Abstract
An insulating interlayer between conductive filler and polymer matrix is essential for inhibiting the dielectric loss of polymer composites. To suppress the dielectric loss of nickel (Ni)/poly(vinylidene fluoride) (PVDF) composites while still having a high dielectric permittivity (k) near the percolation threshold, Ni particles were first encapsulated with a layer of insulating NiO shell through direct thermal oxidation method at high temperature, and then were incorporated into PVDF to explore the influence of the NiO coating on dielectric properties of the Ni/PVDF composites. The results indicate that the calcination temperature and the filler loading significantly influence the dielectric properties of the composites. Ni@NiO/PVDF composites have high k and low loss compared with the pristine Ni/PVDF. The NiO insulating interlayer prevents Ni particles from direct contact thereby leading to significantly suppressed conductivity and loss even at high filler loadings. Increasing shell thickness reduces dielectric loss with the k almost unaffected. These Ni@NiO/PVDF composites with a high k and very low loss show promising potential application in the microelectronic and electrical industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-performance dielectric materials are highly desirable and widely used in the development of next-generation miniaturized electronics [1,2,3,4]. In order to maximize the charge that a capacitor can keep, the dielectric material requires a high permittivity (k) and a superior dielectric breakdown strength (Eb) [5, 6]. Currently, commercial dielectrics for high-power applications are dominated by ceramics, which usually perform inherent brittleness and low Eb, as well as the problem of difficult machining. Compared with the traditional ceramics, polymers show clear advantages such as very easy processing, lightweight, low cost, excellent flexibility, and high Eb, etc., thereby making them commonly applied in various industrial occasions [7]. Unfortunately, most of polymers suffer from a rather low k (often less than 10), which would not address the requirements of further miniaturization of electronic components, and limit their many practical engineering applications [1, 7,8,9,10]. To tackle this problem, the composites based on combination of polymers and ceramic or conductive particles always are designed and developed to expect a high k but low loss, as well as good flexibility and other desirable properties [7, 11, 12].
For ceramic/polymer composites, a high loading (often > 50 vol.%) must be adopted to achieve desirable high-k polymer composites [1, 13], and subsequently, the high filler loading unavoidably not only gives rise to increasing loss and conductivity, decreased Eb, but also brings about deteriorated mechanical and processing properties. For conductive/polymer composites, the k of composites experiences a dramatic increase about one or even several orders when the loading of the conductive fillers approaches a percolation transition (fc), but at expense of a immense dielectric loss, thus markedly reducing the energy storage efficiency and even challenging the long term stability of the related products [1, 14,15,16]. Especially, a large quantity of conductive networks will be formed in the composites if the filler loading is greater than fc, which makes the composites no longer to be a dielectric material [14]. Thus, achieving the balance between sufficiently high k and greatly low loss is still a challenge in obtaining composites with improved dielectric performances at present [17].
Recent years have witnessed great progress in developing innovative polymer composites with high k and concurrently restrained dielectric loss. One of the most effective methods is to introduce an insulator interlayer or external shell outside the conductive filler to form a core–shell structured filler [18], which exhibits superior performances due to the combined effects of cores and shells [19]. The conductor core is used for inducing interfacial polarizations and enhancing the k [20], and the insulator shell not only acts as interlayer barriers to prevent cores from direct connection, but also produces excellent interfacial compatibility between the fillers and the matrix and ensures the homogeneous dispersion of fillers in the matrix [21]. Currently, it was reported that core–shell structured particles can be either synthesized by complex chemical synthetic routes with rather high production cost [1], such as CoFe2O4@Mn-MOF [22], CNTs@TiO2 [23], polydopamine (PDA)-SiO2@BaTiO3 nano-particles (BT NPs) or PDA-SiO2@BT nano-fibers (NFs) [24], or by relatively facile sol–gel method (e.g., Ni@SiO2 [25]) and direct thermal oxidation method (e.g., β-SiCw@SiO2 [1]) with rather low cost.
Nickel (Ni) is a promising ferromagnetic material, which has poured a great deal of attention in the applications of catalyst, batteries, electricity, magnet sensors due to its specific electrical, optical, and magnetic properties compared with other metal fillers [25]. Throughout the years, many research efforts have been devoted to developing high-performance polymer composites with Ni particles. For instance, Zhu et al. [26] theoretically and experimentally demonstrated that core–shell structured porous Ni@BaTiO3 scaffolds infiltrated with epoxy was an effective way to achieve balanced k and loss. The k of 4.0 vol.% Ni@BaTiO3/epoxy could be as high as 6397 and a loss remains as low as 0.025 at 10 kHz. In another study, Kum-onsa et al. [27] attempted to improve dielectric properties of poly(vinylidene fluoride) (PVDF) by incorporating high-k Na1/3Ca1/3Bi1/3Cu3Ti4O12 (NCBCTO) ceramic particles and core–shell Ni@NiO particles. The NCBCTO-(f = 0.15) and Ni@NiO filled PVDF composites showed a high k of 279.4 and a low tanδ of 0.075 at 1 kHz. Thus, the Ni@NiO exhibits obvious advantages to form composites with high k and low loss.
Based on the discussion above, we synthesized Ni@NiO (with controlled NiO shell thickness) using the simple and effective thermal oxidation method and investigated the dielectric properties of the corresponding composites. Unlike previous study [28], in this research, the raw Ni was uniformly encapsulated by a layer of NiO with tailored shell thickness through maintaining a constant calcination time and adjusting the oxidation temperature, then the obtained Ni@NiO particles were incorporated to PVDF. The results are expected to provide deep insights into the influence of core–shell structured Ni@NiO and its thickness on dielectric properties towards designing composites with a concurrent increase in k but low loss.
Experimental
Materials
The raw Ni powders (with an average particle size: 1 ~ 3 μm) were purchased from Wuxi Shunda Materials Co., China. PVDF FR903 was supplied by 3F New Materials Co. (Shanghai, China) and served as the polymer matrix. N, N-Dimethylformamide (DMF) and others were purchased from Tianjin Chemical Reagent Co. China.
Preparations
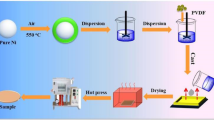
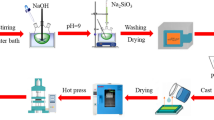
The formation of NiO shell with various thicknesses on the pristine Ni particles was accomplished by a direct thermal oxidation method under an air atmosphere for 3 h at 500 °C, 600 °C, 700 °C, respectively. A certain amount of PVDF was evenly dissolved in DMF solvent by stirring at 60 °C water bath for 30 min. Simultaneously, the Ni or Ni@NiO particles were evenly dispersed in DMF, respectively, under ultrasonication for 30 min. Then, the two above solutions were blended and further vigorously stirred at room temperature for 8 h. Afterwards, the obtained solution was casted onto a clean glass plate and dried in a vacuum oven under 120 °C to evaporate the residual DMF completely. Finally, it was compression-molded at 200 °C for 15 min under a pressure of about 15 MPa [1, 3, 16]. The schematic diagram of the preparation of Ni@NiO particles and their PVDF composites is shown in Fig. 1.
Characterizations
The functional groups of the composite samples were characterized using Fourier transform infrared spectroscopy (FT-IR, Perkin-Elmer, Paragon 1000) over the wavenumber range of 4000 cm−1 to 400 cm−1. The phase structures of Ni and Ni@NiO were performed by a Shimadzu X-ray diffractometer-6000 (XRD) equipped with a graphite homochromatic instrument and a Cu anticathode (40 kV, 30 mA, scanning rate 2°/min). The thermal properties of composites were determined by a thermogravimetric analyzer (TGA, SDTA851, Swiss) with a heating rate of 10 °C /min under air atmosphere from room temperature to 800 °C. The fillers’ distribution in PVDF matrix was investigated by scanning electron microscope (SEM, JSM-7000F, JEOL). Before the measurement, samples were fractured in liquid N2 and the fractured surface was sputtered with a thin layer of gold. The morphology of the core–shell structure for the Ni@NiO or pristine Ni particles was measured by an H-800 transmission electron microscope (TEM) from Hitachi Co (Japan).
The dielectric properties of the samples were characterized by an Agilent 4294A impedance analyzer over a wide frequency range of 40–107 Hz at room temperature.
Results and discussion
Characterizations of core–shell Ni@NiO
TEM images of the as-received raw Ni and core–shell structured Ni@NiO particles with different NiO shell thicknesses are shown in Fig. 2. Compared with the relatively smooth surface of the raw Ni (Fig. 2a), the calcined Ni particles show a typical core–shell structured morphology (Fig. 2b–d). In other words, a thin NiO layer with uniform thickness was formed outside the surface of raw Ni and the thickness of the NiO shell increases with the increase of calcination temperature. For example, the NiO shell’s thickness in Ni@NiO-500 °C/3 h, Ni@NiO-600 °C/3 h and Ni@NiO-700 °C/3 h is measured to be about ~ 5, ~ 8 nm and ~ 12 nm thick, respectively. The TEM measurement works as the strongest evidence to prove the successful synthesis of a NiO layer around core Ni.
The FT-IR spectra for Ni and core–shell structured Ni is depicted in Fig. 3a. Two absorption peaks, ~ 3441 cm−1 and ~ 1634 cm−1, can be identified in both fillers and come from the stretching vibration and bending vibration of hydroxyl groups, indicating the presence of H2O on the filler surface. Compared with the raw Ni, steep IR absorption bands, ranges from 496 to 423 cm−1 were observed due to stretching vibrations of octahedral NiO6 group in face-centered cubic (FCC) structure of NiO in FT-IR spectra [29, 30]. The Ni@NiO filler presents high-intensity absorption bands at ~ 466 cm−1, which can be agree well with the stretching vibration of Ni–O. In addition, the corresponding absorption peaks at ~ 989 and ~ 849 cm−1 for Ni are not all reflected in the spectrum of Ni@NiO because they are significantly weakened. Above results proved that a layer of NiO shell is covered on the Ni surface.
Figure 3b reveals the TGA curve of Ni over the temperature range from 30 °C to 800 °C. There is no apparent mass loss for raw Ni from 30 °C to 90 °C, and after that, a slight weight loss can be noticed owing to the removal of water and small molecules physically absorbed on the surface of Ni. The remarkable increase in the weight occurs when the temperature is above 450 °C, and the reason is attributed to the formation of Ni@NiO oxides. The weight increase becomes marginal upon from 700 °C again.
The XRD measurements for the pristine and modified Ni fillers are shown in Fig. 4c. From the XRD patterns, compared with raw Ni, it is clear that the diffraction peak intensity of Ni particles with calcination distinctly decreased and several new characteristic peaks of NiO can be observed. With the increase of calcination temperature, NiO’s characteristic peak intensity is significantly enhanced. In detail, 2θ values of FCC NiO at 37.06°, 43.02°, 63.06°, 75.31° and 79.37° can be assigned to the (111), (200), (220), (311) and (322) crystal planes, respectively. As a consequence, good crystallinity of the NiO shell is obtained successfully.
The cross-sectional SEM morphologies of raw Ni and core–shell structured Ni particles with different filler loadings are presented in Fig. 4. It is quite clear that the pure Ni particles are slightly smaller in size as compared to Ni@NiO particles in accordance with Fig. 4a, b, e and f. Furthermore, the surface of Ni@NiO particles became a little rough on account of the volume expansion during oxidation process. As presented in Fig. 4c–e, compared with raw Ni, it can be seen that Ni@NiO particles are uniformly distributed across the sample without obvious aggregation, even at high filler loadings, which can be ascribed to the enhanced interfacial interactions between Ni@NiO particles and PVDF.
Dielectric properties
Figure 5 illustrates the variation of k with the increase of filler loading and frequency for the polymer composites based on raw Ni or Ni sintered at different temperature. From Fig. 5a, c–e, we can see that, the k of all the PVDF composites increases with increasing filler loadings within the measured frequency range. After reaching a critical filler loading, the k presents an abrupt increase, accompanied by a strong dielectric relaxation. In the low-frequency range, the dramatic increase in k is more pronounced, which also leads to a significant enhancement in relaxation strength.
Dependence of dielectric permittivity on frequency and filler loading for the PVDF composites containing: (a) pure Ni, (c-e) Ni@NiO with different shell thicknesses, (b, f) Dielectric permittivity of composite (100 Hz) as a function of filler loading for pure Ni and Ni@NiO(-600 oC/3 h)/PVDF composites, respectively, (inset: log–log plot of dielectric constant vs. (fc-f)). (g) Dielectric permittivities of PVDF filled with pure Ni and Ni@NiO with different calcination temperature of 50 wt.% and 60 wt.%
There are a number of theories concentrated on explaining the sharp increase in k for the near-percolated composites. One theory (the microcapacitor model) believes that the increasing k is related to the formation of microcapacitor arrays in the composites. In detail, at low filler loadings, the capacitance of each microcapacitor keeps quite low and the number of microcapacitors is insufficient due to the separation of Ni particles, the dielectric response of the PVDF composites is mainly attributed to the PVDF polarization and the addition of pure Ni particles only provides a weak increase in k, leading to a slight increase in k [28, 31]. With increasing filler loadings, the Ni particles are close to each other so that lots of charge carriers are blocked at the interface between raw Ni and PVDF (namely, the formation of microcapacitors), and Maxwell–Wagner-Sillars (MWS) polarization will occur, resulting in a marked increase in the k of the materials [32]. At high filler loadings, in particular, the k exhibits orders of magnitude enhancement when filler loadings is above 30 wt.%. Such strong increase in the k could be rationalized by the interfacial polarization for the capacitance of microcapacitors. For instance, the k of Ni/PVDF composites can achieve 107 level when the filler loading is 60 wt.% (Fig. 5a). But, the high filler loadings will cause Ni particles to easily agglomerate, and the voids and defects at the interface of the composites will also increase, which is not conductive to the formation of micro-capacitors [25]. For example, an abnormal dielectric behavior occurs at a filler content of 70 wt.% (Fig. 5a). Another theory (the charge diffusion model) considers the contribution of electron transport along the filler networks and the corresponding charge displacement. When the percolation threshold fc is near and the fillers are close together, electrons could tunnel through the filler gaps and explore different filler aggregates that were once electrically isolated [33, 34]. The polarization level of the filler can be greatly improved because more charge carriers can be separated, which can be evaluated by the degree of charge displacement [35]. When the filler content is below 30 wt.%, the k of composites does not change significantly with the increase of frequency, which can put down to the fact that there is little free charge carriers in the matrix and no hysteresis of dipole steering [1, 35]. When the filler content is over 30 wt.%, the dependence of strong frequency and a sharp downward trend in k are more evident in the frequency range from 40 to 105 Hz, which are attributed to the formation of the percolation network caused by the long range movement of free charge carriers [30].
The k at 100 Hz of the 50 wt.% Ni/PVDF and 60 wt.% Ni/PVDF show values of 629 and 517, respectively. From Fig. 5g, the k at 100 Hz of 50 wt.% Ni@NiO/PVDF and 60 wt.% Ni@NiO/PVDF at 500 °C immediately drop to 98.93 and 124.54; while the composites filled with 50 wt.% Ni@NiO/PVDF-600 °C and 50 wt.% Ni@NiO/PVDF-700 °C demonstrate the k of 187.55 and 101.50, and the composites filled with 60 wt.% Ni@NiO/PVDF-600 °C and 60 wt.% Ni@NiO/PVDF-700 °C demonstrate the k of 310.20 and 133.93, respectively. The results clearly manifest that the k is greatly reduced when the NiO shell is first formed. Generally speaking, the higher the sintering temperature, the thicker the NiO shell is. What is noteworthy is that the k of Ni@NiO/PVDF is not directly proportional to shell thickness in this study. The k of the composites shows a maximum value at 600 °C and then a decreasing trend is observed with further increasing temperature. In detail, the k for the composites first keeps on rising as the NiO shell thickness increases due to the gradually improved interface compatibility and the contribution of the new interface between Ni and PVDF, promoting the enhancement of interfacial polarization (IP), followed by a mild reduction in k because further thickening NiO shell would reduce the possibility of electron tunneling through the shell. That is to say, the highest k of the Ni@NiO/PVDF at 600 °C is reached from the enhanced IP resulting from the optimal shell thickness. From the view of the microcapacitor model, the modification of fillers would not produce much variation in the k of polymer composite. Based on the charge diffusion theory, the above discussion is completely consistent with it. In conclusion, it seems that the charge diffusion model is more applicable to describing the polymer composites filled with conductive fillers.
According to percolation theory, the variations of k with frequency follow a power law for polymer-based composites filled with conductive fillers.
where, km stands for the k of the polymer matrix, f stands for the filler volume fraction, fc stands for the percolation threshold, and s stands for an exponent of about 1. The log–log curves of k vs. (fc-f) are plotted in the Fig. 5b, f, the best fit of Eq. 1 yields a fc ≈ 4.76 vol.% and fc ≈7.89 vol.% for the raw Ni/PVDF and Ni@NiO/PVDF-600 °C, respectively. It shows that the formation of the NiO interlayer does not shift the percolation threshold obviously. As a result, it confirms the charge diffusion model in the polymer composites filled with conductive fillers.
Figure 6 depicts the dielectric loss of PVDF with Ni and Ni@NiO with various filler loading over the measured frequency range. At low frequencies, a rapid change with the increasing filler loading in loss was observed, corresponding to the electron transport along the filler network. The phenomena should be ascribed to changes in dipole polarization and interface polarization faster than the external alternating current (AC) electric field [25]. Consequently, the loss at low frequency is dominated by leakage direct current (DC) conduction loss \(\left({k}_{dc}^{\prime\prime}\right)\). With the increase of frequency, the loss of Ni/PVDF and Ni@NiO/PVDF composites slightly increased as fNi or fNiO, which puts down to the dielectric response caused by both the C-F dipole orientation polarizations in PVDF and the segmental motion of PVDF chains [36].
Dependence of dissipation factor on frequency and filler loading with different mass fractions for the PVDF composites containing: (a) pure Ni, (c-e) Ni@NiO with different shell thicknesses, (b) dissipation factor of PVDF filled with pure Ni and Ni@NiO with different calcination temperature of 50 wt.%, (f) dissipation factor v.s. Ni@NiO filler loading at 100 Hz for PVDF with different fillers, and (g) dissipation factor as a function of calcination temperature
As depicted in Fig. 6a, for the dielectric loss of Ni/PVDF composites, the tendency with filler concentration is almost increasing. There was a sharp increase in the value of loss near the fc, mainly owing to the DC conduction produced by the percolation effect. Under the circumstances, the electrons can travel along Ni fillers unconstrainedly and the Ni will establish particle clusters or form partial connectivity, which accounts for high loss at a high Ni loading [37]. Notably, the loss at 100 Hz of the 20 wt.% Ni/PVDF shows a value of 0.04, while this value is remarkably increased to 2350 for 30 wt.% Ni/PVDF. Afterward, the loss has no obvious change with increasing the Ni loading up to 70 wt.% at low frequencies. Figure 6c–e shows the frequency dependent loss for Ni@NiO/PVDF with different calcination temperatures. We can see that, the loss of all samples changed sharply with the increase of filler loading. Nevertheless, it is interesting to keep a value less than 1 for the loss of all the Ni@NiO/PVDF. The significant reduction in loss agrees with the expectation that the insulating NiO layer would prevent Ni against direct contact and avoid the formation of DC leakage across the composites. The NiO shell interlayer can effectively block the charge carries transportation, which inhibits the leakage conduction loss. The losses of PVDF filled with 50 wt.% pure Ni and Ni@NiO at different calcination temperatures were compared in Fig. 6b. It is clearly observed that the Ni/PVDF exhibits a high loss of 629, while the loss is reduced to 0.243, 0.255 and 0.47 for the Ni@NiO/PVDF-500 °C, Ni@NiO/PVDF-600 °C and Ni@NiO/PVDF-700 °C, respectively. It can be concluded that the NiO insulating shell performs a crucial role in suppressing loss of the Ni@NiO/PVDF composites.
Figure 6f, g summarize the evolution of loss (measured at 100 Hz) with filler loading and calcination temperature (the shell thickness). The formation of NiO interlayer can significantly reduce loss of composites. Moreover, at high filler loading, the losses are kept at a rather low level as the temperature rises gradually again. Interestingly, in connection with the previous statement, both k and loss are maintained at a good level under high filler loading. Namely, the composites can maintain a rather low loss at the same time can have a high k.
As a composite, it is generally recognized that the loss \(\left({k}^{\prime\prime}\right)\) is mainly consisted of three parts [26, 38]:
where, the \(\left({k}_{MW}^{\prime\prime}\right)\) stands for the interfacial polarization loss and \({k}_{D}^{\prime\prime}\) stands for the dipole polarization loss.
The conduction loss \({k}_{dc}^{\prime\prime}\)is described as:
where, \({\sigma }_{dc}\) and f are related to dc conduction and f, respectively.
Based on Eqs. (2–3), the \({k}^{\prime\prime}\) of Ni@NiO/PVDF is much lower than that of Ni/PVDF because NiO shell inhibits the generation of \({k}_{dc}^{\prime\prime}\). As a result, the \({\sigma }_{dc}\) can have a remarkable decrease.
Figure 7 illustrates the frequency dependence of AC conductivity for the Ni/PVDF and Ni@NiO/PVDF composites. Over the measured frequency range, the AC conductivity of Ni/PVDF augments linearly with increasing frequency when Ni content is below 20 wt.% in Fig. 7a. In this case, the conductivity of the Ni/PVDF is almost close as low as that of pure PVDF, because the Ni particles are too far apart to form conductive pathways for the electrons to tunnel. Along with the increase of Ni content, it is noted that the value of the conductivity is almost unaffected by the frequency. This means the formation of a conductive path among the Ni particles, the composite material becomes a conductor nature and electrons can be transported as if in a conductor.
Figure 7c–e presents the AC conductivity of composites based on Ni@NiO oxidized at different temperatures. It is obvious that three composites show similar linear trends with increasing frequency. Compared with Ni/PVDF, the conductivity of Ni@NiO/PVDF composites decrease significantly under the equivalent filler loading, which is owing to the insulating NiO shell among Ni particles. The interlayer effectively stops the Ni from contacting with each other and prevents the generation of electric current across the composites. In other words, it is not easy to form conductive path in the Ni@NiO/PVDF composites. Therefore, there is no distinct percolation observed in the Ni@NiO/PVDF composites. By comparing the conductivity of the Ni@NiO/PVDF composites at different temperatures, it is found that the conductivity of the Ni@NiO/PVDF decreases gradually with the increase of the NiO shell thickness, which is consistent with the expectation of low conductivity of the composites with good dielectric properties. Figure 7b summarizes AC conductivity of PVDF filled with 50 wt.% pure Ni and 50 wt.% Ni@NiO with different calcination temperatures. We can clearly see that the conductivity of Ni@NiO/PVDF is much lower than that of Ni/PVDF under the same filler loading.
Figure 8 vividly reveals the formation of conductive network and the influence of shell thickness on the dielectric properties of Ni/PVDF and Ni@NiO/PVDF. In detail, at a certain filler content, the raw Ni is in close contact with each other and the electrons can travel along Ni particles almost unconstrainedly, which is very conducive to the formation of a conductive path. According to charge diffusion theory, the enhanced charge separation results in both high k and loss for conductive fillers [37]. Encapsulation of the conductive Ni particle with an insulating NiO shell can effectively prevent charge transfer, and inhibition effect is obvious with the increase of shell thickness, so the loss of composites is greatly reduced. However, most of charges will be trapped at the interface between Ni core and NiO insulating shell for the Ni@NiO/PVDF. Although some charge carriers can still tunnel through the shell and polymer gap, this motion has much slower dynamics than the instantaneous charge separation within the conductive filler. In general, the NiO shell layer improves the dielectric properties of Ni/PVDF.
Conclusion
In this work, core–shell structured Ni@NiO was prepared by a facile thermal oxidation strategy, and the NiO shell was characterized by TEM, FT-IR, TGA, XRD and SEM measurements. The results verify the presence of a layer of crystalline NiO shell outside of the surface of Ni. The insulating NiO shell’s thickness is seen to increase with increasing the calcination temperature, which is proven to have an obvious influence on dielectric of Ni particles and their PVDF composites.
The k and loss increase with increasing filler loading for composites at the whole frequency range. Compared with the Ni, Ni@NiO prominently suppresses the loss of the composites at the equivalent filler content. The reason can be ascribed to the formation of an insulating NiO shell layer on the surface of Ni which acts as an interlayer among the Ni, thereby stopping them from contacting with each other, and inhibiting the dissipation factor into capacitor-tolerated value levels. We go one step further to reveal that a composite with concurrent high k and low loss can be obtained by increasing and further rationalizing the thickness of the NiO shell under high loadings of core–shell fillers. Thus, the obtained Ni@NiO/PVDF composites with appealing dielectric performances, can be potentially used for embedded passive components and other related microelectronic and electrical industries.
References
Zhou WY, Li X, Zhang F, Zhang CH, Li Z, Chen FX, Cai HW, Liu XR, Chen QG, Dang ZM (2020) Concurrently enhanced dielectric properties and thermal conductivity in PVDF composites with core-shell structured β-SiCw@SiO2whiskers. Compos Part A 137:106021
Li B, Yuan MX, Zhang SH, Rajagopalan R, Lanagan MT (2018) Abnormal high voltage resistivity of polyvinylidene fluoride and implications for applications in high energy density film capacitors. Appl Phys Lett 113:193903
Zhou WY, Chen QG, Sui XZ, Dong LN, Wang ZJ (2015) Enhanced thermal conductivity and dielectric properties of Al/β-SiCw/PVDF composites. Compos Part A 71:184–191
Tong Y, Talebinezhad H, Lu X, Cheng ZY, Hill C, Tucker D (2019) Microstructure and enhanced dielectric properties of BaTiO3-SiO2 nanocomposites using hydrogen treated nanoparticles. IET Nanodielectrics 2(1):41–47
Yao ZH, Song Z, Hao H, Yu ZH, Cao MH, Zhang SJ, Lanagan MT, Liu HX (2017) Homogeneous/inhomogeneous‐structured dielectrics and their energy‐storage performances. Adv Mater 29(20):1601727
Xu ZP, Qiang H, Chen Y, Chen ZQ (2017) Microstructure and enhanced dielectric properties of yttrium and zirconium co-doped CaCu3Ti4O12 ceramics. Mater Chem Phys 191:1–5
Zhou WY, Kou YJ, Yuan MX, Li B, Cai HW, Li Z, Chen FX, Liu XR, Wang GH, Chen QG, ZM Dang (2019) Polymer composites filled with core@double-shell structured fillers: effects of multiple shells on dielectric and thermal properties. Compos Sci Technol 181:107686
Jiang YC, Wang JB, Zhang QL, Yang H, Shen D, Zhou FM (2019) Enhanced dielectric performance of P(VDF-HFP) composites filled with Ni@polydopamine@BaTiO3 nanowires. Colloid Surface A 576:55–62
He XF, Zhou J, Jin LY, Long XY, Wu HJ, Xu L, Gong Y, Zhou WY (2020) Improved dielectric properties of thermoplastic polyurethane elastomer filled with core-Shell structured PDA@TiC particles. Materials 13:3341
Yuan MX, Zhang G, Li B, Chung TM, Rajagopalan R, Lanagan MT (2020) Thermally stable low-loss polymer dielectrics enabled by attaching cross-linkable antioxidant to pPolypropylene. ACS Appl Mater Interfaces 12:14154–14164
Wang DR, Huang MY, Zha JW, Zhao J, Dang ZM, Cheng ZY (2014) Dielectric properties of polystyrene based composites filled with core-shell BaTiO3/polystyrene hybrid nanoparticles. IEEE Trans Dielectr Electr Insul 21(4):116–119. https://doi.org/10.1109/TDEI.2013.004329
Fan BY, Liu FH, Yang G, Li H, Zhang GZ, Jiang SL, Wang Q (2018) Dielectric materials for high-temperature capacitors. IET Nanodielectrics 1(1):32–40
Zhang QP, Zhu WF, Liang DM, Wu XL, Chen RC, Sun N, Li YT, Zhou YL (2019) Core-shell structured CaCO3@CNF for enhanced dielectric properties of polymer nanocomposites. Appl Surf Sci 487:77–81
Chen JW, Wang XC, Yu XM, Yao LM, Duan ZK, Fan Y, Jiang YW, Zhou YX, Pan ZB (2018) High dielectric constant and low dielectric loss poly(vinylidene fluoride) nanocomposites via a small loading of two-dimensional Bi2Te3@Al2O3 hexagonal nanoplates. J Mater Chem C 6(2):271–279
Zhou WY, Zhang F, Yuan MX, Li B, Peng JD, Lv YQ, Cai HW, Liu XR, Chen QG, Dang ZM (2019) Improved dielectric properties and thermal conductivity of PVDF composites filled with core-shell structured Cu@CuO particles. J Mater Sci-Mater EL 30(20):18350–18361
Wang TX, Liang GZ, Yuan L, Gu AJ (2014) Unique hybridized graphene and its high dielectric constant composites with enhanced frequency stability, low dielectric loss and percolation threshold. Carbon 77:920–932
Zhou WY, Xu L, Jiang LY, Peng JD, Gong Y, Liu XR, Cai HW, Wang GH, Chen QG (2017) Towards suppressing loss tangent: Effect of SiO2 coating layer on dielectric properties of core-shell structure flaky Cu reinforced PVDF composites. Alloy Compd 710:47–56
Bi JY, Gu YZ, Zhang ZC, Wang SK, Li M, Zhang ZG (2016) Core-shell SiC/SiO2 whisker reinforced polymer composite with high dielectric permittivity and low dielectric loss. Mater Design 89:933–940
Zhou WJ, Cao XH, Zeng ZY, Shi WH, Zhu YY, Yan QY, Liu H, Wang JY, Zhang H (2013) One-step synthesis of Ni3S2 nanorod@Ni(OH)2 nanosheet core-shell nanostructures on a three-dimensional graphene network for high-performance supercapacitors. Energ Environ Sci 6(7):2216–2221
Chen ZH, Li HF, Xie GY, Yang K (2018) Core-shell structured Ag@C nanocables for flexible ferroelectric polymer nanodielectric materials with low percolation threshold and excellent dielectric properties. RSC Adv 8(1):1–9. https://doi.org/10.1039/C7RA12686G
Shen Y, Lin Y, Li M, Nan CW (2007) High dielectric performance of polymer composite films induced by a percolating interparticle barrier layer. Adv Mater 19(10):1418–1422
Ebrahimi AK, Sheikhshoaie I, Salimi S, Arkaban H (2021) In-situ facile synthesis of Superparamagnetic porous core-shell structure for Dye adsorption. J Mol Struct 1228:129797
Wu C, Huang XY, Wu XF, Yu JH, Xie LY, Jiang PK (2012) TiO2-nanorod decorated carbon nanotubes for high-permittivity and low-dielectric-loss polystyrene composites. Compos Sci Technol 72(4):521–527
Feng MJ, Zhang CH, Zhou GT, Zhang TD, Feng Y, Chi QG, Lei QQ (2020) Enhanced energy storage characteristics in PVDF-based nanodielectrics with core-shell structured and optimized shape fillers. IEEE Access 8:81542–81550
Li T, Zhou WY, Li Y, Cao D, Wang Y, Cao GZ, Liu XR, Cai HW, Dang ZM (2021) Synergy improvement of dielectric properties and thermal conductivity in PVDF composites with core-shell structured Ni@SiO2. J Mater Sci-Mater EL 32(4):4076–4089
Zhu XT, Yang J, Dastan DD, Garmestani H, Fan RH, Shi ZC (2019) Fabrication of core-shell structured Ni@BaTiO3 scaffolds for polymer composites with ultrahigh dielectric constant and low loss. Compos Part A 125:105521
Kum-onsa P, Phromviyo N, Thongbai P (2020) Na1/3Ca1/3Bi1/3Cu3Ti4O12-Ni@NiO/poly(vinylidene fluoride): Three-phase polymer composites with high dielectric permittivity and low loss tangent. Results Phys 18:103312
Zhou WY, Gong Y, Tu LT, Xu L, Zhao W, Cai JT, Zhang YT, Zhou AN (2017) Dielectric properties and thermal conductivity of core-shell structured Ni@NiO/poly(vinylidene fluoride) composites. Alloy Compd 693:1–8
Rahal HT, Awad R, Abdel-Gaber AM, Bakeer DE (2017) Synthesis, characterization, and magnetic properties of pure and EDTA-capped NiO nanosized particles. J Nanomater 2017:7460323
Yousaf S, Zulfiqar S, Shahi MN, Warsi MF, Al-Khalli NF, Aboud MF, Shakir I (2020) Tuning the structural, optical and electrical properties of NiO nanoparticles prepared by wet chemical route. Ceram Int 46(3):3750–3758
Zhong SL, Dang ZM, Zhou WY, Cai HW (2018) Past and future on nanodielectrics. IET Nanodielectrics 1(1):41–47
Streibl M, Werner S, Kaschta J, Schubert DW, Moos R (2020) The influence of nanoparticles and their functionalization on the dielectric properties of biaxially oriented polypropylene for power capacitors. IEEE Trans Dielectr Electr Insul 27(2):468–475
Li B, Camilli CI, Xidas PI, Triantafyllidis KS, Manias E (2017) Structured polyethylene nanocomposites: effects of crystal orientation and nanofiller alignment on high field dielectric properties. MRS Adv 2(6):363–368
Li B, Manias E (2017) Increased dielectric breakdown strength of polyolefin nanocomposites via nanofiller alignment. MRS Adv 2(6):357–362
Chen SY, Cheng YH, Xie Q, Xiao B, Wang ZD, Liu JY, Wu GL (2019) Enhanced breakdown strength of aligned-sodium-titanate-nanowire/epoxy nanocomposites and their anisotropic dielectric properties. Compos Part A 120:84–94
Xia W, Zhang Z (2018) PVDF-based dielectric polymers and their applications in electronic materials. IET Nanodielectrics 1(1):17–31
Wang S, Li Q (2018) Design, synthesis and processing of PVDF-based dielectric polymers. IET Nanodielectrics 1(2):80–91
Dang ZM, Yuan JK, Zha JW, Zhou T, Li ST, Hu GH (2012) Fundamentals, processes and applications of high-permittivity polymer-matrix composites. Prog Mater Sci 57(4):660–723
Funding
The authors gratefully acknowledge the financial supports from the National Natural Science Foundation of China (Nos. 51937007, 51577154), and acknowledges the Analytic Instrumentation Center of XUST.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interests
The authors declare no confict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, D., Zhou, W., Li, T. et al. Tailoring dielectric performance of Ni/poly(vinylidene fluoride) composites through constructing NiO shell as an interlayer. J Polym Res 28, 234 (2021). https://doi.org/10.1007/s10965-021-02594-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02594-y