Abstract
Developing a high dielectric constant (εr) polymer dielectrics with low dielectric loss and high thermal conductivity (TC) is still continuous demands for advanced electrical power systems. Herein, nickel (Ni) particles were encapsulated by silica (SiO2) via a sol–gel process using sodium silicate as a precursor, and the obtained core–shell Ni@SiO2 powders were blended into poly(vinylidene fluoride) (PVDF) to investigate the effects of SiO2 insulating layer and its thickness on dielectric properties and TC of composites. Compared with pristine Ni, the Ni@SiO2/PVDF composites exhibit a superior εr, and remarkably suppressed loss and conductivity, attributable to the SiO2 interlayer between the core Ni particles which effectively prevents them from direct contacts and significantly reduces the leakage loss. Moreover, the Ni@SiO2/PVDF composites still possess a high TC owing to the restrained thermal interfacial resistance and enhanced interfacial compatibility between the fillers and the matrix. The developed Ni@SiO2/PVDF composites with high k and TC but low loss are potential for microelectronic industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dielectric materials with excellent dielectric performances, thermal conductivity and mechanical properties have caught extensive attention in recent years owing to their ever-increasing demand in microelectronics, electric accessories, high power microwaves, kinetic weapons [1,2,3,4], etc. Among the common dielectric materials, traditional inorganic ferroelectric ceramic materials, such as BaTiO3, CCTO, PZT have high dielectric constant (εr) and high thermal stability, but they are inherently brittle and processed at very high temperature [5]. While polymers possess excellent physical properties including easy processability, high dielectric breakdown field (Eb), low dielectric loss and lower processing cost, but they often suffer from rather low dielectric constants (< 10) and thermal conductivity (TC, ~ 0.2 W/m K) [6, 7]. Therefore, a single dielectric material can no longer meet the current development needs of power electronic equipment [8]. In order to address this issue, polymer-based composite dielectrics have been introduced by taking polymer as matrix and filling ceramic or conductive particles as fillers [9,10,11,12]. Some critical properties of composites including high εr, low loss, high Eb and good TC are highly desired [13,14,15,16].
For ceramic/polymer composites, high loading (> 50 vol.%) ceramic fillers are usually needed to achieve a desirable εr, which inevitably deteriorate the mechanical flexibility and processing properties [17]. Unfortunately, ceramic/polymer composites still exhibit a low εr with value between 50 and 100 [2]. What calls for special attention is that incorporating a small quantity of ceramic fillers with giant εr (> 104) into polymer matrix could significantly increase the εr of composites, but the agglomeration of inorganic particles is hard to avoid, which will increase dielectric loss and reduce Eb [18, 19]. For conductive particles/polymer composites, a very high εr occurs when the loading of conductive fillers reaches near to the percolation threshold (fc) [20]. However, the materials are inevitably accompanied by a sharp increase in dielectric loss due to the leakage current generated by a plenty of conductive networks when the conductive fillers loading approaches and exceeds the fc [21, 22]. Obviously, it is really a considerable challenge for percolation composition to preciously control the filler loading. Hence, the key issue faced by the two kinds of polymer-based composites is to synergistically obtain high εr and low dielectric loss.
Recently, core–shell structured particles have been explored to effectively deal with the current problems faced by polymer dielectrics [23,24,25]. A variety of insulator external shells, such as TiO2, Al2O3, SiO2, and polymers were introduced to reduce the dielectric loss between the conductive fillers/polymer composites, in that they not only serve as a barrier layer preventing adjacent metal cores from direct connects with each other, but also improve the interface compatibility between core and matrix [26,27,28,29]. Among them, silica (SiO2) is expected to be an excellent candidate for a surfaced shell of fillers due to its easy-processing, facile modification, and stable chemical properties [29]. For example, Ag nanoparticles with SiO2 coating layer effectively reduced the leakage loss caused by high electric field via blocking the aggregation of nanoparticles and reducing leakage current between the core shells [30]. In another case, an insulating SiO2 interlayer was created on the surfaces of flaky Cu by a sol–gel method, and realized very lower loss tangent ( tan δ) in the f-Cu@SiO2/PVDF composite by the virtue of SiO2 shell’ effective suppression on mobility of charge carriers [31].
Nickel (Ni) is a promising conductive filler for polymer-based composites that has drawn wide attention in the applications of batteries, catalyst, magnet sensors, electricity because of its specific electrical, optical, and magnetic properties compared with other conductive fillers, such as Au, Sn, Ag [21, 32,33,34]. However, it has been widely recognized that Ni/polymer possesses a high loss near fc as a result of the leakage current [21, 34]. According to previous studies, although the coated layer of NiO outside of Ni particles was effective in suppressing the dielectric loss, it didn’t ensure the stable existence of the NiO shell [21]. In the current studies, Zhou et al. firstly prepared Ni nanowires (NW) by solution casting method, and then doped it to P(VDF-HFP) with different orientations by magnetic field induction method. An improved dielectric constant (εr = 41 at the 1 kHz) was obtained at teeny filling amount (1.3 vol.%) when Ni NWs is paralleled to the direction of applied electric field as compared to that in the unaligned direction (εr ≈ 15 at the 1 kHz). Meanwhile, the tan δ of Ni NWs/P(VDF-HFP) nanocomposites was lower than 0.1 at the 1 kHz [35]. Zhu et al. synthesized Ni@BaTiO3/epoxy composites, manifesting a remarkable rise of εr from 2855 to 6397 at 10 kHz as the content of the BaTiO3 particles increased from 1.2 to 4 vol.%, and low tan δ ≈ 0.04 at 10 kHz [1].
In this work, we aim to construct a highly insulating silica shell on the surface Ni to suppress the loss and conductivity. So, we first successfully fabricated a SiO2 layer onto the Ni particles by sol–gel process, and then the obtained Ni@SiO2 core–shell particles were added into PVDF (poly(vinylidence fluorider)). The effects of filler content and SiO2 thickness on the dielectric properties and TC of Ni@SiO2/PVDF composite films were analyzed and discussed deeply. And the results will provide a deep insight into the design and preparation of polymer composites with a high εr but low loss, as well as enhance TC.
2 Experimental
2.1 Materials
PVDF FR903 was supplied by 3F New Materials Co. (Shanghai, China) and served as the polymer matrix. Sphere nickel powders (average diameter 1–2 µm) were provided by Shenyang Hangda Materials Co. Sodium silicate solution (Na2SiO3, composition in wt%) was purchased from Tianjin Baishi Chemical Co. ltd, China. N, N-Dimethylformamide (DMF), and others were obtained from Tianjin Chemical Reagent Co. China.
2.2 Preparations
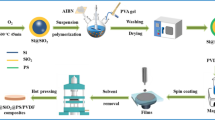
Figure 1 shows the schematic experimental procedures for the fabrication of Ni@SiO2 powders and Ni@SiO2/PVDF composites, which is depicted as follows. First, 50 g Ni was dispersed in 200 mL deionized water under rapid stirring to form a homogeneous solution. Then, the solution of nickel powder was moved to a thermostat water bath at a constant temperature of 90 oC. Subsequently, the pH of the mixture was adjusted to 9 by sodium hydroxide solution in the case of rapid stirring. Next, different amounts of Na2SiO3 were added into the above solution at 80 °C for 5 h. After the accretions of Na2SiO3, the as-obtained products were collected by centrifugation and washed with absolute ethanol at least three times. Finally, Ni@SiO2 hybrids were obtained by drying at 70 °C for 5 h under oven. The resultant samples are named as Ni@SiO2-1, Ni@SiO2-2, Ni@SiO2-3, Ni@SiO2-4, respectively. Here, the Ni@SiO2-1, Ni@SiO2-2 and others mean the weight ratio of the Na2SiO3 to Ni is 5%, 10%, 15% and 20%, respectively.
The Ni@SiO2/PVDF composite was fabricated by solution blending and hot-pressing. Firstly, PVDF was dissolved into the DMF solvents. Then different amounts of Ni@SiO2 were dispersed into above solution, which was continuously dispersed by sonication at 60 °C until forming homogenous slurry. Next, the obtained mixture was treated 6 h of agitation and followed by evaporating the majority of DMF at 120 °C. Subsequently, the resulting composites were heated at 110–120 °C in a vacuum oven for the sake of removing the residual solvent. In the end, it was compression-molded at 200 °C for 15 min under a pressure of about 10 MPa.
2.3 Characterizations
The surface chemistry of the samples was qualitatively measured with a Fourier transform infrared (FT-IR) spectrometer (Perkin-Elmer, Paragon1000) over a spectrum range of 4000/cm to 400/cm. The weight loss of Ni@SiO2/PVDF composites upon heating were measured by a thermo gravimetric analyzer (TGA, SDTA851, Swiss). Under the condition of air flow rate of 40 mL/s, the test temperature range is 200 °C to 800 °C, and the heating rate is 10 °C/min.
The phase structures of raw Ni and the Ni@SiO2 particles were identified with a Shimadzu X-ray diffractometer-6000 (XRD) equipped with a graphite homochromatic instrument and a Cu anticathode (40 kV, 30 mA, scanning rate 2°/min). The microstructure of the core–shell structured Ni@SiO2 particles was performed on the H-800 transmission electron microscope (TEM) from Hitachi Co (Japan). A JEOL JSM-7000F scanning electron microscope (SEM) was employed to observe the fracture morphology of samples. The composites need to be fractured in liquid nitrogen and the fracture surface is made by sputtering gold in vacuum prior to observation.
Dielectric properties of samples were carried out in the frequency range of 40 Hz to 10 MHz at room temperature by an Agilent 4294A impedance analyzer. The thermal conductivity of the composites was performed using a Hot Disk thermal analyzer (TPS 2500S, Sweden).
3 Results and discussion
3.1 Characterizations of core–shell Ni@SiO2
Figure 2a displays the FT-IR spectra of pure Ni and Ni@SiO2 particles, separately. The broad absorption peak appears at about 3450 cm−1 cm and 1610 cm−1, respectively, corresponding to the characteristic peaks for the stretching vibration of hydroxyl groups (–OH) from the H2O absorbed in the Ni and Ni@SiO2 filler particles. As for Ni@SiO2, the corresponding absorption peak intensity of pure Ni decreased, but new peak at 782 cm−1 was observed, which could be attributed to the symmetric stretching vibration of the Si–O–Si. In addition, the obviously enhanced signal at 1400 cm−1 for Ni@SiO2 is assigned to stretching vibration of the Si–OH group compared with pure Ni. The above results confirm the wrapping of SiO2 on the surfaces of Ni particles.
Figure 2b presents the XRD patterns of pure Ni and Ni@SiO2 particles. Both of the XRD patterns exhibit three well-defined diffraction peaks at 2θ values of 44.4°, 51.7° and 76.3°, corresponding to the (111), (200) and (220) lattice planes, separately. The results are consistent with the spectrum of Ni. In comparison with the pure Ni, no new peak visible pertaining to SiO2 is observed in the XRD patterns of Ni@SiO2 specimens, indicating the morphology of SiO2 layer on the surface of Ni is almost completely amorphous.
Figure 2c compares the TGA curves of pure Ni and Ni@SiO2 samples at air atmosphere. For the pure Ni, no obvious mass variant is observed in the temperature range of 200–450 °C. Then, the weight increase starts at 450 °C and becomes constant from 750 °C, which is attributed to the formation of its own oxides. In contrast, the Ni surface reacts with O2 when the temperature is close to 500 °C for Ni@SiO2 samples, and the peak of mass increase is significantly lower than that of pure Ni, thereby suggesting that the encapsulated Ni possesses good oxidation-proof capability, owing to the coating of SiO2 layer on the surface of core Ni particles. Therefore, TGA curves further demonstrate the existence of SiO2 and the important effect of SiO2 on the dielectric and thermal properties of Ni/PVDF composites.
3.2 Microstructures
Figure 3 shows the SEM images of pure Ni, Ni@SiO2 and their PVDF-based composites at various filler loadings. From Fig. 3a, b, it can be observed that the diameter of the pure Ni particles is about 1–2 µm, and that the particle surface is smooth. While the Ni@SiO2 particles become larger in diameter and rougher in surface as the content of SiO2 coating increases, which mirrors a variation in the thickness of SiO2 shell. Moreover, there is a slight aggregation for the pure Ni particles in comparison with Ni@SiO2 particles due to its own strong magnetism. The insets in Fig. 3a and b represent the TEM images for pure Ni and Ni@SiO2 particles, respectively. It is clearly observed that for the Ni@SiO2 there is an amorphous shell layer of SiO2 on the surface of Ni, acting as an insulator barrier layer between Ni particles. However, no clear external shell can be noticed for the pure Ni from the inset in Fig. 3a. From Fig. 3c–f, both fillers are uniformly dispersed in the polymer matrix without serious aggregation. What is noteworthy is that the coating of SiO2 on the surface of Ni would enhance the phase interfacial interactions of Ni to PVDF compared with the pure Ni particles.
3.3 Dielectric properties
The dielectric permittivity (εr) of Ni/PVDF and Ni@SiO2/PVDF composites with different filler contents were tested from 40 to 107 Hz at room temperature and depicted in Fig. 4. From Fig. 4a, c–f, when the filler loading is lower than 40 wt%, the dielectric constant of all samples shows a weak frequency dependence and approaches a certain constant value in the frequency range of 40 Hz to 105 Hz, suggesting that the dipoles possess enough time to orient with the alternation of frequency [36, 37]. Beyond 40 wt%, the dielectric constant exhibits a strong frequency dependence and keeps on reducing in the whole frequency range, indicating that the lagging effects of polarization with the external electrical field and the occurrence of relaxation of dipole moments in PVDF [19, 21, 38, 39]. Moreover, it is observed that the εr almost increases with increasing the filler loading in PVDF for all the two systems, and the εr of Ni@SiO2/PVDF is visibly lower than that of pure Ni. For example, at 30 wt%, the εr of Ni@SiO2/PVDF is about 117 (Ni@SiO2-1), 50 (Ni@SiO2-2), 34.4 (Ni@SiO2-3) and 25.6 (Ni@SiO2-4) at 100 Hz, respectively, whereas the one of Ni/PVDF with the same Ni loading is 135.3.
Frequency-dependent changes of dielectric permittivity of PVDF composites loaded with different mass fractions of a pure Ni, c–f Ni@SiO2. b, g Dielectric permittivity dependence on filler loading for the Ni/PVDF and Ni@SiO2/PVDF composites (Ni@SiO2-1) at 100 Hz, and the inset is the logarithmic bit of the permittivity by Eq. (1), respectively. h Dielectric permittivity of PVDF filled with pure Ni and different types of Ni@SiO2 under the same filler loading of 30 wt%
The εr of the Ni/PVDF increases relative to pure PVDF due to the interfacial polarization (IP) effect and micro capacitance [1, 12, 21]. On the one hand, the metallic Ni exhibits a quite high conductivity in comparison to the PVDF matrix, and the great difference in conductivity implies strong IPs effect, which leads to the enhanced εr of polymer composite [1, 40]. On the other hand, when an electric field is applied, an adjacent pair of Ni will form a micro capacitor. At first, by blending a small amount of Ni particles into the PVDF matrix, no micro capacitors are formed due to the distance between Ni particles in the polymer matrix [41]. The capacitance of each micro capacitor is rather low and the number of micro capacitors is inadequate, thus resulting in a slight increase in εr. As the pristine Ni loading increases, the formation of a large number of micro capacitors brings about a sharp increase in εr. Meanwhile, the agglomerations of Ni particles and the resultant defects at the interfaces within the composites also increases, these make against to form micro capacitors [21, 31, 39]. For instance, the εr of Ni/PVDF shows an abnormal behavior after the introduction of 50 wt% Ni particles as shown in Fig. 4a.
Compared with the Ni/PVDF series, the Ni@SiO2/PVDF exhibit visibly lower εr, the reasons for which are as follows: First, the insulation of SiO2 incorporated into the conductive Ni surface effectively decreases the formation of traps, resulting in a decline in space charge polarization [42]. Second, the introduction of amorphous SiO2 constrains and suppresses the contribution of IP in Ni@SiO2 particles and Ni@SiO2/PVDF composites. To further explore the effect of the SiO2 shell on the dielectric behavior of Ni@SiO2/PVDF composites, the εr of PVDF filled with pure Ni and different types of Ni@SiO2 under the same filler loading of 30 wt% is provided in Fig. 4h. Obviously, it can also be seen from Fig. 4h that the higher mass fraction ratio of Na2SiO3 to Ni, the lower the dielectric permittivity of the composite, and mass fraction ratio of Na2SiO3 to Ni at 5% and 10% is suitable to obtain relatively high εr [31]. We know that the Na2SiO3 content is positively related to the thickness of the SiO2 interlayer, leading to increase of the distance between the neighboring Ni cores and reduction of micro capacitors [1, 37]. So, a decrease trend can be found in the εr of Ni@SiO2/PVDF composites with increased mass fraction ratio of Na2SiO3 to Ni.
It is worth noting that Ni/PVDF and Ni@SiO2/PVDF composite vary enormously in magnitude at 30 wt% and 50 wt%. This huge variation of the εr near the fc is described by the power law in Eq. (1):
where εm is the εr of the matrix and s is a critical exponent of about 1 [1, 12, 20]. The log-log curves of ε and f are plotted in the insets of Fig. 4b, g and the best linear fitting of the εr shows that the fc values of Ni/PVDF and Ni@SiO2/PVDF composites are 7.89 vol.% and 11.7 vol.%, respectively, while s values are about 0.61 and 0.71.
The frequency dependence (from 40 to 107 Hz) of tan δ of the Ni/PVDF and Ni@SiO2/PVDF composites with different filler contents at room temperature are depicted in Fig. 5. Generally, the dielectric loss is counted as a measure of energy loss from the reorientation of the dipoles in the composites [31]. At low frequency, the decreasing trend and the minimum of tan δ are observed for all samples, which are caused by electric leakage and IP. Electric leakage is dominant for the energy loss of conductive particles/polymer composites, and the IP is a typical behavior of low frequency dielectric response, resulting from the existence of macro heterogeneity interface in different diphase structures [21, 31]. Except for Ni/PVDF, the tan δ for all the Ni@SiO2/PVDF composites increases sharply to a peak at frequencies higher than 105 Hz. The main reason is that a Debye relaxation behavior produced due to the C–F dipole orientation polarizations from the PVDF matrix [1, 8, 31].
From Fig. 5a, the variation in tan δ for the Ni/PVDF composites basically has no dependency on the Ni concentration at an f < fc where the neighbor fillers are distant from each other [1, 39]. While it is found that a significant enhancement of tan δ is observed when the Ni content is near or above fc for the Ni/PVDF composites. Correspondingly, the Ni/PVDF composite with Ni content of 50 wt% shows much higher tan δ = 2350 at 100 Hz compared with the same composite with Ni content of 20 wt% (tan δ = 0.04, at 100 Hz). This phenomenon could attribute to the agglomeration and direct contact of high levels of Ni in the PVDF substation, thus leading to the accumulation of charge carriers at the filler-matrix interface and electron conduction process [39]. Further, the dielectric loss values decrease over the entire frequency range in the same filler loading range, which is put down to the inability of the low mobility charges to keep pace with the external electric filed with increasing frequency, bringing about a lack of electronic oscillations [32]. Consequently, the loss trend of Ni/PVDF composite shows a remarkable different frequency dependence behavior compared with the Ni@SiO2/PVDF composites.
The loss trend curves of Ni@SiO2/PVDF with different types of Ni@SiO2 are indicated in Fig. 5f. In summary, all the four composites samples show similar variation trend. In one respect, no appreciable variation in the dielectric loss values with increasing filler loading at the entire frequency range was observed until the filler loading exceeds 40 wt%. In other respect, all of them exhibit much lower dielectric loss than those of Ni/PVDF at low frequency (< 105 Hz). At the same filler loading (30 wt%), the tan δ at 100 Hz for the Ni@SiO2/PVDF (Ni@SiO2-4) and Ni/PVDF are 0.05 and 2.24, respectively. Moreover, the loss factor increases significantly within the frequency range of 105 to 107 Hz. The main mechanisms for variation in tan δ of the Ni@SiO2/PVDF composites can be given from the following three aspects. First, the dielectric loss is almost independent of filler loading (< 40 wt%), indicating that the existence of SiO2 between Ni particles makes them isolated from each other, resulting in nonoccurrence of percolation behavior even at an f \(\ge\) fc [12, 21]. Second, when different amounts of Na2SiO3 added into the solution, Ni particles would be covered by a layer of insulating SiO2 shell with different thicknesses, which plays a crucial role in restraining the migration of free charge carriers between conductive Ni cores and reducing the leakage current to a certain extent [1, 21]. But, for the pure Ni particles, the SEM images have proved that the Ni@SiO2 in PVDF matrix present better dispersity compared with the pristine Ni particles. Therefore, the poor dispersity accelerates the formation of the internal conductive network path in Ni/PVDF composites, causing a violent electron conduction process [1, 39]. Third, the effect of adding Ni@SiO2 particles on tan δ is negligible at high frequencies on account of high frequency relaxation of the dipoles [1, 12, 31].
By observing Fig. 5a, higher coating amount of SiO2 is conducive to higher suppression degree of conduction loss. Generally, the following equation is used to describe the dielectric loss (ε″) of a composite:
where \({\varepsilon }_{\text{dc}}^{\prime\prime}\) and \({\varepsilon }_{\text{MW}}^{\prime\prime}\) stand for conduction and IP, respectively, and \({\varepsilon }_{\text{D}}^{\prime\prime}\) relates with the dipole loss factor [39].
The conduction loss factor is determined as follows:
where \({\sigma }_{\text{dc}}\) and f represent the direct current (DC) conductivity and frequency, respectively [39].
According to Eqs. (2) and (3) and experimental results, we could infer that conduction loss make a tiny contribution to the total loss for the Ni@SiO2/PVDF. Hence, comparison of Ni/PVDF and Ni@SiO2/PVDF systems indicates that the DC conductivity is significantly suppressed by SiO2 shell and the conduction loss is dominant factor producing a large dielectric loss at the low frequencies [1, 39].
The alternating current (AC) conductivity as a function of frequency of the Ni/PVDF and Ni@SiO2/PVDF composites with various Ni and Ni@SiO2 mass fractions are presented in Fig. 6. Clearly, the AC conductivities of all composites increase linearly with frequency. Typically, AC conductivity is directly proportion to frequency [31, 43]. The results of our experiment are consistent with this theory. However, the dependence of AC conductivity is different for the Ni/PVDF composites with various filler loading. It is considered that electron tunneling or direct contact between conductive particles is the main mode of electrical transmission of polymer-based composites [10, 29, 44]. When the Ni content is less than the fc (7.89 vol.%), the slight increase in AC conductivities with increasing Ni loading is associated with the occurrence of a small amount of electron tunneling, which could be visually reflected by Fig. 6g. Once the fNi \(\to\) fc, conductivity values undergo a sharp increase in the low frequencies, and a DC conduction independent of frequency appears in the system, which means that the Ni particles start to come into contact with each other and form conductive networks in the PVDF matrix, leading to the transition from insulator to conductor and a large a large dielectric loss [1, 21, 31]. Beyond fc the variation in conductivity can be ignored because the polymer was endowed with a conductive nature.
Electric conductivity dependence on frequency and filler loading for PVDF filled with: a pure Ni, b–e Ni@SiO2. f Electric conductivity vs. filler loading at 100 Hz for pure Ni and Ni@SiO2 with various fillers. g–h Schematic representation of the formation of a conductive network for Ni/PVDF and Ni@SiO2/PVDF [32]
As presented in Fig. 6b–e, all AC conductivities of Ni@SiO2/PVDF composite exhibit a slight rise with increasing filler concentration, and appear almost linear growth with frequency, suggesting the insulating nature of the Ni@SiO2/PVDF composites on account of the presence of insulation of SiO2, which plays a decisive role in restraining the formation of conductive networks by means of the isolation of adjacent Ni particles. The filler distribution of Ni@SiO2 particles in PVDF can be visually illustrated in Fig. 6h. As a result, no distinct percolation is found in the Ni@SiO2/PVDF composites. Under the circumstances, the interaction between Ni particles makes little contribution to electrical transmission of composites [1, 30, 32]. While the electron tunneling between adjacent Ni particles is dominant for electrons transport [21]. Furthermore, as shown in Fig. 6f, a common feature that can be observed in four composites is that a piecemeal decline in AC conductivity under the same filler content. It is attributed to the increased proportion of SiO2, resulting in a gradual decrease in the path of electron conduction and the amount of free charge [45].
Based on the analysis above, the improvement in the dielectric properties in Ni@SiO2/PVDF composites is attributed to the core–shell Ni@SiO2. The coating of SiO2 shell significantly reduces the AC conductivity and dielectric loss of the system. Combined with the results of dielectric properties, it can be found that mass fraction ratio of Na2SiO3 to Ni at 10% is conducive to lower the dielectric loss and AC conductivity of Ni@SiO2/PVDF composites while still keeping a fairly high εr [34].
3.4 Thermal conductivity
To further investigate the effect of the SiO2 shell on the thermal conductivity (TC) of composites. Figure 7 compares the TC of PVDF filled with pure Ni and different types of Ni@SiO2 particles under different filler concentration. Obviously, all the samples exhibit a gradual growing trend in TC with filler loading. A relatively low TC of pristine PVDF can be enhanced through a blend of Ni particles with a high TC (~ 90 W/m K) and the PVDF matrix [21]. Generally speaking, there exists a relationship between TC and microstructure of composites, which is determined by the filler loading [46]. According to the amount of Ni particles, the increase in TC can be analyzed in two processes. First, when increasing the Ni filler loading from 10 to 30 wt%, the TC of the Ni/PVDF composites is slowly increased owing to a weak interaction among Ni particles at a lower filler loading, which produces a high interface thermal resistance between the Ni particles and PVDF matrix, and thus results in some obstacles to phonon propagation [1, 31]. Subsequently, when the Ni loading increases from 30 to 50 wt%, a markedly increasing TC trend is observed. In this case, Ni particles are easy to form partial connection or networks, thus suppressing and inhibiting the interfacial thermal resistance significantly and creating paths for phonons transmission [1, 47]. Therefore, the TC of the composites exhibits a rapid enhancement. For instance, the 40 wt% Ni/PVDF displays a TC of 0.58 W/m K, which is nearly threefold the TC of a pristine PVDF.
It is found that the SiO2 layer’ thickness has an influence on TC of composites through altering the interface structure of polymer composites. Compared with Ni/PVDF, a slightly higher TC is observable from some types of Ni@SiO2/PVDF composites at the same filler concentration, while a slightly reduced TC is also found in other types of systems. For multi-component polymer composites, thermal pathways not only depend on the connection and distribution of the fillers, but also depend on the number of defects and voids at different phase interface boundary zones [9, 46, 47]. If there are fewer defects and voids at the phase interface, the thermal interface contact resistance will be very small, which is conducive to the phonons transfer. In this study, the SiO2 shell outside of core Ni has some hydroxyl groups (–OH), which can react with the F atoms in PVDF to form hydrogen bond action and enhance the interfacial adhesion and compatibility between the Ni particles and the PVDF matrix, thereby leading to the reduction of interfacial defects [46, 48]. Thus, the TC of Ni@SiO2/PVDF can be improved thanks to the suppression of the interface thermal resistance. Moreover, the SiO2 has much higher intrinsic TC than that of air at the interfacial area. The presence of SiO2 at the Ni@SiO2/PVDF interface replaces the corresponding air and voids as comparison with the Ni/PVDF. Therefore, the reduced interface phonon mismatch and phonon scattering promote the transfer of phonons across the entire interface area [1].
However, with further increasing the thickness of interlayer shell the TC is seen to slightly decrease. The reason could be ascribed to the fact that the thicker interlayer constrains the heat conduction capacity of highly thermal conductive Ni owing its rather low TC, subsequently resulting in higher thermal interface resistance and leading to a suppressed TC of composites [49]. Only at a moderate interlayer thickness, TC can be enhanced thanks to the formation of good interlayer promoting phonon transition across the interface boundary. The highest TC is achieved in the PVDF with Ni@SiO2-2 for all composites with various filler loading. For example, at a fixed filler loading of 50 wt%, the TC of the four Ni@SiO2/PVDF composites is measured as 0.77 (Ni@SiO2-1), 0.85 (Ni@SiO2-2), 0.73 (Ni@SiO2-3) and 0.70 (Ni@SiO2-4), respectively, as comparison with 0.75 for pristine Ni/PVDF. The results indicate that the interlayer thickness of the transition shell should be carefully tuned to achieve an enhanced TC of composites.
4 Conclusions
In summary, core–shell structured Ni@SiO2 particles were synthesized by a sol–gel process using sodium silicate as a precursor, and the Ni@SiO2/PVDF were prepared. The results from FT-IR, TGA and XRD confirm the formation of a layer of SiO2 shell outside of Ni particles. Compared with the Ni/PVDF composites, the εr of Ni@SiO2/PVDF composites decreases at the same filler loading due to the suppressed contribution from IP, and the tan δ is significantly restrained to very low level due to the insulating SiO2 layer. The SiO2 shell between the adjacent Ni particles effectively prevents them from direct contacts with each other, and remarkably reduces the leakage current, thereby leading to the distinct reduction in both dielectric loss and conductivity. Moreover, with further increasing the interlayer thickness the suppression on the loss and conductivity of all composites is much prominent. In addition, a slightly higher TC is observed in the Ni@SiO2/PVDF composites compared with Ni/PVDF because the SiO2 interlayer could improve the interfacial compatibility and restrain the interfacial thermal resistance, thus promoting the phonon transfer across the composites. The SiO2 thickness is found to exert an influence on the TC of composites. Therefore, results demonstrate that the thickness of SiO2 shell should be logically regulated so as to have an optimal effect on dielectric properties and TC. The prepared Ni@SiO2/PVDF composite with high-εr, low loss and enhanced TC exhibit potential application in microelectronic and electrical industries.
References
X.T. Zhu, J. Yang, D. Dastan, H. Garmestani, R.H. Fan, Z.C. Shi, Compos. A Appl. Sci. Manuf. 125, 105521 (2019)
J.W. Zha, S.C. Yao, Y. Qiu, M.S. Zheng, Z.M. Dang, IET Nanodielectr. 2(3), 103–108 (2019)
L.H. Zhao, L. Yan, C.M. Wei, Q.H. Li, X.L. Huang, Z.L. Wang, M.L. Fu, J.W. Ren, J. Phys. Chem. C 124, 12723–12733 (2020)
P. Wang, W.D. Wei, Z.Q. Li, W. Duan, H.L. Hana, Q. Xie, J. Mater. Chem. A 8(6), 3509–3516 (2020)
J.C. Ma, U. Azhar, C.Y. Zong, Y.B. Zhang, A.H. Xu, C.C. Zhai, L.Q. Zhang, S.X. Zhang, Mater. Des. 164, 107556 (2019)
Z.D. Wang, Y.H. Cheng, M.M. Yang, J.L. Huang, D.X. Cao, S.Y. Chen, Q. Xie, W.X. Lou, H.J. Wu, Compos. B Eng. . 140, 83–90 (2018)
M.S. Zheng, C. Zhang, Y. Yang, Z.L. Xing, X. Chen, S.L. Zhong, Z.M. Dang, IET Nanodielectr. 3(3), 94–98 (2020)
W.Y. Zhou, F. Zhang, M.X. Yuan, B. Li, J.D. Peng, Y.Q. Lv, H.W. Cai, X.R. Liu, Q.G. Chen, Z.M. Dang, Mater. Electron. 30(20), 18350–18361 (2019)
Z.H. Yao, Z. Song, H. Hao, Z.Y. Yu, M.H. Cao, S.J. Zhang, M.T. Lanagan, H.X. Liu, Adv. Mater. 29(20), 1601727 (2017)
V.K. Prateek, R.K. Thakur, Gupta, Chem. Rev. 116(7), 4260–4317 (2016)
D.L. He, Y. Wang, S.L. Song, S. Liu, Y. Deng, ACS Appl. Mater. Interfaces 9(51), 44839–44846 (2017)
Z.M. Dang, J.K. Yuan, J.W. Zha, T. Zhou, S.T. Li, G.H. Hu, Progress Mater. Sci. 57(4), 660–723 (2012)
Y.C. Jiang, J.B. Wang, Q.L. Zhang, H. Yang, D. Shen, F.M. Zhou, Colloids Surf. A 576, 55–62 (2019)
S.L. Zhong, Z.M. Dang, W.Y. Zhou, H.W. Cai, IET Nanodielectr. 1(1), 41–47 (2018)
M.X. Yuan, G. Zhang, B. Li, T.C. Mike Chung, R. Rajagopalan, M.T. Lanagan, ACS Appl. Mater. Interfaces 12(12), 14154–14164 (2020)
M.X. Yuan, B. Li, S.H. Zhang, R. Rajagopalan, M.T. Lanagan, ACS Appl. Polym. Mater. 2(3), 1356–1368 (2020)
Y.C. Jiang, Z. Zhang, Z. Zhou, H. Yang, Q.L. Zhang, Polymers 11, 1541 (2019)
T. Hu, J. Juuti, H.L. Jantunen, T. Vilkman, J. Eur. Ceram. Soc. 27(13–15), 3997–4001 (2007)
K. Meeporn, P. Thongbai, Appl. Surf. Sci. 481, 1160–1166 (2019)
Q.P. Zhang, W.F. Zhu, D.M. Liang, X.L. Wu, R.C. Chen, N. Sun, Y.T. Li, Y.L. Zhou, Appl. Surf. Sci. 487, 77–81 (2019)
W.Y. Zhou, Y. Gong, L.T. Tu, L. Xu, W. Zhao, J.T. Cai, Y.T. Zhang, A.N. Zhou, J. Alloys Compd. 693, 1–8 (2017)
T. Huang, C.G. Ma, P.B. Dai, J. Zhang, Compos. Sci. Technol. 176, 46–53 (2019)
G.L. Wu, Y.H. Cheng, Y.Y. Ren, Y.Q. Wang, Z.D. Wang, H.J. Wu, J. Alloys Compd. 652, 346–350 (2015)
A.S. Zeraati, M. ArjmandOrcid, U. Sundararaj, ACS Appl. Mater. Interfaces 9(16), 14328–14336 (2017)
S.H. Liu, J. Wang, H.S. Hao, L.M. Zhao, J.W. Zhai, Ceram. Int. 44, 22850–22855 (2018)
F. Liang, L. Zhang, W.Z. Lu, Q.X. Wan, G.F. Fan, Appl. Phys. Lett. 108, 072902 (2016)
G.F. Liu, Y. Chen, M.J. Gong, X.Y. Liu, Z.K. Cui, Q.B. Pei, J.L. Gu, C. Huang, Q.X. Zhuang, J. Mater. Chem. C 6, 10829–10837 (2018)
L. Hu, Y.T. Xia, Q.Q. Wang, H.Y. Yang, Q.L. Zhang, J. Mater. Sci. Mater. Electron. 29, 1269–1279 (2017)
S. Kargar, D. Elhamifar, A. Zarnegaryan, J. Phys. Chem. Solids. 146, 109601 (2020)
L. Weng, X.M. Wang, X.R. Zhang, L.Z. Guan, L.Z. Liu, H.X. Zhang, W.W. Cui, Polym. Compos. 41(6), 2245–2253 (2020)
W.Y. Zhou, L. Xu, L.Y. Jiang, J.D. Peng, Y. Gong, X.R. Liu, H.W. Cai, G.H. Wang, Q.G. Chen, J. Alloys Compd. 710, 47–56 (2017)
D.E. Abulyazied, H.M. Abomostafa, G.M. El komy. J. Inorg. Organometall. Polym. Mater. 30, 2335–2346 (2020)
M. Panda, V. Srinivasa, A.K. Thakur, Appl. Phys. Lett. 92(13), 3804 (2008)
L. Xu, W.Y. Zhou, B. Li, Y.J. Kou, H.W. Cai, F.X. Chen, G.H. Wang, D.F. Liu, Z.M. Dang, J. Elastomers Plast. 52(4), 304–321 (2020)
Y. Zhou, S. Chen, D. Wu, L.H. Liu, H. L, D. Zhang, Compos. Commun. 16, 11–19 (2019)
Y. Tong, H. Talebinezhad, X. Lu, Z.Y. Cheng, C. Hill, D. Tucker, IET Nanodielectr. 2(1), 41–47 (2019)
V.P. Anju, S.K. Narayanankutty, Mater. Sci. Eng. B 249, 114418 (2019)
Z.H. Chen, H.F. Li, G.Y. Xie, K. Yang, RSC Adv. 8(1), 1–9 (2018)
W.Y. Zhou, X. Li, F. Zhang, C.H. Zhang, Z. Li, F.X. Chen, H.W. Cai, X.R. Liu, Q.G. Chen, Z.M. Dang, Compos. A Appl. Sci. Manuf. 137, 106021 (2020)
W.Y. Zhou, Q.G. Chen, X.Z. Sui, L.N. Dong, Z.J. Wang, Compos. A Appl. Sci. Manuf. 71, 184–191 (2015)
S.A. Mirkhani, A.S. Zeraati, E. Aliabadian, M. Naguib, U. Sundararaj, ACS Appl. Mater. Interfaces 11(20), 18599–18608 (2019)
Z.B. Pan, L.M. Yao, J.J. Liu, X.Y. Liu, F.P. Pi, J.W. Chen, B. Shen, J.W. Zhai, J. Mater. Chem. C 7(2), 405–413 (2019)
S.Y. Chen, Y.H. Cheng, Q. Xie, B. Xiao, Z.D. Wang, J.Y. Liu, Composites Part A 120, 84–94 (2019)
B. Li, F. Salcedo-GalanFelipe, P.I. Xidas, E. Manias, ACS Appl. Nano Mater. 1(9), 4401–4407 (2018)
Y. Zhang, C.H. Zhang, Y. Feng, T.D. Zhang, Q.G. Chen, Q.G. Chi, L.Z. Liu, X. Wang, Q.Q. Lei, Nano Energy 66, 104195 (2019)
W.Y. Zhou, Y. Zhang, J.J. Wang, H. Li, W.H. Xu, B. Li, L.Q. Chen, Q. Wang, ACS Appl. Mater. Interfaces 12, 46767–46778 (2020)
J.W. Ren, Q.H. Li, L. Yan, L.C. Jia, X.L. Huang, L.H. Zhao, Q.C. Ran, M.L. Fu, Mater. Des. 191, 108663 (2020)
L.H. Zhao, L. Yan, C.M. Wei, Q.H. Li, X.L. Huang, Z.L. Wang, M.L. Fu, J.W. Ren, J. Phys. Chem. C 124(23), 12723–12733 (2020)
Z.M. Shen, J.C. Feng, Compos. Sci. Technol. 170, 135–140 (2019)
Acknowledgements
The authors gratefully acknowledge the financial supports from the National Natural Science Foundation of China (Nos. 51937007, 51577154), and acknowledges the Analytic Instrumentation Center of XUST.
Funding
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, T., Zhou, W., Li, Y. et al. Synergy improvement of dielectric properties and thermal conductivity in PVDF composites with core‐shell structured Ni@SiO2. J Mater Sci: Mater Electron 32, 4076–4089 (2021). https://doi.org/10.1007/s10854-020-05149-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-05149-x