Abstract
An electroactive series of polyamides was synthesized from 4,4′-diamino-4′′-phenoxy triphenylamine (1), 4,4′-diamino-4′′-benzyloxy triphenylamine (2) and terephthaloyl or isophthaloyl chloride. The FTIR (Fourier Transform Infrared) spectral characterization and solubility test of the subsequent polyamides (PA I-IV), UV-vis, photoluminescence spectral analysis (PL) and cyclic voltammetry were utilized to assess photophysical and electrochemical properties of the materials. The oxidative-thermal stability of the polyamides as judged by thermogravimetric analysis was found to be in the range 438-532 °C with the char yield more than 53% at 800 °C. Introduction of aryloxy triphenylamine units along with polymer backbone flexibility improved the organosolubility of the synthesized polyamides (PA I-IV). The appreciable organosolubility of the (PA I-IV) is enough to be used in coating applications such as inkjet printing. Bluish green light emission from our synthesized materials upon excitation at 375 nm is credited to the triphenylamine linked amide-based polymer chain. Both pendent phenoxy and benzyloxy groups at para position in (PA I-IV) have also elevated the HOMO energy levels and hence lowered the onset oxidation potential. Additionally, the computational analysis was also conducted to get optimized ground-state geometry by DFT method (B3LYP) with 6-31G basis set. The 3-dimensional distributions of both HOMO (Highest Occupied Molecular Orbital) and LUMO (Lowest Unoccupied Molecular Orbital) of the polymers were obtained. The computational data of the polymers also augmented the experimental data suggesting their future use as redox-active materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triarylamine-based luminogens and aromatic polyamides (PA) have attracted considerable interest as thermally stable fluorescent materials for variety of organic light emitting applications [1]. The triphenylamine (TPA) consist of π electron rich character with propeller like spatial arrangement, is frequently reported as luminogens [2]. However, the dimerization of electrogenerated TPA cation radical via anodic oxidation to generate tetraphenyl benzidine is responsible for the loss of aromaticity in the system [3]. Afterwards, it has been reported that the dimerization of radical cation can be avoided by the use of p-blocked TPA, which is capable of forming stable radical cation, unable to react further, but is appropriate for various optoelectronic applications [4]. Furthermore, it has been observed that the electronic nature and bulkiness of the p-substituent affect its electrochemical properties [5, 6]. The electron donating groups (methoxy, naphthoxy) were previously reported to affect the oxidation potential of the materials and responsible for the elevation of the HOMO (highest occupied molecular orbital) energy levels [7, 8]. Therefore, feasibility of chemical modifications for improvement in emission efficiencies as well as adjustment of the energy levels makes TPA based PA attractive for the optoelectronic applications. However, the difficulty in processing of the wholly aromatic PA in common organic solvents, restricted its application as film forming and coating materials. The chemical modification was employed to improve organosolubility of light emitting polyamides [9,10,11].
Recently, aggregation-induced emission enhancement (AIEE) and aggregation-induced emission (AIE) based active molecules have attracted much attention of the researchers [12]. As AIE-active materials are widely applied in biomedical imaging, mechanochromic luminescence (MCL) and organic light emitting diiodes (OLEDs), their mechanisms have been proposed in various studies, showing that the restriction of intramolecular rotations by bulkiness of the group is one of the most important mechanism. The π-π interaction is responsible for the aggregation caused quenching (ACQ), which could be avoided by the use of bulky pendant on para position of TPA based polyamide chain [13].
Thus, in this contribution, we synthesized a series of p-aryloxy substituted polyamides from two TPA based diamines: 4,4′-diamino-4′′-phenoxy triphenylamine (1), 4,4′-diamino-4′′-benzyloxy triphenylamine (2). These TPA based diamines could not only preserve the favorable thermal stability due to high aromatic content in the system but also impart AIEE. The computational analysis of the dimers of TPA-PAs was carried out to correlate/augment/validate various parametres with experimental observations. Appreciable organosolubility, render these materials better processability which could be beneficial for spin coating and inkjet printing. Fluorescent, stable electroactive nature of TPA-PAs as evaluated from their PL spectral and cyclic voltammetry analysis render these materials useful for hole transport in multiple applications in future.
Experimental
Chemicals and reagents
Terephthaloyl chloride, isophthaloyl chloride, 4-fluoronitrobenzene, cesium fluoride, acetonitrile, 4-benzyloxy aniline and DMSO were purchased from Merck, Germany. The diamine monomer 4,4′-diamino-4′′-phenoxy triphenylamine (1) was prepared as reported in literature [14].
Methods and measurements
The elemental analyses were accomplished on the CHNS analyzer, Flash 2000 series and FTIR spectra were collected on Bruker α-Alpha-P model by ATR method. The Bruker spectrometer, working at 300 MHZ in DMSO-d6 was used to record NMR spectra. The computation and optimization of geometry ground-state and energy levels of HOMO and LUMO were obtained by DFT (density functional theory) using B3LYP method with 6-31G basis set using Guassian 03 software. The thermal stability of the polymers was evaluated on NETZSCH TGA analyzer, model TG209 F3 in an oxidative environment at rate of 10 °C/min. The Cary, 100 Conc UV-vis and PC1 photon counter spectrophotometers were used to collect UV-vis and PL spectra respectively. The BAS-100 B electrochemical analyzer comprising of carbon, platinum wire and Ag/AgCl as electrodes was used for CV experiments.
Synthesis of 4,4′-diamino-4′′-benzyloxy triphenylamine (3)
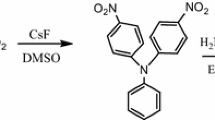
The synthesis of 4,4′-diamino-4′′-benzyloxy triphenylamine (3) was accomplished in two steps via cesium fluoride facilitated nucleophilic arylation to (2) and reduction to diamine (3) via palladium-charcoal catalysis. [8, 14]
Synthesis of 4,4′-dinitro-4′′-benzyloxy triphenylamine (2)
4,4′-Dinitro-4′′-benzyloxy triphenylamine (2) was prepared by cesium fluoride (1 mmole) mediated nucleophilic arylation of 4-benzyloxyaniline (0.5 mmole) by 4-fluoro-nitrobenzene (1 mmole) in 20 mL DMSO. The reaction mixture was heated at the 120 °C for 24 h, monitored by thin layer chromatography. The product was isolated by stirring a cold reaction mixture in methanol, filtered washed with plenty of water, dried, recrystallized from ethanol as an orange solid.
Yield: 69%. Rf = 0.68 (n-Hexane: EtOAc, 6:1), m.p. 217–219 °C; Anal. Calcd for C25H19N3O5 C, 68.02; H, 4.34; N, 9.52; observed C, 68.01; H, 4.32; N, 9.51 FTIR ῡ/cm−1: 1329 (C-N of TPA), 1229 (C-O-C) and 1489, 1569 (NO2). 1H-NMR (DMSO-d6) δ/ppm Benzyloxy Protons: 7.4 (5H, m, 4,6,7,8), 5.14 (2H, s, 4), Aromatic Protons: 8.18 (1, dt, J = 9, 3.3 Hz, 4H), 7.21 (2, dd, J = 5.1, 2.1, 4H),7.15 (3, d, J = 8.4, 2H) and 7.24 (4, dd, J = 6.6, 2.5, 2H).
Reduction of 2a into diamine 4,4′-diamino-4′′-benzyloxy triphenylamine (3)
The diamine 3 was prepared by reduction of 4.54 mmol 2 (2.0 g) using hydrazine monohydrate (7 mL) and catalyst 10% Pd/C (0.2 g) in 50 mL ethanol, following a well-known procedure and purified by recrystallization as slightly grey solid. Yield: 74%. Rf = 0.41 (n-Hexane: EtOAc,1:1) m.p. 196 °C. Anal. Calcd for C25H23N3O C, 78.71; H, 6.08; N, 11.02; observed C, 78.68; H, 6.05; N, 11.00. FTIR ῡ/cm−1: 3459, 3339 (N-H), 1335 (C-N of TPA) and 1219 (ether). 1H-NMR (DMSO-d6) δ/ppm 5.2 NH2 (4, s, 4H), Benzyloxy(BZ): 5.2 (2H, s, 5), 7.5 (5H, m, 6,7,8), Aromatic protons: 6.49 (4H, d, J = 8 Hz, 1), 6.78 (2, d J = 8 Hz, 4H), 6.68 (3, dd, J = 7.2 Hz, 2H), 6.88 (4, d J = 7.5 Hz, 2H) 13CNMR (DMSO-d6) δ/ppm 115.28 (1), 117.48 (2), 118 (3), 115.18 (4), 70.6 (5), 119 (6), 122.7 (7), 120.62 (8), 136.8 (9), 127.3 (10), 130.2 (11), 145.7 (12) and 130.2 (13).
Synthesis of polyamides PA (I-IV)
Low temperature polycondensation (0 °C to room temperature) of diacid chlorides and diamines (1,3) was used for the preparation of polyamides (PA I-IV). A 100 mL round bottom flask, furnished with condenser, dropping funnel and ice bath, was charged with 1.88 mmol of diamine monomer, 15 mL THF and 10 mL triethylamine under N2 atmosphere. Corresponding diacid chloride solution in THF (1.92 mmol/10 mL) was added very slowly via dropping funnel for 30 min. It was heated gradually to room temperature, stirred well for 1 h and refluxed for an additional hour. The viscous solution turned gradually into sticky product. The synthesized polyamide was filtered, washed with plenty of THF and methanol and kept under vacuum for 24 h.
Polyamide (PA-I)
The synthesis of PA-I was carried out by the polycondensation of 1.88 mmol (0.69 g) diamine monomer (1) and 1.92 mmol (0.39 g) terephthaloyl chloride in THF. FTIR (ῡ/ cm−1) 3285 (N-H, amide), 1645 (carbonyl of amide), 1225 (ether).
Polyamide (PA-II)
The polycondensation of 1.88 mmol (0.69 g) diamine monomer (1) with 1.92 mmol isophthaloyl chloride (0.39 g) in THF was conducted following aforementioned procedure. The polymer PA-II was obtained as grey solid. FTIR (ῡ/ cm−1) 3282 (N-H, amide), 1650 (carbonyl of amide), 1224 (ether).
Polyamide (PA-III)
The polymer PA-III was prepared as dark green solid by the reaction of 0.74 g, 1.88 mmol diamine monomer (3) and 1.92 mmol, 0.39 g terephthaloyl chloride using the aforementioned method. FTIR (ῡ/ cm−1) 3272 (N-H, amide), 1645 (carbonyl of amide), 1235 (ether) (Fig. 1).
Polyamide (PA-IV)
The reaction of 0.74 g, 1.88 mmol diamine monomer (3) with 0.39 g, 1.92 mmol of isophthaloyl chloride yielded PA-IV as dark brown solid. FTIR (ῡ/ cm−1) 3261 (N-H, amide), 1651 (carbonyl of amide), 1247 (ether) (Fig. 2).
Results and discussion
Synthesis of monomer diamine (3)
The newly synthesized diamine (3) and dinitro compounds were analyzed by FTIR, and NMR spectral methods. The FTIR spectra of both 2 and 3 are depicted in Fig. 3. It displayed distinguishing peak at 1569 cm−1 assigned to N-O stretching vibrations of nitro group in 2. Furthermore, triphenylamine (TPA) formation was evidenced from the absorption band at 1491 cm−1, assigned to C-N stretching vibrations. The palladium-charcoal catalyzed reduction of 2 into diamine 3 is revealed from new spike shaped absorption band in FTIR spectrum at 3459 and 3339 cm−1. This may be credited to the N-H stretching vibrations of NH2 group [15].
Further confirmation of structures of both 2 and 3 was carried out by NMR spectroscopy. The 1H-NMR spectral analysis of 2 revealed a downfield resonance signal at 8.18 ppm, consistent with the electron withdrawing nature of the adjacent nitro groups at para position. (Annex) This indicated the formation of TPA containing nitro groups. The 1H and 13C NMR spectra of diamine (3) are displayed in Fig. 4a and b respectively. From Fig. 4a, the complete conversion of 2 into 3 was confirmed by distinguishing a broad resonance singlet at 4.87 ppm. This may be credited to the primary amine proton. Additionally, an upfield shift of the aromatic protons (H1, H2, H3 and H4) provided considerable indication for the successful synthesis of diamine. The benzyl (Bz) group is verified by the resonance signals at 4.99 and 7.4 ppm, this corresponded to methylene and phenyl protons respectively. The 13C-NMR spectral analysis further confirmed our aforementioned discussion. (Fig. 4b) [15].
Synthesis of polyamides (I-IV)
All the polyamides (PA I-IV) were synthesized in THF solution from diamine monomers 1 and 3 by triethylamine catalyzed polycondensation with terephthaloyl and isophthaloyl chloride. The method is outlined in Fig. 2. As shown in organosolubility data in Table 1., the synthesized polyamides (PA I-IV) can be solution-cast into films, indicating the formation of high molecular weight polymers. The structures of the PA I-IV were evaluated by FTIR spectroscopy and CHN elemental analysis. Figure 5 shows the FTIR spectra of PA I-IV. All the prepared polyamides (PA I-IV) exhibited distinctive absorption bands of the -CONH group at 3315 cm−1 (N − H stretching) and 1664 cm−1 (carbonyl group). The elemental analyses also indicated the complete polymerization of the corresponding diamine with diacid chloride into corresponding polyamide [16].
Organosolubility of PA I-IV
All the synthesized polyamides were evaluated for their processability in common organic solvents. Approximately 10 mg polymer sample was placed in 1 mL organic solvent and checked for its organosolubility at room temperature and on heating for 2–3 h. The solubility behavior of the PA I-IV is summarized in Table 1. It is obvious that the synthesized polymers are appreciably soluble in common organic solvents. This is the manifestation of the triphenylamine unit as well as linked aroyl substituent on one hand and amide unit on the other hand.
Thermal stability of PA I-IV
The thermal strength of these polyamides (PA I-IV) was studied by thermogravimetric analysis (TGA). The TGA curve of a representative polyamide (PA-I) in an oxidative atmosphere is displayed in Fig. 6. The thermal stability was assessed in terms of Td, char yield and limiting oxygen index (LOI). The thermal behavior data obtained from TGA curves is tabulated in Table 2. In general, the PA (I-IV) exhibited excellent thermal stability with negligible weight loss up to 438 °C in oxidative atmosphere. The maximum weight-loss temperatures of the polymers in air were observed in the range of 501–573 °C. The char yield (amount of carbonized residue) at 800 °C of these materials was observed in the range 53–60%. High char yields of these PA (I-IV) can be attributed to their high aromatic contents. The LOI values ranging from 37.5 to 41.5, were greater than normal amount of oxygen in air (21%) indicating the fire retardancy and flame-retardant feature of the synthesized materials. The fire-retardant properties play an important role in the reduction of combustibility of the polymers and toxic fume production. Furthermore, appreciable thermo-oxidative ability renders these materials, low flammability and retention of molecular strength at elevated temperature. Therefore, synthesized materials are capable of working at elevated temperature if fabricated in devices [17, 18].
Photophysics of PA I-IV
All the synthesized polymers (PA I-IV) were evaluated for UV-vis absorption and photoluminescence (PL) emission in NMP. The absorption and emission profiles of the PA I-IV are depicted in Figs. 7 and 8 respectively. The relevant absorption and emission data are summed up in Table 3. In the UV-vis absorption spectra all the polyamides exhibited prominent absorption bands in the range 290–294 nm. The high energy absorption bands in the range 336–358 nm are assigned to be arising from triphenylamine (TPA) to amide n–п* transitions. Whereas, the absorption peaks in the range 290–294 nm are the manifestation of the п–п* transitions of the aroyl pendant groups.
The HOMO-LUMO energy gaps (Eg) were calculated from the onset wavelength λonset using the following Eq. (1).
The values of Eg are in the range 2.76–3.02 eV such low band gap values are the manifestation of the highly conjugated polyamide backbone. A significant portion of the solar radiations can be absorbed by the materials with such low band gap. The lowest Eg value of PA-III (2.76 eV) is attributed to its symmetrical extended conjugated polymeric chain.
The synthesized polyamides PA I-IV exhibited similar emission profiles in NMP solutions with emission maxima in the range 532–570 nm. This range indicates green light emission, except for the polymer PA-IV, which emit bluish green light in the range 449–550 nm. The emission profile clearly indicates that the emission initiates from a similar excited state in all the polyamides. From which it is obvious that the emission mainly comes from the TPA-aroyl core because it has a lower energy gap [19].
The electrochemical properties of polyamides PA (I-IV)
The electrochemical properties of synthesized polymers (PA I-IV) were explored by cyclic voltammetry (CV) for the cast film on an ITO-coated glass substrate as working and platinum wire as supporting electrode in dry acetonitrile containing 0.1 M of LiClO4 as electrolyte salt under inert atmosphere of nitrogen. A well-defined reversible redox wave was detected in the first CV scan of these polymers. Figure 9 indicates the cyclic voltammograms of the synthesized polyamides (PA I-IV) recorded at a scan rate of 50 mV/s. All the CV curves for PAI-IV; indicated a distinct redox wave, with onset oxidation potentials (Eonset) of 0.35 V, 0.40 V, 0.25 V and 0.3 V respectively. The single oxidation peak, in each CV plot can be attributed to oxidation of the electron-rich nitrogen atom in the TPA core. After numerous oxidative cyclic scans between 0 and 2.0 V at scan rate of 50 MV/s, the polymer films still well-maintained superior redox reversibility. Figure 9 compares the CV curves of structurally similar polyamides I-IV. As expected, the onset oxidation potential of PA-III is the lowest value because of benzyloxy substitution at para position of the TPA unit on one hand and symmetrical terephthalic acid amide unit on the other hand. This result indicated that the presence of the benzyloxy substituent in combination with terephthalic acid amide unit stabilizes the oxidation state. However, in PA-IV the isophthalic acid amide unit being unsymmetrical unit may be responsible for the disruption of planarity and hence the elevation of onset oxidation potential. However, the onset oxidation potentials for the structurally similar TPA linked polyamide with -OCH3 at ortho, meta and para positions were reported to be 0.61 V, 0.66 V and 0.55 V respectively by Lee et al. [7]. These values are higher than the onset oxidation of our synthesized redox-active polyamides This lower onset oxidation potential (0.25–0.4 V) of synthesized PA as compared to the previously reported polyamide, is beneficial for their multiple applications in optoelectronics [7, 19].
The energy levels of HOMO and LUMO (lowest unoccupied molecular orbital) of our synthesized polyamides can be calculated from the Eonset or half-wave potentials (E1/2) and the results are summed up in Table 4. The ferrocene/ferrocenium (Fc/Fcþ) was used as an external standard with a redox potential at 0.44 V vs Ag/AgCl in dry acetonitrile. We can assess the HOMO energy levels of synthesized polyamides (I-IV) by assuming HOMO of ferrocene at 4.40 eV with respect to zero vacuum level. The values of HOMO energy levels as calculated from CV experiments are −4.75, −4.80, −4.65 and − 4.70 eV for PA (I-IV) respectively. The highest HOMO energy level for the PA-III authenticated the previous discussion about the benzyloxy substituent. The reported polyamides with analogous structure PA-V (-OCH3 substituent, terephthalic acid amide) and PA-VI (-OCH3 substituent, isophthalic acid amide) exhibited HOMO energy level at −4.99 eV [19]. The elevation of HOMO energy levels and redox stability of synthesized polyamides is quite beneficial for their future use in optoelectronic applications [20].
Computational analysis of PA I-IV
In order to get more comprehension into different energy levels of the present TPA-based polyamides, molecular simulation on the basic unit was conducted by DFT/B3LYP/6-31G with the Gaussian 03 program as shown in Fig. 10. The optimization of the dimers of the polyamide’s ground state was carried out to calculate HOMO and LUMO energy levels. The calculated values as summarized in Table 4, are in the range 2.39–4.65 eV and 0.76–2.31 eV for HOMO and LUMO respectively.. The simulation results are not valid for the polymers PA-II and PA-IV, the reason may be the steric factor Fig. 10 represents the HOMO-LUMO orbital diagram of dimers of PA-I and PA-III. From this figure, it is obvious that the HOMO-LUMO electron densities of the polyamides are mainly located on triphenylamine moiety and amide linkage. The HOMO-LUMO band gap was found in the range 1.60–2.34 eV. From computational analyses it is obvious that practically measured values are higher than the calculated one. It may be attributed to the fact that the values were calculated for dimers rather than polymer (software was unable to deal long polymer chain), containing large number of TPA units elevating HOMO and LUMO levels. These results may also suggest that the synthesized polyamides could be used as an organic layer in OLEDs as hole transport materials in future [20].
Conclusion
A series of light emitting, electroactive polyamides have been readily prepared from the polycondensation of newly synthesized diamine monomer, 4,4′-diamino-4′′-phenoxy triphenylamine and 4,4′-diamino-4′′-benzyloxy triphenylamine with the terephthaloyl/isophthaloyl chloride. Introduction of the bulky electron-donating phenoxy and benzyloxy group to the polymer main chain not only affords good thermal stability but also enhanced organosolubility of the polyamides. The new series of polyamides are emitting light in the blue to green region. All the synthesized polyamides reveal valuable electrochemical characteristics such as low oxidation potential, shallow HOMO energy level. Thus, these features indicate the incorporation of para substituents benzyloxy and phenoxy groups on TPA is a good approach for easy oxidation and the prepared polyamides have great potential for applications in optoelectronic applications.
References
Iwan A, Sek D (2011) Polymers with triphenylamine units: photonic and electroactive materials. Prog Polym Sci 36:1277–1325. https://doi.org/10.1016/j.progpolymsci.2011.05.001
Lin HY, Liou GS, Lee WY, Chen WC (2007) Poly (triarylamine): its synthesis, properties, and blend with polyfluorene for white-light electroluminescence. J Polym Sci A Polym Chem 45:1727–1736. https://doi.org/10.1002/pola
Chiu KY, Su TX, Li JH, Lin TH, Liou GS, Cheng SH (2005) Novel trends of electrochemical oxidation of amino-substituted triphenylamine derivatives. J Electroanal Chem 575:95–101. https://doi.org/10.1016/j.jelechem.2004.09.005
Yen HJ, Guo SM, Liou GS (2010) Synthesis and unexpected electrochemical behavior of the triphenylamine-based aramids with ortho-and Para-trimethyl-protective substituents. J Polym Sci A Polym Chem 48:5271–5281. https://doi.org/10.1002/pola.24326
Wang HM, Hsiao SH (2009) Electrochemically and electrochromically stable polyimides bearing tert-butyl-blocked N, N, N′,N′-tetraphenyl-1, 4-phenylenediamine units. Polymer 50:1692–1699. https://doi.org/10.1016/j.polymer.2009.02.009
Hsiao SH, Liao WK, Liou GS (2017) Synthesis and electrochromism of highly organosoluble polyamides and polyimides with bulky trityl-substituted triphenylamine units. Polymers 9:511. https://doi.org/10.3390/polym9100511
Lee MJ, Kwak YJ, Seok WC, Lee SW (2016) Synthesis, characterization and electrochromic properties of polyamides having triphenylamine derivatives. Polym Bull 73:2427–2438. https://doi.org/10.1007/s00289-016-1670-y
Khalid N, Park OO, Akhter T, Siddiqi HM (2017)) Fluorescent, electroactive, thermally stable triphenylamine-and naphthalene-based polyimides for optoelectronic applications. Journal of Applied Polymer Science 134:44526. https://doi.org/10.1002/app.44526
Liou GS, Hsiao SH, Ishida M, Kakimoto M, Imai Y (2002) Synthesis and characterization of novel soluble triphenylamine-containing aromatic polyamides based on N, N′-bis (4-aminophenyl)-N,N′-diphenyl-1, 4-phenylenediamine. J Polym Sci A Polym Chem 40:2810–2818. https://doi.org/10.1002/pola.10364
Yen HJ, Liou GS (2012) Solution-processable triarylamine-based electroactive high performance polymers for anodically electrochromic applications, polymer chemistry 2:255-264.DOI. https://doi.org/10.1039/c1py00346a
Debaditya B, Bandyopadhyay P, Ghosh S, Banerjee S, Padmanabhan (2015) Highly gas permeable aromatic polyamides containing adamantane substituted triphenylamine. J Membr Sci 474:20–31. https://doi.org/10.1016/j.memsci.2014.09.032
Ningwei S, Su K, Zhou Z, Yu Y, Tian X, Wang D, Zhao X, Zhou H, Chen C (2018) AIE-active polyamide containing diphenylamine-TPE moiety with superior electrofluorochromic performance, ACS applied materials & interfaces 10:16105-16112.DOI. https://doi.org/10.1021/acsami.8b01624
Yuan WZ, Lu P, Chen S, Lam JW, Wang Z, Liu Y, Kwok HS, Ma Y, Tang BZ (2010) Changing the behavior of chromophores from aggregation-caused quenching to aggregation-induced emission: development of highly efficient light emitters in the solid state. Advanced Materials 22:2159–2163. https://doi.org/10.1002/adma.200904056
Yanqiu W, Liang Y, Zhu J, Bai X, Jiang X, Zhang Q, Niu H (2015) High coloration efficiency and fast switching speed of poly (amic acid-imide) s containing triphenylamine in acidic electrolyte. RSC Adv 5:11071–11076. https://doi.org/10.1039/c4ra12970a
Liou GS, Lin KH (2009) Synthesis and characterization of a novel electrochromic aromatic polyamide from AB-type triphenylamine-based monomer. J Polym Sci A Polym Chem 47:1988–2001. https://doi.org/10.1002/pola.23286
Chang CW, Liou GS, Hsiao SH (2007) Highly stable anodic green electrochromic aromatic polyamides: synthesis and electrochromic properties. J Mater Chem 17:1007–1015. https://doi.org/10.1039/B613140A
Chattopadhyay DK, Webster DC (2009) Thermal stability and flame retardancy of polyurethanes. Prog Polym Sci 34:1068–1133. https://doi.org/10.1016/j.progpolymsci.2009.06.002
Guardia L, Mark J, Hale RC, Harvey E (2006) Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol 40:6247–6254. https://doi.org/10.1021/es060630m
Hsiao SH, Liou GS, Kung YC, Hsiung TJ (2010) Synthesis and properties of new aromatic polyamides with redox-active 2, 4-dimethoxytriphenylamine moieties. J Polym Sci A Polym Chem 48:3392–3401. https://doi.org/10.1002/pola.24124
Jiwei C, Niu H, Wang C, Ma L, Bai X, Wang W (2012) Tuning the bandgaps of polyazomethines containing triphenylamine by different linkage sites of dialdhyde monomers. Electrochim Acta 76:229–241. https://doi.org/10.1016/j.electacta.2012.04.117
Declaration of interest
Financial support of this project was provided by the Higher education Commission of Pakistan under the National Research Program for Universities research project 20–3821/NRPU/R&D/HEC/14.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalid, N., Shumail, S., Siddiqi, H.M. et al. Triphenylamine based redox-active, fluorescent polyamides: synthesis and photophysics. J Polym Res 27, 51 (2020). https://doi.org/10.1007/s10965-020-2029-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-2029-5