Abstract
In the present study, low density polyethylene (LDPE) has been crosslinked at 170 °C with three different systems by a) using peroxide, b) peroxide and accelerator and c), peroxide, accelerator and sulfur. The effect of chemical crosslinking on LDPE structure has been investigated using torque measurements, Fourier transform infrared spectroscopy (FTIR), wide angle X-ray diffraction (WAXS), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). Therefore, effects of each crosslinking system on the structural and thermal properties of the material in terms of crystallinity, thermal transitions and stability have been discussed. The reversible crosslinking of LDPE allow the recyclability of polyolefins, increasing the thermal properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyolefins are today the most widely produced polymers in the plastic industry [1]. Polyethylene, especially low density polyethylene (LDPE) [2] was found to exhibit a very wide range of properties such as low weight, good mechanical properties, excellent electrical isolation, good resistance to chemicals and easy processing [3]. Because of these properties, it is used as insulator material for several applications [4], such as electrical as coating for wires [5], food industry as packaging films [6], trash bags, and recently in greenhouse films [7]. Because of its nonpolar character, polyethylene is well known to exhibit a very low compatibility and adhesion with several substrates such as metals and polar polymers. The incorporation of polar groups into the polymer chain increases its compatibility and enlarges its possibility of interaction, giving rise to the formation of adhesive bonds with the mentioned substrates, thus allowing interesting applications [8]. In some others, it is essential to modify LDPE to enhance certain properties [9].
Crosslinking method is largely used to modify the polymer properties [10, 11]. Several studies have been conducted on polymer crosslinking with different peroxides and other crosslinking agents such as silanes [12]. In addition to external aspects such as crosslinking temperature and time, some structural characteristics of the polymer strongly influence both, the crosslinking ability and the resulting network [13]. Several ways such as chemical methods, lead to crosslinked polymers. The most common method is to add peroxide as crosslinking agent to the resin. When heated above their decomposition temperatures, peroxides provide free aggressive oxy radicals capable of extracting hydrogen from the polyethylene backbone, thus transferring the free radical site to polyethylene [14–16]. When this occurs, polyethylene chains can crosslink together. A second method of crosslinking polyethylene is through the use of irradiation. In this case, the free radical formed on the polyethylene backbone results from an electron beam irradiation [17–20]. The third methodology to provoke crosslinking involves the grafting of a silane upon polyethylene and, subsequently, the crosslinking reaction through condensation of the silane grafts by moisture [21–23].
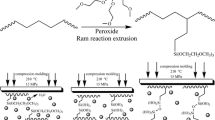
Moreover, it is well known that crosslinking is capable of influencing the mechanical properties of polymers [24]. During the past 20 years, a growing interest has appeared with the application of Diels–Alder (DA) reaction in macromolecular chemistry [25]. Diels–Alder reaction is well-known as [4 + 2] cycloaddition reaction of dienophile and diene giving covalent bonds, which could be easily broken down [26]. In the recent years, these thermally-reversible crosslinked polymers [27–29] are widely studied to explore their applications as encapsulating systems, structural materials and coatings as well as enrich of recyclability. In the last decade Bouhelal et al. [30–32] have developed for the polyolefines materials an original method of reversibly crosslinking reaction (RXR) in melting state using DA reaction. RXR technique for preparing reversibly crosslinked polymers consists in mixing a polymer with crosslinking agents based on organic peroxide, accelerator and sulfur at high temperatures, to form macro-radicals able to hand up crosslinked materials in solid state. The simultaneous coupling of the macro-radicals with sulfur leads to an optimum reversible crosslinking degree of the final product (see Scheme 1) [30]. The main advantage of this crosslinking process is that it can be straightforward applied to all kind of thermoplastics processing techniques (extrusion, injection or calendering) without addition costs. In the present study, RXR technique was used to modify low density polyethylene crosslinked by three different systems. In the first system, LDPE has been vulcanized with peroxide, in the second with peroxide and accelerator, and the third with peroxide, accelerator and sulfur. The main objective of this study is to compare the effects of each crosslinking system on the structural and thermal properties of the final material. Various techniques has been used including torque measurements, Fourier transform infrared spectroscopy (FTIR) and wide angle X-ray diffraction (WAXS). The thermal properties have been also determined using a differential scanning calorimeter (DSC) and a thermogravimetric analyzer (TGA).
Experimental
Sample preparation
Low density polyethylene (LDPE) was supplied by «Exxon Mobil», Saudi Arabia. Peroxide, di(ter-butyl peroxyisopropyl) benzene (DTBPIB), also called Perkadox 14–40-B-gr, was provided by Akzo Nobel Polymer Chemicals B.V. Amersfoort, Netherlands. The accelerator, tetramethylthiuram disulfide (TMTDS), was provided by Rhodia, France. The sulfur was supplied by Wuxi Huasbeng Chemical Additives Factory, China. All the products were used without further purification.
Formulations were prepared using LDPE and various crosslinking agents. The samples were heated and melted in a Brabender at 170 °C during 30 min, then, compressed into a dreher-type Brabender before undergoing the diverse tests. The films were obtained by compression molding using a POLYLAB manual press between hot plates at 230 °C and at a pressure of 1.5 MPa for 5 min. A quench was applied to the films from the melt to room temperature.
Analysis of polymers
For the Dynamic Rheological Analysis, a plastograph-type Brabender was used. The processability of LDPE blends has been evaluated by torque measurements (torque = moment of force) required to mix the molten components in a heated chamber at 170 °C, at a rotor speed of 30 rpm. To clarify the role of each component in the blends, the torque–time evolution was first measured for the neat peroxide, then for the couple peroxide/accelerator and finally for peroxide/sulfur/accelerator [33].
The spectroscopic analyses were conducted in a Perkin-Elmer FTIR device. The morphological structure of the different systems was measured by wide-angle X-ray scattering (WAXS) using a Bruker D8 Advance diffractometer working in parallel beam geometry. By using a Göbel mirror, the originally divergent incident X-ray beam from a line focus X-ray tube (Cu, operating at 40 kV and 40 mA) is transformed into an intense and parallel beam that is free of Kβ radiation. The parallel beam optic required in the secondary beam path is achieved by an equatorial axial Soller slit of 0.2°. The linear detector Lynxeye DE used presents an active area of 14.4 mm × 16 mm. Measurements were performed in reflection (θ-2θ configuration) varying the scattering angle 2θ from 4 to 30° with a step of 0.05°. The measuring time employed was 10 s/point.
The differential scanning calorimetry measurements were performed Thermal analysis was performed in a Q2000 TA instruments. Samples (±10 mg) weighed to 0.002 mg with an electronic autobalance (Perkin-Elmer AD4). The first heating scan was performed at 10 °C/min under dry nitrogen (50 cm3/min) after decreasing the temperature from room temperature to −70 °C, then samples were heated up to 200 °C and maintained at this temperature during 5 min. Consecutive to the heating process, samples were cooled down to 25 °C at 5 °C/min, and after that a new successive heating and cooling runs were performed as previously.
The thermal stability of these samples was analyzed by using a TA Q500 from TA Instruments, calibrate in weight and temperature. Samples were heated from 25 °C to 800 °C and heating rate of 10 °C/min. All the samples were previously dried at 60 °C.
Results and discussion
The formulations were prepared to analyze the effect of the each component on their properties. In Table 1 are collected the amount values of each component used.
Figure 1 represents the variation of torque as a function of time for different formulations. The curve F0 represents unmodified LDPE behavior. An abrupt increase in torque was recorded up to point D, probably due to the high resistance provided by the solid pellets. Beyond this point, the torque begins to decrease, because of the LDPE melting from a minimal value to the point A, which is assigned to the complete melting of LDPE. After the point A, the torque remains constant before the appearance of an equilibrium stage C, which indicates the final viscosity of the polymer [33]. The other curves show the different behavior of crosslinking agents used to modify LDPE. The torque was observed to decrease to achieve the minimum value TA (point A) at which the phase change from solid to melt state occurs, followed by an increase of torque up to a maximum value TB (point B), which means that the maximum crosslinking reaction is taking place. Then, a partial reduction of torque is observed related to a partial destruction of network formed, until a stage appears for torque with a constant plateau value TC (value at the point C), which is higher than TA. The TB and TC values depend on working conditions and crosslinking agents. The principle of the reaction consists in creating macro-radicals through peroxide and accelerator decomposition process, then stabilizing them by active sulfur groups. In the first system (in presence of peroxide), the torque is observed to be higher compared to the other systems. Besides, TB is constant until TC according to their curves. The second system (with peroxide and accelerator) is lower than the third system (with peroxide, accelerator and sulfur), which presents an intermediate stage between the previous systems. The same behavior is observed in each series at all crosslinking agent contents (see Table 2). In addition, in all the cases the torque increases as amount of crosslinking agent does.
To evidence the reversibility, films were performed by compression molding between hot plates at 230 °C and at a pressure of 1.5 MPa during 5 min. In the first system series, in all the contents used, the samples did not melt when heated to prepare films but they decomposed. In Fig. 2, it is clearly observed in the picture that the sample F4 film degrades. Consequently, this circumstance indicates that the crosslinking is traditional. On the contrary, second and third sample series are able to form films, as can be also seen in Fig. 2 for samples F8 and F12. Therefore, they can be reprocessed, which demonstrates their reversibility. From these results, the following characterizations were carried out only in the samples of second and third systems, which are able to be molten again and form films.
To confirm the torque measurements and then, the crosslinking process, the gel content of these samples was determined according to standard ASTM D2765–01 [34] in refluxing xylene for 12 h. The obtained values are also collected in Table 2 and show that the use of sulfur induces higher crosslinking and it increases with the content of crosslinking agent.
Figure 3 displays the FTIR spectra of different samples performed with the second crosslinking system. The observed characteristics are similar in all the systems used. Absorbance bands appear near 2900 and 1470 cm−1 due to C-H stretching and CH2 deformation vibration, respectively. A specific strong sharp doublet in the 730–700 cm−1 region indicates CH2 rocking motion. This doublet band appears only when crystalline ethylene units are present in the blend, while single band is observed for amorphous polyethylene systems [35]. A clear difference is observed in intensity and in shape between the LDPE spectrum and the spectra of modified LDPE materials. The band observed at ca. 3400 cm−1, assigned to the -OH stretching frequency, increases as a function of the crosslinking agent content, being more noticeable in the case of modification with peroxide and accelerator than with peroxide, accelerator and sulfur.
In this third system, new characteristic weak bands of S-S bonding of disulphide around 500 cm−1 and C-S at 1150 cm−1 appear (see Fig. 4), which do not appear in the second system. New weak bands are also observed in both systems at 875 cm−1 due to the swinging of N-H, emerged from the reaction with the accelerator. A trans-vinylene absorption band is observed at 965 cm−1, and 1110 cm−1 assigned to C = S stretch. The bands at 475 cm−1 corresponds to the S-S stretch vibration in aryl-S-S-aryl, respectively, which varies proportionally with the content of crosslinking agent.
Figure 5 shows the WAXS patterns of LDPE blend and representative crosslinked LDPE samples for both systems. Three main peaks can be observed: two sharp peaks at ca. 21.5° and 23.8°, and a broad halo. The two sharp peaks, assigned to (110) and (200) crystalline reflection peaks of the polyethylene orthorhombic crystal, slightly decrease as crosslinking agent content increases. Then, the broad halo, attributed to the amorphous part of the polymer [36], increases with the increment of crosslinking agent used. From the WAXS profiles, the degree of crystallinity (χc) can be measured using the following equation (Eq. 1) [37, 38]:
where Ic and Ia are the integrated intensities corresponding to the crystalline and amorphous phases, respectively. Deconvolutions of profiles were performed to integrate the different peaks.
The crystallite dimension, Lc WAXS, of unmodified and LDPE crosslinked samples are calculated by Scherrer equation (Eq. 2) [39]:
λ is the wavelength of X-ray (λ = 0.154 nm), β is the full width at half maximum (FWHM) intensity in diffraction profile, and θ is Bragg’s angle. The obtained crystallite size and intensity are collected in Table 3. It is clearly observed that the degree of crystallinity is lower in the case of second system and decreases as the crosslinking agent content increases. Similar trend is found in the case of crystallite size, which is due to the restricted mobility of the polyethylene chains caused by the presence of crosslinked points [36].
Figure 6 displays the DSC thermographs of unmodified and LDPE crosslinked samples, and the results are collected in Table 4. The temperatures of melting or crystallization seem to be relatively maintained with the crosslinking in comparison with the unmodified LDPE. However, the enthalpic energy decreases with the addition of crosslinking agent [24]. This effect is more pronounced in the case of introducing sulfur in the crosslinking reaction.
Additionally, the crystallinity of these samples was calculated using the melting enthalpies according to the following equation (Eq. 3) [24]:
where ∆Hm is the melting enthalpy obtained from the DSC melting endotherms and ∆H0 m = 293 J/g is the melting enthalpy of 100 % crystalline polyethylene at the equilibrium melting point, T0 m = 418.5 K.
The crystallite sizes can be also calculated using the melting temperature by means of Thomson-Gibbs’ equation[40] (Eq. 4) as follows:
where σ = 93 mJ/m is the fold surface free energy and ρc = 1 g/cm3 is the crystal phase density [27]. The crosslinking introduces an increment in the crystalline degree in comparison with the neat LDPE. However, this crystalline degree decreases as the crosslinking proportion increases, especially in the modified samples with the incorporation of sulfur into the process. Moreover, the crystal size does not significantly change with the increment of crosslinking agent although a slightly diminishment is noticeable. This variation is more evident in the cooling process where the temperature is shifted to lower temperatures. Network sites in the crosslinked polyethylene disturb the chain motion of the macromolecules, preventing polyethylene chains from forming regular sized spherulites during crystallization [36]. In the case of sulfur series, these variations are larger than that of the second system. It is important to mention that obtained values are in concordance with those previously estimated, although DSC data are slightly higher than those estimated with WAXS analysis.
Having this in mind, TGA analysis was conducted on the samples of LDPE in two crosslinking systems to evaluate the thermal stability of the materials. Figure 7 shows the TGA curves, from which, a slight difference is observed between the unmodified and crosslinked LDPE in the second system. However, a significant difference is observed between the unmodified and crosslinked LDPE in the third system. The latter exhibiting a high thermal stability. The curves are almost similar for all the samples, though the decomposition of the crosslinked samples occurred at higher temperatures than for neat LDPE, as can be seen in Fig. 7. This increase may be explained by the presence of sulfur in the third system, contrary to the second system.
The differential thermogravimetry (DTG) curves, also presented in Fig. 7, show a single decomposition process and the temperatures involving the maximal decomposition velocity for each material. A slight difference is also observed between the pure LDPE and crosslinked LDPE in the second system. However, a significant difference in decomposition process is clearly appreciated between the pure and the crosslinked LDPE in the third system. Table 4 also gathered the values of TOnset, Tmax, and -dw/dt. In general, all the samples enhance the thermal stability with the crosslinking process being more pronounced in those with sulfur component. The variation of initial temperature of degradation as a function of crosslinking agent content is displayed for clarity in Fig. 8. Here it is easily noticeable the drastic increase of stability of LDPE treated with sulfur.
Conclusions
Reversible crosslinked LDPE with different degree of crosslinking were prepared by RXR technique using low peroxide contents and accelerator with or without sulfur addition. The structure of LDPE was modified in terms of crystalline degree and crystal size as well as the thermal properties, principally, the thermal stability. These effects are remarkably superior in LDPEs prepared with the incorporation of sulfur in the formulation. From this new preparation method and looking TC values, it can be clearly stablished that the second and third systems offer the possibility to control the viscosity with higher values, as demonstrated by the torque values, being of great interest from the industrial point of view. Therefore, these crosslinked polymers can be used in the preparation of recyclable materials.
References
Chum PS, Swogger KW (2008) Olefin polymer technologies—History and recent progress at The Dow Chemical Company. Prog Polym Sci 33(8):797–819. doi:10.1016/j.progpolymsci.2008.05.003
Moez AA, Aly SS, Elshaer YH (2012) Effect of gamma radiation on low density polyethylene (LDPE) films: Optical, dielectric and FTIR studies. Spectrochim Acta, Part A 93:203–207. doi:10.1016/j.saa.2012.02.031
Saki TA (2015) Reactive melt blending of low-density polyethylene with poly (acrylic acid). Arab J Chem 8(2):191–199. doi:10.1016/j.arabjc.2011.05.021
Basfar AA (2002) Flammability of radiation cross-linked low density polyethylene as an insulating material for wire and cable. Radiat Phys Chem 63(3–6):505–508. doi:10.1016/S0969-806X(01)00545-X
Jana RN, Nando GB (2003) Thermogravimetric analysis of blends of low-density polyethylene and poly(dimethyl siloxane) rubber: the effects of compatibilizers. J Appl Polym Sci 90(3):635–642. doi:10.1002/app.12599
AlMaadeed MA, Nógellová Z, Janigová I, Krupa I (2014) Improved mechanical properties of recycled linear low-density polyethylene composites filled with date palm wood powder. Mater Des 58:209–216. doi:10.1016/j.matdes.2014.01.051
Basfar AA, Idriss Ali KM, Mofti SM (2003) UV stability and radiation-crosslinking of linear low density polyethylene and low density polyethylene for greenhouse applications. Polym Degrad Stab 82(2):229–234. doi:10.1016/S0141-3910(03)00216-7
Priola A, Bongiovanni R, Gozzelino G (1994) Solvent influence on the radical grafting of maleic anhydride on low density polyethylene. Eur Polym J 30(9):1047–1050. doi:10.1016/0014-3057(94)90197-X
Smedberg A, Hjertberg T, Gustafsson B (1997) Crosslinking reactions in an unsaturated low density polyethylene. Polymer 38(16):4127–4138. doi:10.1016/S0032-3861(96)00994-9
Krupa I, Luyt AS (2000) Thermal properties of uncross-linked and cross-linked LLDPE/wax blends. Polym Degrad Stab 70(1):111–117. doi:10.1016/S0141-3910(00)00097-5
Khonakdar HA, Jafari SH, Wagenknecht U, Jehnichen D (2006) Effect of electron-irradiation on cross-link density and crystalline structure of low- and high-density polyethylene. Radiat Phys Chem 75(1):78–86. doi:10.1016/j.radphyschem.2005.05.014
Kang T-K, Ha C-S (2000) Effect of processing variables on the crosslinking of HDPE by peroxide. Polym Test 19(7):773–783. doi:10.1016/S0142-9418(99)00048-3
Andersson LHU, Hjertberg T (2006) The effect of different structure parameters on the crosslinking behaviour and network performance of LDPE. Polymer 47(1):200–210. doi:10.1016/j.polymer.2005.11.023
Anbarasan R, Babot O, Maillard B (2004) Crosslinking of high-density polyethylene in the presence of organic peroxides. J Appl Polym Sci 93(1):75–81. doi:10.1002/app.20390
Kim KJ, Ok YS, Kim BK (1992) Crosslinking of polyethylene with peroxide and multifunctional monomers during extrusion. Eur Polym J 28(12):1487–1491. doi:10.1016/0014-3057(92)90140-W
Suyama S, Ishigaki H, Watanabe Y, Nakamura T (1995) Crosslinking of polyethylene by dicumyl peroxide in the presence of 2,4-diphenyl-4-methyl-1-pentene. Polym J 27(4):371–375. doi:10.1295/polymj.27.371
Qu B, Rårby B (1995) Radiation crosslinking of polyethylene with electron beam at different temperatures. Polym Eng Sci 35(14):1161–1166. doi:10.1002/pen.760351406
Tretinnikov ON, Ogata S, Ikada Y (1998) Surface crosslinking of polyethylene by electron beam irradiation in air. Polymer 39(24):6115–6120. doi:10.1016/S0032-3861(98)00075-5
Salehi SMA, Mirjalili G, Amrollahi J (2004) Effects of high-energy electron beam on low-density polyethylene materials containing EVA. J Appl Polym Sci 92(2):1049–1052. doi:10.1002/app.20079
Gheysari D, Behjat A (2001) Radiation crosslinking of LDPE and HDPE with 5 and 10 MeV electron beams. Eur Polym J 37(10):2011–2016. doi:10.1016/S0014-3057(01)00084-2
Kuan H-C, Kuan J-F, Ma C-CM, Huang J-M (2005) Thermal and mechanical properties of silane-grafted water crosslinked polyethylene. J Appl Polym Sci 96(6):2383–2391. doi:10.1002/app.21694
Sirisinha K, Chuaythong P (2014) Reprocessable silane-crosslinked polyethylene: property and utilization as toughness enhancer for high-density polyethylene. J Mater Sci 49(14):5182–5189. doi:10.1007/s10853-014-8226-z
Shah GB, Fuzail M, Anwar J (2004) Aspects of the crosslinking of polyethylene with vinyl silane. J Appl Polym Sci 92(6):3796–3803. doi:10.1002/app.20381
Hlangothi SP, Krupa I, Djoković V, Luyt AS (2003) Thermal and mechanical properties of cross-linked and uncross-linked linear low-density polyethylene–wax blends. Polym Degrad Stab 79(1):53–59. doi:10.1016/S0141-3910(02)00238-0
Magana S, Zerroukhi A, Jegat C, Mignard N (2010) Thermally reversible crosslinked polyethylene using Diels–Alder reaction in molten state. React Funct Polym 70(7):442–448. doi:10.1016/j.reactfunctpolym.2010.04.007
Teramoto N, Arai Y, Shibata M (2006) Thermo-reversible Diels–Alder polymerization of difurfurylidene trehalose and bismaleimides. Carbohydr Polym 64(1):78–84. doi:10.1016/j.carbpol.2005.10.029
Liu YL, Hsieh CY, Chen YW (2006) Thermally reversible cross-linked polyamides and thermo-responsive gels by means of Diels-Alder reaction. Polymer 47(8):2581–2586. doi:10.1016/j.polymer.2006.02.057
Defize T, Thomassin J-M, Alexandre M, Gilbert B, Riva R, Jérôme C (2016) Comprehensive study of the thermo-reversibility of Diels–Alder based PCL polymer networks. Polymer 84:234–242. doi:10.1016/j.polymer.2015.11.055
Yoshie N, Saito S, Oya N (2011) A thermally-stable self-mending polymer networked by Diels–Alder cycloaddition. Polymer 52(26):6074–6079. doi:10.1016/j.polymer.2011.11.007
Bouhelal S, Lewis KW (2009) Method to make reversibly cross-linked isotactic polypropylene. US7517942B1. US7517942B1
Khellaf S, Khoffi F, Tabet H, Lallam A, Bouhelal S, Cagiao ME, Benachour D, BaltáCalleja FJ (2012) Study of iPP crosslinking by means of dynamic and steady rheology measurements. J Appl Polym Sci 124(4):3184–3191. doi:10.1002/app.34996
Bouhelal S, Cagiao ME, Khellaf S, Benachour D, Baltá Calleja FJ (2008) Structure and properties of new reversibly crosslinked iPP/LDPE blends. J Appl Polym Sci 109(2):795–804. doi:10.1002/app.28194
Bouhelal S, Cagiao ME, Benachour D, Calleja FJB (2007) Structure modification of isotactic polypropylene through chemical crosslinking: toughening mechanism. J Appl Polym Sci 103(5):2968–2976. doi:10.1002/app.25406
ASTM D2765-01 (2001) Standard test methods for determination of gel content and swell ratio of crosslinked ethylene plastics. ASTM International, West Conshohocken, http://www.astm.org
The infrared spectra atlas of monomers and polymers (1983) Saddler Research Laboratories. Lewiston, NY
Yu S, Park C, Hong SM, Koo CM (2014) Thermal conduction behaviors of chemically cross-linked high-density polyethylenes. Thermochim Acta 583:67–71. doi:10.1016/j.tca.2014.03.018
Mishra JK, Raychowdhury S, Das CK (2000) Effect of interchain crosslinking on the shrinkability of the blends consisting of grafted low-density polyethylene and carboxylated nitrile rubber. Mater Lett 46(4):212–218. doi:10.1016/S0167-577X(00)00172-5
Matthews JL, Peiser HS, Richards RB (1949) The X-ray measurement of the amorphous content of polythene samples. Acta Crystallogr 2(2):85–90. doi:10.1107/S0365110X49000199
Patterson AL (1939) The scherrer formula for X-Ray Particle Size determination. Phys Rev 56(10):978–982
Acknowledgments
This work has been supported by MINECO (MAT2013-47902-C2-1-R).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saci, H., Bouhelal, S., Bouzarafa, B. et al. Reversible crosslinked low density polyethylenes: structure and thermal properties. J Polym Res 23, 68 (2016). https://doi.org/10.1007/s10965-016-0965-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-0965-x