Abstract
Polypropylene (PP) grafted with p-hydroxy-N-phenyl maleimide (pHPMA) was prepared by melt reactive extrusion in a twin screw extruder in presence of dicumyl peroxide as initiator. The content of pHPMA was varied from 1.0 up to 4.0 wt%. The grafted PP was characterized by FTIR, melt flow index (MFI), contact angle measurements, mechanical testing and thermogravimetric analysis (TGA). The non-isothermal crystallization kinetics of the neat PP and grafted PP were investigated using differential scanning calorimetry. The results showed that the MFI of the grafted PP (PP-g-pHPMA) was higher than that of PP homopolymer. The contact angle of the grafted PP was found to be decreased by increasing the monomer percent up to 3.0 wt%. Further increase of the monomer leads to an increase in the contact angle which may be attributed to the non-homogeneous distribution of the pHPMA grafted chains onto the PP surface. The thermal stability was enhanced by increasing the content of pHPMA. Grafting has not significantly affected either the tensile strength or elongation at a break point. A noticeable change in crystallization behavior of PP matrix has been observed upon grafting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer research has been changed significantly in the last few decades, as the number of new polymers introduced into polymer research field has been reduced and this led to increase the need to improve the performance of the existing polymers. Several techniques, such as chemical modification, blending, and reinforcement, have been applied in order to improve them.
Recently, polypropylene (PP) has been extensively used in a wide range of applications due to its versatility and low cost although it has some limitations such as low thermal stability and non-polar nature which limit its interaction with other products. So, the chemical modification of polypropylene through grafting with reactive monomers is necessary to enhance its properties and increase its compatibility by imparting a variety of functional groups to its surface [1] without affecting the nature of its backbone [2,3,4,5,6]. Grafting reactions are usually in solution [2,3,4,5,6,7,8,9], in the solid state [10, 11] or in the melt [12,13,14,15,16,17,18,19,20] and they require the presence of organic peroxides, such as dicumyl peroxide or benzoyl peroxide, which act as initiators to the grafting reaction.
Nowadays, melting by reactive extrusion is the most widely applied industrial method for grafting reactions due to its low cost compared to the solution method which costs a lot due to large quantities of the solvent consumed; which limits its use in large scale productions. In the reactive extrusion method, the polymer, monomer, and initiator are mixed together in an extruder at above the polymer softening temperature. On the other hand, the reactive extrusion method has a disadvantage of secondary reactions for the PP like β-scissions due to the elevated temperatures used in the extrusion process [12, 17,18,19,20], which lead to decrease of the molecular weight compared to those obtained from the solution method.
Maleic anhydride (MA) and its iso-structural analogues such as N-substituted maleimides are successfully used as reactive monomers for the graft modification of a wide range of polyolefins such as polypropylene, as the introduction of MA or one of its derivatives to the non-polar backbone of polypropylene, overcomes the disadvantage of low surface energy of the polymer, improves its surface hydrophilicity, increases its compatibility with polar materials, and enhanced its thermal stability, hence increases its applicability [12, 17,18,19,20,21].
Maleimide and its N-substituted derivatives proved to have the ability to be polymerized free radically, in spite of a 1,2-disubstituted ethylenic structure, to give thermally stable polymers, particularly polymers derived from monomers containing aromatic substituent [21,22,23,24,25,26].
In the present work, polypropylene (PP) was grafted with p-hydroxy-N-phenyl maleimide (pHPMA) via reactive extrusion, using dicumyl peroxide (DCP) as an initiator, which can improve the polarity and the thermal properties of the PP. The pHPMA feed was varied from 1.0 to 4.0 wt%, while DCP was fixed at 0.1 wt%. The prepared PP-g-pHPMA copolymers were characterized by melt flow index (MFI), contact angle, tensile test and thermogravimetric analysis (TGA) to investigate the effect of pHPMA content on all these properties. The non-isothermal crystallization of PP-g-pHPMA was studied by DSC.
Experimental
Materials
Polypropylene (PP) was provided by Rabigh Refining & Petrochemical Co., it showed to have MFI of 5.5g/10 min (230 °C/ 2.16Kg). Dicumyl peroxide, bis(1-methyl-1-phenyl ethyl) peroxide, was purchased from Sigma-Aldrich (Gillingham, UK). Maleic anhydride was purchased from ALPHA CHEMIKA (India). p-Hydroxy aniline, acetic anhydride, anhydrous sodium acetate and n-hexane were obtained from Sigma-Aldrich (Gillingham, UK) and used as received without further purification.
Synthesis of p-hydroxy-N-phenyl maleimide (pHPMA)
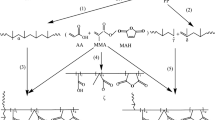
p-Hydroxy-N-phenylmaleimide (pHPMA) was prepared according to Scheme 1 as described previously [26,27,28]. In a brief, 0.1 mol of p-hydroxy aniline dissolved in 100 cm3 of diethyl ether was added drop-wise to 0.1 mol maleic anhydride dissolved in 100 cm3 of toluene. The reaction mixture was stirred at room temperature for 1 h, and the stirring was continued for 24 h. The canary yellow precipitate was filtered, washed with acetone and water, then dried. The melting point (mp) of the obtained product (p-hydroxy maleanilic acid) is 201-202 °C. A mixture of 10.0 g p-hydroxy maleanilic acid, 5 g freshly fused sodium acetate, and 50 ml of acetic anhydride was heated for 0.5 h at 90 °C. Then the reaction mixture was precipitated by pouring slowly into an ice-water. The precipitate was filtered, washed with sodium bicarbonate solution and water, and then dried. The obtained solid was recrystallized twice from 2-propanol and finally dried in a vacuum oven at 65 °C for 24 h to give a pale yellow powder of p-hydroxy-N-phenyl maleimide (mp 158 °C).
Reactive extrusion
Grafting of pHPMA onto PP was carried out in a brabender plasticorder (torque rheometer) twin head extruder. Polypropylene pellets containing 1.0, 2.0, 3.0 and 4.0 wt% pHPMA and 0.1 wt% dicumyl peroxide, as initiator, were introduced in barrel and kept at 200 °C for 10 min, and the screw speed was 70–80 rpm.
All grafted PP copolymers were refluxed in xylene for 4 h and then precipitated in acetone to remove the non-reacted monomers, excess of initiator or oligomers if formed during processing. After that, they were dried in a vacuum oven at 70 °C for 24 h to constant weights. The percentage of grafting (%G) was determined gravimetrically from the following relation:
where Wo and Wg are the weights of the original and grafted PP, respectively.
The calculated percentages of grafting were found to be 0.94, 1.91, 2.83 and 3.93%, which are in good agreement with feed ratio of pHPMA based on PP, and labeled here as PP-g-pHPMA-1, PP-g-pHPMA-2, PP-g-pHPMA-3 and PP-g-pHPMA-4, respectively.
Characterization of grafted PP
FTIR
The Fourier transform infrared (FTIR) of the grafted PP thin films was conducted with a FTIR spectrometer (Shimadzu 800, Kyoto, Japan) in the range 500–4000 cm−1.
Melt flow index
Melt flow indices (MFI) of pure PP and PP graft copolymers were obtained by MP1200-melt indexer at 230 °C and 2.16 kg load. The basic unit has dimensions of 458 mm width × 394 mm depth × 521 mm height of basic unit; it uses a three-zone band heater, instead of the more common two zone band heater, in order to increase the temperature stability of the machine. Samples were prepared from extruded polymers by compression using a hot press at 200 °C at 60 MPa for 1 min, then cooled under pressure to ambient temperature.
Contact angle measurement
The sessile drop technique was used to measure the contact angles using KYOWA contact angle meter equipped with software for drop-shape analysis. A 4.0 μL droplet of filtered, deionized distilled water was placed on the surface of the samples at room temperature. The angle between the baseline of the drop and the tangent at the drop boundary was measured, using a direct reading goniometry telescope, with a magnified image of the droplet displayed on a screen. The drop shape was solved numerically and fitted by means of mathematical functions. Each reported value is the average of five independent measurements. The contact angle was evaluated by applying the following equation:
where, θ is the contact angle, h is the height and w is the width of the droplet that were measured from the photograph and from the geometric considerations.
Mechanical testing
The tensile strength of all samples was measured two times for each sample at room temperature according to ASTM D638–1 using dumbbell specimens on LMF-L20KN universal testing machine with a cross head speed of 50 mm/min.
Thermogravimetric analysis
Thermogravimetric analysis was performed on TGA Q500 thermal analyzer under nitrogen atmosphere. Samples weight of 2–4 mg were measured from room temperature to 600 °C at a heating rate of 10 °C/min.
Differential scanning calorimetry
Non-isothermal crystallization was carried out using a TA Q20 differential scanning calorimeter under a purge of nitrogen. The instrument was calibrated using high purity indium and zinc standards. Approximately 12 mg samples were melted at 195 °C for 3 min in order to eliminate any previous thermal history; then they were cooled to 50 °C with cooling rates 5.0, 7.5, 10, 15 and 20 °C/min. The exothermic crystallization peaks were recorded as a function of temperature. Melting of the investigated samples was also studied in the temperature range from 30 to 190 °C at a heating rate of 10 °C/min.
Results and discussion
FTIR analysis
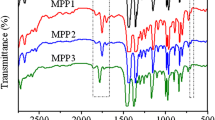
Figure 1 shows the FTIR spectra of neat PP and grafted PP with 1.0, 2.0, 3.0 and 4.0 wt% pHPMA. Compared with neat PP, the obvious appearance of new absorption bands in the grafted PP at 1709 and 1508 cm-l, corresponding to the stretching vibrations of –C=O of imide ring and C=C of aromatic ring, respectively, could be assigned to pHPMA grafted chains. Further, the intensity of these bands increases with increasing the percentage of grafting, confirming the grafting of pHPMA onto PP skeleton since the unreacted monomer and homopolymer had been removed by purification. The extent of grafting of PP can be expressed by carbonyl index (CI), which represents the ratio of absorbance at 1709 cm−1 (A1709), characteristic of the carbonyl of imide groups, to that at 1167 cm−1 (A1167), characteristic of the CH3 groups of PP repeating units. The obtained CI values were found to be 0.64, 0.77, 0.98 and 1.36 for PP-g-pHPMA-1, PP-g-pHPMA-2, PP-g-pHPMA-3 and PP-g-pHPMA-4 copolymers, respectively. The mechanism of grafting of the pHPMA onto PP could be assumed as shown in Scheme 2.
Rheological characterization
The melt flow index (MFI) of the neat PP and grafted PP was measured and the results are shown in Table 1. It can be observed that the MFI increased from 5.7 for PP up to 114.1 g/10 min upon grafting of PP with 1.0% wt pHPMA (sample PP-g-pHPMA-1), indicating that the grafting process causes a great reduction in the viscosity. By increasing the grafting of PP with 2% wt pHPMA, the MFI declined to 52.6 g/10 min then increased with increasing the amount of pHPMA up to 4.0 wt%. Generally, MFI is a direct indication of melt viscosity and, hence, depends on average molecular weight. MFI can also be influenced by other factors such as molecular weight distribution, degree of long chain branching and degree of cross-linking. However, the impact of feed pHPMA on MFI is not clear. The increment of the MFI values of PP upon grafting, which depends on the content of pHPMA, may be attributed to the decrease of the molecular weight of PP as the result of main chain secession that occurred during free radical initiated pHPMA-grafting PP. The observed reduction in the MFI of the graft PP with 2.0 up to 4.0 wt% pHPMA may be due to some sort of crosslinking formation between PP matrix polymeric chains. This is consistent with the previous finding of grafting of maleic anhydride onto PP [29, 30]. Generally, it was concluded that the melt flow properties of grafted PP, irrespective of pHPMA content, is better than neat PP.
Contact angle measurement
The polarity of the neat PP and graft PP films was measured by the contact angle of distilled water on the sample films and the results are shown in Table 2. As can be seen, the contact angle for a neat PP film was found to be 73.2 ± 1.1o. The contact angle of the graft PP decreased to 58.3 ± 0.1o with increasing feed of pHPMA up to 3.0 wt%, but it increased up to 81.8 ± 0.3o with increasing pHPMA to 4.0 wt%. These results suggested that the functionalization of polypropylene surface with pHPMA grafted chains has led to an increase of the hydrophilic character of the PP, while the increase of contact angle of PP grafted with 4.0 wt% pHPMA may be due to pHPMA homopolymerization [31, 32] and some sort of the crosslinking formation of PP polymeric chains.
Mechanical testing
The tensile strength and elongation % at the break point of PP and its graft products are shown in Table 3. The results show that the tensile strength and the elongation of PP slightly decreased with increasing the grafting of PP up to 2.0 wt% of pHPMA. This is probably due to the slight degradation of the PP matrix, resulting essentially from chain-scissions that occurs during grafting in the molten state. With the increase of pHPMA content up to 4.0 wt%, the tensile strength and the elongation slightly showed to an increase. Such behavior could be ascribed potentially to the cross-linking of polymeric chains of PP matrix.
Thermogravimetric analysis
The TGA and their corresponding derivative curves (DTGA) of the PP and grafted PP are presented in Fig. 2. As can be seen, the thermograms of the neat and grafted PP exhibited the same profile and the thermal degradation occurs via one step. The values of the initial decomposition temperature (Ti), the maximum rate of decomposition temperature (Tmax) and the temperature at which half of the material decomposes (T50) of the investigated samples are summarized in Table 4. As obvious, a considerable enhancement in the thermal stability of PP matrix was occurred upon grafting, as mirrored from the shift of Ti, Tmax. and T50. Such behavior might be attributed to the effect of crosslinking of polymeric chains that occurs upon grafting and/or the presence of a relatively more stable poly (pHPMA) grafting chains onto the PP skeleton, which improved the thermal stability [27, 33,34,35]. In the case of PP-g-pHPMA-4, the onset degradation temperature is increased by about 100 °C compared to neat PP.
Melting and non-isothermal crystallization
DSC was used to characterize the influence of pHPMA content on crystallinity and transition temperatures of grafted PP copolymers. The heating and cooling DSC traces for neat PP and grafted PP recorded at a rate of 10 °C/ min are shown in Fig. 3. From these curves, several parameters; namely, the melting point maximum temperature (Tm), the exothermic temperature peak of crystallization (Tc), melting enthalpy and crystallization enthalpy are derived and summarized in Table 5. It can be seen that the melting temperature of the PP matrix decreases, while the crystallization temperature increases with the pHPMA grafting chains and an increase in its content. The increase of Tc upon increasing the content of pHPMA suggests that the pHPMA grafting chains exerts a nucleating effect in the PP crystallization process and lowers the nucleation energy. On the other hand, the decrease of melting may be due to decrease in size and imperfection of PP spherulites as the result of pHPMA grafting onto PP matrix. Similar observations are found in several PP blends and PP graft copolymers [36,37,38,39,40]. In terms of the melting and crystallization enthalpies, the pHPMA content does not significantly change their values, indicating that all PP graft copolymers, irrespective of PP content, exhibit comparable degree of crystallinity (Table 5).
Typical DSC non-isothermal crystallization curves of neat PP and PP-g-pHPMA-4 at various cooling heating rates are shown in Fig. 4. The crystallization data at different cooling rates are summarized in Table 6. For both neat PP and PP-g-pHPMA-4 copolymer, increasing cooling rate results in the decrease of Tc which may be due to a larger supercooling at higher cooling rates [40, 41]. At a given cooling rate, Tc of grafted PP is higher than that of pure PP. This indicates that the grafted PP can crystallize more easily.
The relative degree of crystallinity as a function of temperature was obtained by integration of the exothermic peaks during the non-isothermal scans in Fig. 4. The results are represented graphically in Fig. 5. It can be seen that the higher the cooling rate, the shorter is the time for the completion of crystallization. Furthermore, all curves for PP and grafted PP exhibit only one stage (primary crystallization); the secondary crystallization is less significant. This is consistent with the previous findings [42,43,44].
The crystallization half-time, t1/2, can be estimated from the time at which the extent of crystallization is half completed (Table 6). The result clearly indicates that the value of t1/2 decreased with increasing cooling rates, suggesting that the crystallization rate for all the grafted samples increased with increasing cooling rates (Fig. 6). It is also shown that, at any given cooling rate, the t1/2 value of the grafted PP is lower than that of neat PP. Such short crystallization time suggests that the pHPMA grafted chains likely plays a role in nucleation effect. These results are consistent with the observation reported in many grafted PP systems [45, 46].
The crystallization rate parameter (CRP) is used to quantitatively compare non-isothermal crystallization rates, which can be determined from the slope of a plot of 1/t1/2 versus the cooling rate (Fig. 6); obtained CRP values are listed in Table 6. The higher value of CRP means a higher rate of crystallization. As seen in Table 6, the CRP value of the grafted PP is higher than that of pure PP.
The non-isothermal crystallization kinetics was analyzed using the Avrami equation, proposed by Jeziorny [43, 44]:
where n is the Avrami exponent, Kt the Avrami rate constant and Xt the relative crystallinity at time t. At a given cooling rate, the values of n, Kt can be determined from the slope and intercept of the plots of log[− ln (1 − Xt)] against logt (Fig. 7). The obviously good linearity for all plots reflects the adequacies of the modified Avrami equation to describe the non-isothermal crystallization for neat PP and its graft copolymers. Considering the influence of cooling rate (ϕ), Jeziorny [44] proposed a corrected rate parameter Kt, which is expressed in the eq. (3):
Based on eqs. (2) and (3), the values of n and Kc were calculated and the results are presented in Table 6. The average n values of neat PP and PP-pHPMA-4 are found to be 4.2 and 3.7, respectively, suggesting that the non-isothermal melt crystallization of the grafted PP copolymer follows a heterogeneous, instantaneous nucleation and the three-dimensional spherulitic growth, as occurring in PP homopolymer [47, 48]. Furthermore, the Kc values of the PP-g-pHPMA-4 were enhanced compared to the neat PP, suggesting acceleration of crystallization of the PP upon grafting.
Friedman [49] and Vyazovkin [50,51,52] developed the differential isoconversional method for evaluating the effective activation energy for the non-isothermal crystallization process. According to Friedman, the effective activation energy (ΔEXt) can be calculated as a function of the relative crystallinity according to the following equation:
where dXt/dt is the instantaneous crystallization rate for a given relative crystallinity Xt, ΔEXt is the effective activation energy for the crystallization process, R is the universal gas constant, and T is the temperature. At a given Xt, the ΔEXt can be calculated from the slope of the plot of ln(dXt/dt) versus 1/T for a set of cooling rates (Fig. 8). The ΔEXt values determined for various values of Xt, in the range 0.05 to 0.95, are derived and represented graphically in Fig. 9. It is obvious that the ΔEXt has a negative value, indicating that the crystallization rate increases with decreasing temperature, which is the characteristic feature of the crystallization region that corresponds to temperatures higher than that of the maximum crystallization rate, and that the crystallization process of polymer is a barrierless and spontaneous process [52, 53]; the higher the ΔEXt, the more difficult is the transport of macromolecular segments to the growing surface. In Fig. 9, the plots show that the effective activation values of PP homopolymer and PP graft copolymer are comparable, inferring that both polymers may proceed with similar crystallization mechanism.
Conclusions
In this work, PP grafted with various amounts of pHPMA (1.0, 2.0, 3.0 and 4.0 wt%) were prepared by reactive extrusion using brabender plasticorder twin head extruder. The obtained PP grafted copolymers were characterized by MFI, contact angle measurements, tensile test, TGA and DSC. The results showed that the graft PP exhibits high MFI compared with neat PP and whose values depend on the amount of pHPMA. The contact angle of the graft PP gradually decreases with pHPMA content up to 3.0 wt%, suggesting that the modification of PP surface with pHPMA grafted chains could lead to increase the hydrophilic character of the PP. Unexpectedly; graft PP sample with 4.0 wt% pHPMA exhibited a high value of the contact angle compared to neat PP. This may be due to the non-homogeneous distribution of the grafted chains of pHPMA on the PP surface. It was observed that the tensile strength and the elongation slightly decrease with increasing pHPMA content up to 3.0 wt% and then slightly increase upon grafting of PP with 4.0 wt% pHPMA. The reduction in the tensile properties was probably due to a slight degradation of PP matrix that occurs upon grafting in melt state. TGA results showed that the thermal stability is enhanced upon the incorporation of pHPMA grafted chains. The DSC results showed that the melting temperature of the graft PP decreases, while the crystallization temperature increases and becomes narrow with increasing the extent of grafting. No considerable change in the crystallinity was observed for the graft PP compared with that of neat PP.
The non-isothermal crystallization of PP grafted with 4.0 wt% pHPMA (PP-g-pHPMA-4) as well as PP were investigated using DSC at different cooling rates 5, 7.5, 10, 15, and 20o C/min, and have been described using Avrami model. The average n values of neat PP and PP-g-pHPMA-4 are found to be 4.19 and 3.66, respectively, suggesting that the non-isothermal melt crystallization of the graft PP follows a heterogeneous nucleation and three-dimensional spherulitic growth, as occurring in the neat PP. The effective activation energy of the crystallization process (ΔEXt) was calculated by Friedeman isoconversional method. It was found that the ΔEXt values are increased with the advance of the crystallization process.
References
Bhattacharya A, Misra BN (2004) Grafting: a versatile means to modify polymers techniques, factors and applications. Prog Polym Sci 29:767–814
Abudonia KS, Saad GR, Naguib HF, Eweis M, Zahran D, Elsabee MZ (2018) Surface modification of polypropylene film by grafting with vinyl monomers for the attachment of chitosan. J Polym Res 25:125
Wang Y, Ni Q, Liu Z, Zou J, Zhu X (2011) Grafting modification and properties of polypropylene with pentaerythritol tetra-acrylate. J Polym Res 18:2185–2193
Wang H, Wei J, Li S, Chen Y, Ren Z, Qiu S (2013) Preparation and characterization of acrylic acid(AA) and 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS) grafted polypropylene by two-steps electron beam irradiation for filtration of cigarette smoke. J Polym Res 20:44
Wang H, Wei J, Li S, Chen Y, Ren Z (2013) Qiu S (2013) preparation and characterization of acrylic acid(AA) and 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS) grafted polypropylene by two-steps electron beam irradiation for filtration of cigarette smoke. J Polym Res 20:44
Wei X, Wu S (2013) Preparation of novel PP-g-BMA sorbent by two-step irradiation method for elimination of homopolymerization in grafting process. J Polym Res 20:292
Kong Z, Wu X, Wei J, Zhang H, Cui L (2016) Preparation and characterization of hydrophilicity fibers based on 2-(dimethyamino)ethyl mathacrylate grafted polypropylene by UV- irradiation for removal of Cr(VI) and as(V) J Polym res 23:199
Krause-Sammartino LE, Lucas JC, Reboredo MM, Aranguren MI (2013) Maleic anhydride grafting of polypropylene: peroxide and solvent effects. Plast Rubber Compos 35:117–123
García-MartínezJM LO, Collar EP (1998) Chemical modification of polypropylenes by maleic anhydride: influence of Stereospecificity and process conditions. J Appl Polym Sci 68:483–495
Rengarajan R, ParameswaranV R, Vicic M, Lee S, Rinaldi PL (1990) N.M.R analysis of polypropylene- maleic anhydride copolymer. Polymer 31:1703–1706
Qian J, Huang Z, Dang S, Xu Y(2011) Improvements of polypropylene grafted maleic anhydride with ultrasonication, pre-irradiation and co-irradiation methods. J Polym Res18:1557–1565
GüldoğanY ES, Rzaev ZMO, Pişkin E (2004) Comparison of maleic anhydride grafting onto powder and granular polypropylene in the melt by reactive extrusion. J Appl Polym Sci 92:3675–3684
Burton E L, Woodhead M, Coates P, GoughT (2010) Reactive grafting of glycidyl methacrylate onto polypropylene. J Appl Polym Sci 117:2707–2714
Berzin F, Flat JJ, Vergnes B (2013) Grafting of maleic anhydride on polypropylene by reactive extrusion: effect of maleic anhydride and peroxide concentrations on reaction yield and products characteristics. J Polym Eng 33:673–682
Khunova V, Zamorsky Z (1993) Studies on the effect of reactive polypropylene on the properties of filled polyolefin composites, part I. Advantages of solid-phase-grafted maleated polypropylene over melt-phase-modified polymers. Polym-Plast Techn Eng 32:289–298
Hu GH, Flat JJ, Lambla M (1993) Exchange and free radical grafting reactions in reactive extrusion. Macromol Symp 75:137–157
Ho RM, Su AC, Wu CH, Chen SI (1993) Functionalization of polypropylene via melt mixing. Polymer 34:3264–3269
Bettini SHP, Agnelli JAM (1999) Grafting of maleic anhydride onto polypropylene by reactive processing. I Effect of maleic anhydride and peroxide concentrations on the reaction J Appl Polym Sci 74:247–255
Oromiehie A, Ebadi-Dehaghan H, Mirbagheri S (2014) Chemical modification of polypropylene by maleic anhydride: melt grafting, characterization and mechanism. Int J Chem Engin Appl 5:117–122
Cao K, Shen Z, Yao Z, Wei B, Pang X, Lu Z, Yan L, Chen Z (2009) New insight into the action of supercritical carbon dioxide for grafting of maleic anhydride onto isotactic polypropylene by reactive extrusion. Chem Eng Sci 65:1621–1626
Baker W, Scott C, Hu GH (1999) Synthesis of polyolefin graft copolymers by reactive extrusion. Prog Polym Sci 24:81–142
LiY XXM, Guo BH (2001) Study on styrene-assisted melt free-radical grafting of maleic anhydride onto polypropylene. Polymer 42:3419–3424
Elsabee MZ, Sabaa MW, Naguib HF, Furuhata K (1987) Copolymerization of methyl methacrylate with N-Phenylmaleimide in different solvents. J Macrom Sci PartA-Chem 24:1207–1221
Matsumoto A, Kubota T, Otsu T (1990) Radical polymerization of N-(alkyl-substituted phenyl)maleimides: synthesis of thermally stable polymers soluble in nonpolar solvents. Macromolecules 23:4508–4513
Onimura K, Matsushima M, Yamabuki K, Oishi T (2010) Synthesis and properties of N-substituted maleimides conjugated with 1,4-phenylene or 2,5-thienylene polymers. Polym J 42:290–297
CavaMP DAA, Muth Kand Mitchell M (1961) N-phenylmaleimide. J Organ Syn 41:93–95
Mokhtar SM, Mostafa TB (2000) Gama radiation-induced graft copolymerization of N-p-hydroxyphenylmaleimide onto polypropylene films. J Polym Res 7(4):215–219
Ahmed I, Mustapha A (2010) Synthesis of new azo compounds based on N-(4- Hydroxypheneyl)maleimide and N-(4-Methylpheneyl)maleimide. Molecules 15:7498–7508
Moad G (1999) The synthesis of polyolefin graft copolymers by reactive extrusion. Prog Polym Sci 24:81–142
Boaen NK, Hillmyer MA (2005) Post-polymerization functionalization of polyolefins. Chem Soc Rev 34:267–275
Sanchez-Valdes S, Guerrero-Salazar C, Ramosde Valle LF, Lopez-Quintanilla M, Yasfiez-Flores I, Orona-Villarreal F, Ramirez-Vergas R (1997) Characterization of LLDPE-LLDPEgMA blends by contact angle and FTIR-ATR studies. J Polym Eng 17:257–267
Kubota H (1993) Photografting of acrylonitrile and Methacrylic acid on polyethylene film under air atmosphere. J Appl Polym Sci 48:1717–1721
Liu G, Li X, Zhang L, Qu X, Liu P, Yang L, Gao J (2002) Thermal analysis of solution copolymers of styrene with N-Phenylmaleimide. J Appl Polym Sci 83:417–422
Yang P, Ratcliffe LBD, Armes SP (2013) Efficient synthesis of poly(methacrylic acid)-block-poly(styrene-alt-N-phenylmaleimide) Diblock copolymer lamellae using RAFT dispersion polymerization. Macromolecules 46:8545–8556
Liu C, Wei D, Zheng A, Li Y, Xiao H (2006) Improving Foamability of polypropylene by grafting modification. J Appl Polym Sci 101:4114–4123
Brandrup S, Immergut EH (1975) Polymer handbook. New York: Interscience 5:24
Wang Y, Mingtao Run M (2009) Non-isothermal crystallization kinetic and compatibility of PTT/PP blends by using maleic anhydride grafted polypropylene as compatibilizer. J Polym Res 16:725–737
Zhang Z, Yu F, Yu W, Zhang H (2015) Non-isothermal crystallization behavior of dynamically vulcanized long chain branched polypropylene/ethylene-propylene-diene monomer blends. J Polym Res 22:198
Yuan Q, Awate S, Misra RDK (2006) Nonisothermal crystallization behavior of polypropylene–clay nanocomposites. Eur Polym J 42:1994–2003
LiuWJ YHL, WangZ DLSLJJ (2002) Effect of nucleating agents on the crystallization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J Appl Polym Sci 86:2145–2152
Ziabicki A (1974) Theoretical analysis of oriented and non-isothermal crystallization. Colloid Polym Sci 252:433–447
Yu J, He J (2000) Crystallization kinetics of maleic anhydride grafted polypropylene ionomers. Polymer 41:891–898
Avrami M (1940) Kinetics of phase change. II Transformation-Time Relations for Random Distribution of Nuclei. J Chem Phys 8:212–224
Jeziorny A (1978) Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.S.C. Polymer 19:1142–1144
Sathe SN, Rao GSS, Devi S (1994) Grafting of maleic anhydride onto polypropylene: synthesis and characterization. J Appl Polym Sci 53:239–245
Lui H, Wang Q (2000) Solid phase grafting of Hydroxymethyl acrylamide onto polypropylene through Pan milling. J Appl Polym Sci 78:2191–2197
Lorenzo MD, Silvestre C (1999) Non-isothermal crystallization of polymers. Prog Polym Sci 24:917–950
Nandi S, Ghosh AK (2007) Crystallization kinetics of impact modified polypropylene. J Polym Res 14:387–396
Friedman HL (1964) Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic J Polym Sci Polym Symbosia banner 6:183–195
Vyazovkin S, Sbirrazzuoli N (2002) Isoconversional analysis of the non-isothermal crystallization of a polymer melts. Macromol Rapd Commun 23:766–770
Vyazovkin S, Sbirrazzuoli N (2003) Isoconversional analysis of calorimetric data on nonisothermal crystallization of a polymer melt. J Phys Chem B 107:882–888
Vyazovkin S, Dranca I (2006) Isoconversional analysis of combined melt and glass crystallization data. Macromol Chem Phys 207:20–25
Ide F, Hasegawa A (1974) Studies on polymer blend of nylon 6 and polypropylene or nylon 6 and polystyrene using the reaction of polymer. J Appl Polym Sci 18:963–974
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, M.E., Saad, G.R., Eid, A.I. et al. Synthesis and characterization of polypropylene grafted with p- hydroxy-N-phenyl maleimide. J Polym Res 26, 98 (2019). https://doi.org/10.1007/s10965-019-1752-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1752-2