-0. The mass of TPU-0 and TPU-1 was all decreased rapidly and reached 9.3% and 9.4% at the end of experiment, respectively. The reason can be explained as follows: although the char residue layer level of TPU-1 was improved, it is not compact enough to inhibit the combustion of materials and leads to high mass loss. However, the residual mass of TPU-2 was 13.8%, increased by 4.4% compared with that of TPU-1 at the same loading. The main reason is that AMP on the surface of HGM@AMP can decompose and form phosphoric acid and metaphosphate to promote the cross-linking of the composites and char residue formation, creating a good physical protective barrier to prevent heat and mass transferring.
FPI and FGI
The fire performance index (FPI) and the fire growth index (FGI) are calculated after CCT. The FPI is defined as the ratio of the ignition time (ITT) and pHRR, and the FGI is defined as the ratio of peak HRR and the time to PHRR. Usually, the higher the value of FPI is and the lower the value of FGI is, the higher the flame-retardant effect of the material will be. From Fig. 9, it could be seen that the FPI value of TPU-2 (0.096) was higher than those of TPU-0 (0.042) and TPU-1(0.036), and the FGI value of TPU-2 (3.25) was lower than those of TPU-0 (7.25) and TPU-1(6.03), which meant that the TPU-2 has the highest flame retardancy.
Photographs of char residue
Photographs of TPU composites after the CCT are shown in Fig. 10. It could be seen that the surface of the sample of TPU-0 was relatively loose and some large holes or cracks could be observed at the surface of the char layer. After the introduction of 0.5 mass% HGM into the TPU composite, a continuous and compact char layer is formed for TPU-1, but some crevasses and holes still could be seen on the surface of the char residue layer, indicating that it was still not good enough to protect the TPU from burning. However, for the samples with 0.5 mass% HGM@AMP, a compact and smooth char residue layer was found and few cracks and holes were on the surface of the char residue layer. A good char residue layer could act as a barrier to restrain the heat release and smoke generation, which made TPU composites exhibit good flame-retardant performance.
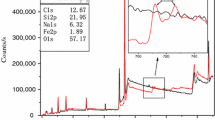
In order to observe the microstructure of the char residue layers and further explore the flame-retardant mechanism, the char residue layers left after CCT were examined by SEM–EDS analysis [27, 28]. The SEM results of TPU-0, TPU-1 and TPU-2 are shown in Fig. 11. It could be seen that the surface of TPU-2 was much denser than those of TPU-0 and TPU-1. Furthermore, there is a black layer formed on the surface of the samples with 0.5 mass% HGM@AMP, indicating that AMP had played an important role in the char formation process. It had been confirmed that HGM would migrate onto the surface of the sample and catalyze TPU carbonization to form a compact char residue layer during the combustion in previous studies. To verify whether the AMP would separate with HGM in the process of heating, melting and migration, the EDS test was used to detect the distribution of elements on inner/outer surface of TPU-2 and the results are shown in Fig. 12. It could be seen that the elements Si, Mo and P exist synchronously, and the distribution density of elements on outer surface was obviously higher than those of the inner, which proved that AMP would migrate onto the surface of the sample with HGM and improve the flame-retardant performance of TPU [17].
Smoke density test
The detailed information about smoke character can be tested by the smoke density test (SDT). And the luminous flux (LF) is an important parameter to measure the smoke production of composites. Figure 13 presents the LF curves of TPU-0, TPU-1 and TPU-2 in the SDT without (A) and with (B) flame. It could be seen from Fig. 13a that the LF of TPU-0 rapidly decreased in the first 500 s and attained the lowest LF value (3.4%) at 1200 s. In the case of TPU-1, the LF value was lower than TPU-0 between 200 s and 400 s and higher than TPU-0 after 400 s and up to 5.2%, which meant that HGM did not significantly reduce smoke generation. On the contrary, it had increased the release of the smoke gas in the prophase. However, in the case of TPU-2 with the same loading HGM@AMP, the generation of smoke particle was dramatically reduced after 250 s and the LF value (7.9%) was higher than TPU-0 and TPU-1. This is because AMP had migrated onto the surface of the sample with HGM and catalyzed the carbonization of composite and formed char residue layer barrier, which could effectively reduce the generation and diffusion of smoke particles. However, it was due to the fact that they were tested in the condition of without flame; the char residue layer was not compact enough, which would lead to the char residue layer collapse and part of internal smoke gas release. So, the improvement in LF was not very obvious at the end of the test. But, it could be seen from Fig. 13b that the samples were tested with flame that the distinction between them was obvious. And the final LF values were 4.4, 7.8 and 16.5%, respectively. The LF value for the sample with 0.5 mass% HGM@AMP was significantly increased compared with TPU-0 and TPU-1. This was the interaction result of the char formation of phosphate acid and smoke suppression effect of molybdate acid from AMP on the surface of HGM. Moreover, it also could be seen that the LF curves of the samples liked saddle shape after about 500 s. This was because there were smoke particulates generating and began to reunite and settle. And the production rate was less than the sedimentation rate. In conclusion, it can be concluded that HGM@AMP has better effect of smoke suppression for TPU than HGM.
Thermogravimetric analysis (TG)
TG and DTG curves of TPU and flame-retardant TPU composites under nitrogen atmosphere are shown in Fig. 14. It could be found from Fig. 14a that the final thermal degradation temperature of TPU-0, TPU-1 and TPU-2 was 330 °C, 327 °C and 300 °C, respectively. Compared with pure TPU, the initial degradation temperature of flame-retardant TPU composites decreased owing to the addition of HGM and HGM@AMP. This indicated that both HGM and HGM@AMP could promote the decomposition of TPU at early temperature and the promoting effect of HGM@AMP was more apparent. And TPU-1 and TPU-2 composites exhibited an enhanced thermal behavior at temperatures ranging from to 700 °C and had higher residues compared with TPU-0, which could protect the TPU matrix from heat and combustion. Comparing TPU-2 with TPU-1, the samples had almost the same residual mass during the thermal degradation process. The reason may be that the quality of the samples used to test was only 10 mg, not enough to further form a continued protective char residue layer at high temperature, and the residue mass decreases quickly after 550 °C. From the DTG curves shown in Fig. 14b, it could be seen that the degradation of TPU-0, TPU-1 and TPU-2 is all composed of two peaks. For the sample of TPU-0, the first peak was assigned to the rupture of the TPU main chains; the second peak was defined as the catalytic carbonization of polyol in TPU. And for the samples of TPU-1 and TPU-2, the first temperature peak values were lower than that of TPU-0, especially for TPU-2, which further confirmed the promoting effect of AMP in TPU; the second peak values of TPU-1 and TPU-2 were lower than that of TPU-0, and TPU-2 had the lowest DTG peak value, which meant the decomposition rate was decreased and was favorable to promote the formation of char residue, further prohibiting thermal decomposition of polymers [29,30,31].
To analyze the results of TG and DTG, the conclusion can be demonstrated that TPU-2 has higher thermal stability at high-temperature range than those of TPU-0 and TPU-1, owing to the effect of AMP compound on the shell of HGM.
TG-IR characterization of volatile products
TG-IR is used to analyze the gas products of TPU and TPU composites during the thermal degradation process. According to the DTG curve, the real-time TG–FTIR spectra of TPU composites at four special temperatures (240 °C, 360 °C, 440 °C and 560 °C) with high mass loss rate was chosen to study and are shown in Fig. 15.
It could be seen from Fig. 15a that the infrared signal was hardly detected at 240 °C for TPU-0 and TPU-1, which meant that the samples did not decompose. However, for the sample of TPU-2, FTIR spectra could be obtained in the regions of 2300–2400 cm−1 and 3400–4000 cm−1 during the thermal decomposition process, which were attributed to the characteristic bands of CO2 and H2O, respectively [32]. This indicated that TPU-2 has released CO2 and H2O at 240 °C, earlier than those of TPU-0 and TPU-1, which was beneficial to inhibit further decomposition of materials. The reason was that AMP could promote the decomposition of TPU at low temperature, which further illustrated the TG results in Fig. 14. When the temperature was raised to 360 °C (Fig. 15b), the thermal degradation components of three samples were gradually detected. The peaks appeared such as nonflammable gases, CO2 and flammable gases, aliphatic hydrocarbons and carboxylic acids, which were attributed to the fracture of the polyurethane backbone. And when the temperature was raised to 440 °C (Fig. 15c), the intensity of absorption peaks was higher than those of samples at 360 °C, indicating that the thermal degradation of materials was gradually strengthened. In Fig. 15d, it could be seen that the absorption peak for TPU-0 was decreased obviously at 560 °C, while TPU-1 and TPU-2 were still in higher thermal decomposition stage, especially for TPU-2, which explained that the addition of AMP could prolong the thermal degradation process on the basis of pure TPU. Combined with the results of TG test, the conclusion also can be drawn that AMP could promote the decomposition of TPU at earlier temperature. As the degradation continues, the AMP migrated onto the surface of TPU with HGM, catalyzing the carbonization and cross-linking of the TPU matrix, which resulted in higher thermal stability.
Conclusions
In this paper, a new type of flame retardant (HGM@AMP) was prepared and its affection on flame-retardant TPU was also tested by series means. The SEM and XRD results confirmed that AMP had been coated on the surface of HGM successfully. The CCT showed that the heat parameters including HRR and THR and the smoke parameters including SPR, TSR, SF and so on were significantly decreased using HGM@AMP as flame retardant than HGM. The SDT indicated that HGM@AMP could significantly improve the LF value compared with HGM and has better effect of smoke suppression for TPU than HGM. And the TG-IR test showed that the AMP could migrate onto the surface of TPU with HGM, catalyzing the carbonization and cross-linking of the TPU matrix, which resulted in higher thermal stability. In short, HGM@AMP represented dramatically better flame-retardant and smoke suppression properties in TPU composites than HGM and would have a wide application prospect in fire safety fields.
References
Saralegi A, Rueda L, Fernández-D’Arlas B, Mondragon I, Eceiza A, Corcuera M. Thermoplastic polyurethanes from renewable resources: effect of soft segment chemical structure and molecular weight on morphology and final properties. Polym Int. 2013;62:106–15.
Chen X, Jiang Y, Jiao C. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J Hazard Mater. 2014;266:114–21.
Finnigan B, Martin D, Halley P, Truss R, Campbell K. Morphology and properties of thermoplastic polyurethane nanocomposites incorporating hydrophilic layered silicates. Polymer. 2004;45:2249–60.
Chattopadhyay D, Webster D. Thermal stability and flame retardancy of polyurethanes. Prog Polym Sci. 2009;34:1068–133.
Lin M, Li B, Li Q, Li S, Zhang S. Synergistic effect of metal oxides on the flame retardancy and thermal degradation of novel intumescent flame-retardant thermoplastic polyurethanes. J Appl Polym Sci. 2011;121:1951–60.
Weil E. Levchik S Current practice and recent commercial developments in flame retardancy of polyamides. Flame Retard. 2009;22:85–104.
Covaci A, Harrad S, Abdallah M. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Env Inter. 2011;37:532–56.
Laoutid F, Bonnaud L, Alexandre M, Lopez-Cuesta J, Dubois P. New prospects in flame retardant polymer materials: from fundamentals to nanocomposites. Mater Sci Eng. 2009;63:100–25.
Ricciardi M, Antonucci V, Zarrelli M, Giordano M. Fire behavior and smoke emission of phosphate-based inorganic fire-retarded polyester resin. Fire Mater. 2012;36:203–15.
Chen X, Ma C, Jiao C. Synergistic effects between iron-graphene and ammonium polyphosphate in flame-retardant thermoplastic polyurethane. J Therm Anal Calorim. 2016;126:1–10.
Liu Y, Wang Q. Preparation of microencapsulated red phosphorus through melamine cyanurate self-assembly and its performance in flame retardant polyamide 6. Polym Eng Sci. 2010;46:1548–53.
Almeras X, Bras M, Hornsby P, Bourbigot S, Marosi G, Keszei S, et al. Effect of fillers on the fire retardancy of intumescent polypropylene compounds. Polym Degrad Stab. 2003;82:325–31.
Park S, Jin F, Lee C. Preparation and physical properties of hollow glass microspheres-reinforced epoxy matrix resins. Mater Sci Eng. 2005;402:335–40.
Hu Y, Mei R, An Z, Zhang J. Silicon rubber/hollow glass microsphere composites: influence of broken hollow glass microsphere on mechanical and thermal insulation property. Compos Sci Technol. 2013;79:64–9.
Jiao Y, Xiao G, Xu W, Zhu R, Lu Y. Factors influencing the deposition of hydroxyapatite coating onto hollow glass microspheres. Mater Sci Eng. 2013;33:2744–51.
Sun L, Wan S, Yu Z, Wang L. Optimization and modeling of preparation conditions of TiO2 nanoparticles coated on hollow glass microspheres using response surface methodology. Sep Purif Technol. 2014;125:156–62.
Jiao C, Wang H, Li S, Chen X. Fire hazard reduction of hollow glass microspheres in thermoplastic polyurethane composites. J Hazard Mater. 2017;332:176–84.
Chen X, Jiang Y, Jiao C. Synergistic effects between hollow glass microsphere and ammonium polyphosphate on flame-retardant thermoplastic polyurethane. J Therm Anal Calorim. 2014;117:857–66.
Liu L, Hu J, Zhuo J, Jiao C, Chen X, Li S. Synergistic flame retardant effects between hollow glass microspheres and magnesium hydroxide in ethylene-vinyl acetate composites. Polym Degrad Stab. 2014;104:87–94.
Wang G, Hu Xiao-Ping LuZ, Song L, Zhang M, Zhang J. Preparation and characterization of ammonium molybdophosphate coated by microcapsule and flame retardancy and smoke suppression of its composites with UPR. Fine Chem. 2007;24:108–12.
Deng Q, Li M. Cheng Z Study of cesium recovery with zirconyl phosphate-ammonium molybdophosphate complex ion exchanger. Nucl Power Eng. 2006;27:94–6.
Ramgobin A, Fontaine G, Penverne C, Bourbigot S. Thermal stability and fire properties of salen and metallosalens as fire retardants in thermoplastic polyurethane (TPU). Mater. 2017;10:665–83.
Kamikawa D, Kuroda K, Inoue M, Kubo S, Yoshida T. Evaluation of combustion properties of wood pellets using a cone calorimeter. J Wood Sci. 2010;55:453–7.
Schartel B, Hull T. Development of fire retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2010;31:327–54.
Sacristán M, Hull T, Stec A, Ronda J, Galià M, Cádiz V. Cone calorimetry studies of fire retardant soybean-oil-based copolymers containing silicon or boron: comparison of additive and reactive approaches. Polym Degrad Stab. 2010;95:1269–74.
Jiao C, Wang H, Zhang Z. Preparation and properties of an efficient smoke suppressant and flame-retardant agent for thermoplastic polyurethane. Polym Adv Technol. 2017;28:1690–8.
Wang H, Jiao C, Zhao L. Preparation and characterization of TiO2-coated hollow glass microsphere and its flame-retardant property in thermoplastic polyurethane. J Therm Anal Calorim. 2018;131:2729–40.
Savas L, Deniz T, Tayfun U, Dogan M. Effect of microcapsulated red phosphorus on flame retardant, thermal and mechanical properties of thermoplastic polyurethane composites filled with huntite & hydromagnesite mineral. Polym Degrad Stab. 2017;135:121–9.
Zhou K, Gui Z, Hu Y. The influence of graphene based smoke suppression agents on reduced fire hazards of polystyrene composites. Composites. 2016;80:217–27.
Carty P, Creighton J, White S. TG and flammability studies on polymer blends containing acrylonitrile–butadiene–styrene and chlorinated poly(vinyl chloride). J Therm Anal Calorim. 2001;63:679–87.
Zhou K, Tang G, Jiang S. Combination effect of MoS2 with aluminum hypophosphite in flame retardant ethylene-vinyl acetate composites. RSC Adv. 2016;6:37672–80.
Chen X, Ma C, Jiao C. Enhancement of flame-retardant performance of thermoplastic polyurethane with the incorporation of aluminum hypophosphite and iron-graphene. Polym Degrad Stab. 2016;129:275–85.
Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (Nos. 51776101, 51206084) the Natural Science Foundation of Shandong Province (ZR2017MB016) and the Project of the State Administration of Work Safety (shandong-0039-2017AQ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiao, C., Wang, H. & Chen, X. An efficient flame-retardant and smoke-suppressant agent by coated hollow glass microspheres with ammonium molybdophosphate for thermoplastic polyurethane. J Therm Anal Calorim 137, 1579–1589 (2019). https://doi.org/10.1007/s10973-019-08044-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08044-8