Abstract
A new type of π-conjugated polymers containing 2-diisopropylamino-1,3,5-triazine were prepared via Sonogashira or Suzuki coupling reaction. The structures of the polymers were performed by FT-IR, 1H-NMR, UV–vis spectroscopy, photoluminescence spectroscopy, gel permeation chromatography, thermal analysis and X-ray diffraction analysis. These derived polymers were soluble in common organic solvents such as tetrahydrofuran, chloroform, toluene, and showed good thermal stability. Polymers containing 1,4-diethynyl-2,5-bis(dialkoxy)benzene unit in polymer main chain emitted blue-green light in solution phase under UV light irradiation except with polymer containing 9,9-dioctylfluorene(blue light). Acidochromic behaviors of polymers were studied in CHCl3-CF3COOH mixtures. 9,9-Dioctylfluorene-containing polymer displayed better acidochromic property and linear relationship between absorbance and concentration with the concentration of CF3COOH from 5.384 × 10−4 to 26.92 × 10−4 mol/L. Electrochemical behaviors of polymers depicted p-doping and some hole-transporting properties. XRD results showed that polymers exhibited certain crystallinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past few decades, π-conjugated polymers have been known as an interesting class of organic materials for optical and electrical applications. A large number of papers have been published on conjugated chains with the linear and rigid structures [1–5]. Although several papers have recently been published on the preparation and properties of the rigid rod conjugated polymers [6–8], more detailed studies seem to be required for revealing their basic properties.
1,3,5-triazine is known as a typical electron-accepting heteroaromatic unit. It has a greater electron affinity (EA) than other typical electron-deficient heteroaromatic compounds, such as pyridine and pyrimidine due to three electron-withdrawing imines in triazine ring. Polymers containing 1,3,5-triazine unit in main chain have also been prepared and their electrical, optical, and thermal properties have been investigated [9]. For instance, the electron-transporting behavior of low-molecular-weight 1,3,5-triazines has been prepared and investigated their utilization in organic light-emitting diodes [10, 11]. In our work on conjugated-systems, we were interested in polymers containing electron-acceptor heterocycles like 2-diisopropyl-amino-1,3,5-triazine. However, examples of such polymers are still limited, and the preparation of new π-conjugated polymers containing 2-diisopropylamino-1,3,5-triazine unit in main chain is anticipated to reveal the utility of the 1,3,5-triazine-based polymers as electronic materials. Protonation of nitrogen-containing heteroaromatic polymers such as poly(vinylpyridine) presents obvious changes in optical properties of the polymer. However, because of low solubility of π-conjugated polymers, examples of such studies on the protonation of π-conjugated polymers are limited [12, 13]. In addition, so far there are no reports about acidochromic behavior of triazine-containing polymer.
In this work, we report the simple routes for synthesis of π-conjugated polymers based on 2-diisopropylamino-1,3,5-triazine unit linked with different aromatic rings and present their optical and electrochemical properties in detail. These polymers containing 2-diisopropylamino-1,3,5-triazine reported in this paper are considered to possess linear molecular structures and expected to show interesting optical and electrical properties [3, 14].
Experimental
Materials
All the chemicals used were of analytical grade. Cynuric chloride, 2,7-dibromo-9H-fluorene, 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 1-bromododecane, 1-bromooctane, CuI, Acetonitrile, tetrahydrofuran (THF), trimethylsilylacetylene(TMSA), hydroquinone, diphenylanthracene, triphenylphosphine (TPP), Pd(OAc)2, n-butyllithium (2.7 M), Alequent336, tricyclohexylphosphane (TCHP) were purchased from ACROS Chemical Co. and used as received. 1,4-diethynyl-2,5-bis(octyl-oxy)benzene (4a) and 1,4-diethynyl-2,5-bis(dodecyloxy)benzene (4b), and 2,2′-(9,9-dioctyl-9H-fluorene-2,7-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) (5) were synthesized according to the papers [15, 16]. Pd(PPh3)2Cl2 was prepared by this method used in this article [17]. Diisopropylamine and tetrahydrofuran (THF) were dried and distilled from metal sodium powder under a nitrogen atmosphere. Other reagents such as methanol, acetone, and chloroform were commercially available and used as received unless otherwise stated.
Characterization and measurements
1H-NMR spectra were taken with a VARIAN INOVA-400 spectrometer (400 MHz). Photoluminescence was recorded on a Shimadzu RF-5301 spectrophotometer. Elemental analytical data was made on a Vario MICRO CHNS elemental analyzer. FT-IR spectra of the polymers were obtained by using a BRUKEREUINOX-55 Fourier transform infrared spectrometer (frequency range 450 ~ 4000 cm−1). UV–vis spectra of the copolymers in film state and solution phase were recorded by using Shimadzu UV-2450 spectrophotometer. The X-ray diffraction studies were performed on a D/Max 2400 X-ray diffractometer by using Cu-Ka radiation source (λ = 0.15418 nm). The scanning rate was 2°/min over a range of 2θ =5 ~ 60°. Molecular weight determinations were made by Waters Alliance GPCV 2000 at a flow rate of 0.94 ml/min in THF using Waters styragel columns with temperature at 35 °C. The thermal stability of the dry sample was determined using Netzsch STA 449C instrument with temperature range of 26 ~ 900 °C at heating rate of 10 °C /min, using a nitrogen purge at a flow rate of 30 ml/min. The cyclic voltammetry (CV) was performed with a CHI660A Electro-chemical Workstation (CH Instruments, China) in a conventional three electrode cell with a counter electrode (Pt) and a reference electrode (Hg/Hg2Cl2) in room temperature.
Mnonomer synthesis
Synthesis of 2-diisopropylamino-4,6-dichlorotriazine (6)
A solution of cynuric chloride (3 g, 2.4 mmol) in acetone (24 mL) was treated with diisopropylamine (1.02 mL) via funnel with slow dropping rate in ice bath and stirred for 3 h at this same condition. The reaction mixture was poured into ice water and formed precipitate. Then, the white precipitate was filtered and stirred in water for 2 h. The product was recovered by filtration and dried in vacuum at 50 °C to afford white solid (2.9 g, 73 %). FT-IR (KBr, cm−1) 2975, 2875, 1575, 1373, 842, 791; 1H-NMR (CDC13) δ: 4.42 (s, 2H), 1.35 (d, 12H). Anal. Calcd for (C9H14Cl2N4) (249.14) : C, 43.39; H, 5.66; Cl, 28.46; N, 22.49. Found: C, 43.71; H, 5.43; Cl, 28.22; N, 22.64.
Polymer synthesis
Synthesis of the polymers containing 1,4-diethynyl-2,5-bis(dialkoxy)benzene ring (P1, P2)
The poly(2,5-didodecyloxy-1,4-diethynylphenylene-alt-2-diisopropylamino-4,6-s-triazine) (P1), poly(2,5-dioctyloxy-1,4-diethynylphenylene-alt-2-diisopropylamino-4,6-s-triazine) (P2) were prepared by modifying the literature method [3] and the procedure for the preparation of P1was given as a typical example:
0.606 mmol of 6, 0.606 mmol of 4a, 29.6 mg of Pd(PPh3)2Cl2, and 29.6 mg of CuI were combined in 30 mL of THF in 100 mL Schlenk-tube and degassed using oil pump then bubbled with N2 for 20 min. 9 mL of diisopropylamine was slowly added via syringe. The reaction mixture was stirred at 55 °C for 24 h. Then, it was poured into 150 mL methanol and stirred overnight, and filtered. The orange yellow precipitate was stirred in acetone overnight and recovered by filtration. After drying overnight at 50 °C in vacuum, P1 was isolated in 75 % yield. GPC analysis (soluble part in THF) gave Mn of 5670, Mw of 6987 and M w /M n = 1.23. FT-IR (KBr, cm−1) 2923, 2851, 2216, 1551, 1498, 1384, 1275, 1033, 856, 806; 1H-NMR(CDC13) δ: 7.06–6.90 (m, 2H), 4.41 (s, 2H), 3.97–3.94 (m, 4H), 1.82–1.24 (m, 36H), 0.86 (t, 6H) . UV–vis (CHC13): λ = 440 nm.
P2: isolated in 81 % yield. GPC analysis (soluble part in THF) gave Mn of 5063, Mw of 5912 and M w /M n = 1.17. FT-IR (KBr, cm−1) 2923, 2851, 2218, 1551, 1501, 1381, 1277, 1027, 860, 812; 1H-NMR (CDC13) δ: 7.07–6.96 (m, 2H), 4.47 (s, 2H), 3.99–3.96 (m, 4H), 1.85–1.26 (m, 52H), 0.87 (t, 6H) . UV–vis (CHC13): λ = 416 nm.
Synthesis of the copolymer containing 9,9-dioctylfluorene ring (P3)
0.366 mmol of 6, 0.366 mmol of 5, 6 mg of tricyclohexylphosphine, 2 mL potassium carbonate solution (2 M) and two drops of Alequent336 were combined in 10 mL of toluene in 100 mL Schlenk-tube and degassed using oil pump then bubbled with N2 for 20 min. 3 mg mmol of Pd(OAc)2 was quickly added into the reaction mixture. The mixture was stirred at 90 ~ 100 °C for 72 h. Then, It was poured into 30 mL water and extracted with toluene. Organic layer was evaporated in reduced pressure and stirred in methanol for 12 h, and filtered. The grey precipitate was stirred in acetone overnight and recovered by filtration. After drying overnight at 50 °C in vacuum, P3 was isolated in 90 % yield. GPC analysis (soluble part in THF) gave Mn of 6901, Mw of 7965 and M w /M n = 1.15. FT-IR (KBr, cm−1) 2927, 2853, 1538, 1501, 1377, 815; 1H-NMR(CDC13) δ: 8.66 -8.59 (m, 2H), 7.96-7.94 (m, 4H), 4.74 (s, 2H), 2.18 (t, 4H), 1.54–0.78 (m, 40H). UV–vis (CHC13): λ = 368 nm.
Results and discussion
Synthesis and characterization of the polymers
The synthetic procedure of the monomers and copolymers is described in Scheme 1.
The monomer 2-diisopropylamino-4,6-dichloro-s-triazine (6) was prepared by diisoprorylamination of cynuric chloride in a high yield (73 %). 1,4-diethynyl-2,5-bis(octyloxy)benzene (4a) and 1,4-diethynyl-2,5-bis(dodecyloxy)benzene(4b) were synthesized successively via alkylation, iodination, trimethylsilylacetylation and detrimethylsilylation steps using hydroquinone as starting compound in good yields as the previously described method. Then, 6 was copolymerized with 4a and 4b in dry THF using Pd(PPh3)2Cl2 and CuI as catalysts via Sonogashira coupling reaction under dry nitrogen atmosphere to prepare polymer P1 and polymer P2, respectively. 2,2′-(9,9-dioctyl-9H-fluorene-2,7-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) (5) was prepared successively via alkylation, lithiation and borylation steps of 2,7-dibromo-9H-fluorene in good yields according to a previously reported method. Then, the polymer P3 was prepared between 6 and 5 via Suzuki coupling reaction using Pd(OAc)2 and tricyclohexylphosphane (TCHP) as catalysts under dry nitrogen atmosphere.
The chemical structures of the monomers and polymers were characterized by FT-IR, 1H-NMR spectroscopy. Figure 1a and b present the 1H-NMR spectrum of P1 and P3 with the assignments of all the protons. As it is shown in Fig. 1a, the peaks corresponding to the aromatic protons are centered at the interval of 7.06–6.90 ppm. The methylidyne protons bonded to nitrogen appear at 4.41 ppm. The protons of alkyl side-chains and the methyl on isopropylamino group are dominating in the region of 0.86–1.82 ppm. The protons of methylene adjacent to oxygen are shifted down field to 3.97-3.94 ppm. In Fig. 1b, the peaks assigned to the aromatic protons are detected at the interval of 7.94–8.66 ppm. The methylidyne protons bonded to nitrogen and methylene protons adjacent to fluorene ring appear at 4.74 ppm and 2.18 ppm, respectively. The peaks corresponding to these protons of alkyl side-chains and the methyl on isopropylamino group are dominating in the region of 0.86–1.82 ppm.
Figure 2 presents the comparison of FT-IR spectra of monomers and polymers. For polymers, the strong peak at 3285 cm−1 corresponding to C ≡ C-H stretching vibration and the weak peak at 2105 cm−1 corresponding to C ≡ C in curve(c) and (d) are absent in curve (e) and (f), but a new strong peak associated to C ≡ C in polymers structure is appeared at 2216 cm−1 in curve (e) and (f) [18]. Comparing to the FT-IR data of monomers in curve(c) and (d), it is very clear that the stretching vibration peak of acetylene group in polymer main chain in curve(c) and (d), which is considered to be affected by the electron-deficient nature of 1,3,5-triazine ring and electron-donor nature of phenyl ring, is red-shifted and stronger. This indicated that the dipoles are instantaneously changed greatly and the stretching vibration of acetylene group is very efficient. The typical stretching vibration of alkyl group is also detected at 2925 cm−1 and 2851 cm−1. The characteristic peak of C-N on diisopropylamino group attached to triazine ring is detected at 1371 cm−1 almost this same position in curve (a), (e), (f) and (g) [19]. The skeleton vibration of the triazine ring detected at 790 cm−1 in curve (a) is shifted to 812 cm−1 in curve (e), (f) and (g). The intensity of peak detected at 1350 cm−1 corresponding to B-O bond in curve (b) is decreased in curve (g) [16]. The medium peak at 842 cm−1 which is associated with stretching vibration of N-Cl doesn’t appear in curve (e), (f) and (g). According to both of 1H-NMR and FT-IR results, theses polymers were successfully prepared.
Optical properties
The optical properties of polymers were measured in CHCl3 solution and in thin film, respectively. The data are summarized in Table 1. Figure 3a depicts the UV–vis spectra of P1, P2 and P3 in CHCl3 solution. All polymers showed a broad absorption maximum in the range of 368–440 nm due to π–π* electron-transition contribution from the expansion of conjugation segments and the electron donor–acceptor effect. P1 and P2 have absorption maximum at 440 nm and 416 nm, which is similar to π-conjugated polymer consisting of 1,4-diethynyl-2,5-dihexadecyloxybenzene unit as reported by Swager et al. [3], in agreement with the extended π-conjugation system along the polymer main chain. In comparison with homopoly(9,9-dialkyl-2,7-fluorene)s as reported [20–25], P3 shows an red-shifted absorption peak at 368 nm due to the contribution from extended π-electron conjugation as well as the donor–acceptor effect. Comparing with P3, P1 and P2 are significantly red-shifted owing to 1,4-diethynyl-2,5-bis(dialkoxy)benzene unit which has the favorable stack of coplanar conjugated main chains. In addition, all polymers presented another absorption maximum in the range of 300 and 330 nm, which may be attributed to the localized π-conjugated system in relation to phenylene-ethynylene or fluorene unit [19].
The polymers’ thin films were prepared by spin coating. Figure 3b shows the UV–vis absorption of polymers in thin film. In comparison with the solution state, all polymers show some red-shifted absorption maximum in thin film. These results may be ascribable to the planarization of the backbones accommodating side-chain packing or the strong interchain interaction of several conjugated chains [26].
The Photoluminescence (PL) properties of polymers in CHCl3 were studied and the data are summarized in Table 1. PL spectra of polymers in solution phase were presented in Fig. 4a. In comparison with P3, which shows blue emission, P1 and P2 exhibit blue-green emission in their solution phase. P1 and P2 show blue-green light with an emission maximum at 478 nm and 474 nm. Much obvious red shift is observed compared to the conjugated polymers of poly(p-(dialkoxylphenylene-ethynylene)s [3]. However, 9,9-dioctylfluoene unit is introduced to replace 1,4-diethynyl-2,5-bis(dialkoxy)benzene unit of P1and P2, a blue-shifted emission maximum at 410 nm (P3) is observed. Obviously, the emission wavelength of the polymers based on 2-diisopropylamino-1,3,5-triazine unit can be tunable through the structural modification of polymer main chain.
The Photoluminescence properties of the polymers in film state were investigated. The emission spectra are shown in Fig. 4b and the data are summarized in Table 1. Comparing with the PL spectrum of polymers in solution, It is very clear that the emission maximums of these polymers are red shifted (1–53 nm). This indicates that the interchain interaction in solid states exist on all polymers.
The relative PL quantum yields of polymers were estimated according to the method described by Davey et al. [4] relative to quinine sulphate (in 1 M H2SO4) for P1, P2 and 9,10-diphenylanthracence (in cyclohexane) for P3 and the data are summarized in Tables 1. Because of strong self-absorption and excimer formation of the polymers, the estimated quantum yields depend on the concentration, solvents and excitation wavelength upon irradiation. All different concentrations of polymers are below 10−5 M. All polymers are found to be emissive with certain quantum yields. In comparison with similar polymers containing pyrimidine unit [27], the quantum yield of polymers are much higher. It is found that 1,4-diethynyl-2,5-bis(dialkoxy)benzene unit is considered to be the best choice to improve the relative PL quantum efficiency for these kinds of polymers containing 2-diisoprpylamino-1,3,5-triazine unit [19].
Acidochromic properties
With the aim of investigating the acidochromic behavior of the polymers, UV–vis spectra of the polymers were measured in CHCl3–CF3COOH mixtures. For P1 and P2, there is no new peaks in longer wavelength except from some absorption changes of original peak (Fig. 5a) or slight changes of original peak’s shifts (Fig. 5b) after small amount of trifluoroacitic acid added each time. However, P3 gives new absorption peak shifted to longer wavelength in CHCl3–CF3COOH solution. These results are attributable to spatial hindrance caused by different linking state of alkoxy groups on phenylene-ethynylene ring and alkyl groups on fluorene ring. Thus, protonation of P1 and P2 containing phenylene-ethynylene unit with alkoxy side chain is harder than P3 due to the spatial hindrance generated by their better coplanar conjugated main chains. Figure 5c depicts the changes in the absorption spectra of P3 with increasing concentration of CF3COOH. From Fig. 5c, addition of CF3COOH (0 ~ 53.84 × 10−4 mol/L) to a CHCl3 solution of P3 (2 × 10−5 mol/L) led to a quick decrease in the intensity of the absorption at 346, 367 nm and, at the same time, an appearance of a new band at 387 nm. It can be seen that P3 displayed linear relationship between absorbance and concentration with the concentration of CF3COOH from 5.384 × 10−4 to 26.92 × 10−4 mol/L. These results suggest that the appearance of new absorption at a longer wavelength in chloroform solution originated from a π–π* transition along the main chain is strongly affected by the protonation of nitrogen in polymer, which causes the expanding of the scope of electron delocalization and enhances of conjugation between 1,3,5-triazine ring and fluorene ring [17, 28].
Changes in the UV–vis spectra of polymers (2 × 10−5 mol/L) in CHCl3-CF3COOH solution. concentration of CF3COOH/(10−4 mol /L); (a) for P1: (1) 0, (2) 1.346, (3) 2.692, (4) 4.038, (5) 5.384, (6) 13.46, (7) 26.92, (8) 40.38, (9) 53.84; (b) for P2: (1) 0, (2) 1.346, (3) 2.692, (4) 4.038, (5) 5.384, (6) 13.46, (7) 26.92, (8) 40.38, (9) 53.84, (10) 132.6, (11) 269.2, (12) 403.8, (13) 538.4; (c) for P3: (1) 0, (2) 1.346, (3) 2.692, (4) 4.038, (5) 5.384, (6) 13.46, (7) 26.92, (8) 40.38, (9) 53.84
In order to approve the speculation for protonation of polymers, molecular models for dimers of all polymers were carried out using Gaussian 09 series of programs with the B3LYP Hartree-Fock and 6-31G(d) basis set (white, gray, red and blue circles are corresponding to hydrogen, carbon, oxygen and nitrogen atoms in molecular models, respectively.). From Fig. 6, it can be seen that 2-diisopropylamino-s-triazine unit in P1 and P2 is fully surrounded by four alkoxy side chains on coplanar conjugated phenylene-ethynylene ring. Comparing to P1 and P2, 2-diisopropylamino-s-triazine unit in P3 containing fluorene ring with alkyl side chains is semi-surrounded by two alkyl side chains which can cause easily protonation of P3 due to less spatial hindrance.
XRD analysis
XRD patterns of polymers are shown in Fig. 7. As shown in Fig. 7, all polymers presented peaks about 18.1° ~ 24.1°. In comparison with P1, the intensity of peaks of P2 is increased. This indicates that tends of crystallinity and the side-to-side distance between the molecular interlayer are increased when the longer alkoxy side chain were introduced into the phenylene ring on the 2- and 5-positions. When a face-to-face stacked assembly is formed by flat plane of the π-conjugated polymers, it usually gives XRD peak corresponding to an interplane distance at about 3.7 Å [29]. Thus, the peaks of P1 and P2 at about 24° should be assigned to a face-to-face packing distance between the interlayer which has a packing-dimension of about 3.7 Å. For P3, it is very clear that two peaks are observed at 18.13° and 20.10°, which are assigned to the main chain inter-molecular distance about 4.9 Å [30]. These XRD patterns results are consistent with thin film UV–vis spectra results that P1 and P2 containing coplanar 1,4-diethynyl-2,5-bis(dialkoxy)benzene unit displayed more conjugation degree than P3.
Thermal properties
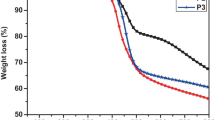
The thermal stabilities of polymers were determined using thermogravimetric analysis (TG) under nitrogen atmosphere. In Fig. 8, these polymers depict very good thermal stability with exhibiting onset decomposition temperature greater than 240 °C under nitrogen atmosphere. For these polymers, the remaining weights at 750 °C are about 36.8, 42.4 and 48.4 %, respectively. In comparison with P1 and P2, P3 exhibited more stability that the onset decomposition temperature is at 370 °C. It indicates that three target polymers depict good thermal stability due to the conjugated backbone containing symmetric 1,3,5-triazine unit [31]. The high decomposition temperature is essential element for many applications in optoelectronic materials.
Molecular weights analysis
Molecular weights were determined by Waters Alliance GPCV 2000 at a flow rate of 0.94 ml/min in THF Waters styragel columns and shown in Tables 2. It is very clear that suitable long-chain alkoxy or alkyl group could improve monomer’s solubility in common organic solvents. In solution phase reaction, molecular weight of polymer is increased by polycondensation of more units which have good solubility in reaction solvents owing to the side chain on monomers. However, steric hindrance originated from the longer side chain could decrease efficient polycondensation rates of monomers and give lower molecular weight [19]. Comparing with P2, P1 and P3 possesses higher molecular weights due to suitable longer alkoxy or alkyl side chain on monomers.
Electrochemical properties
The electrochemical properties of polymers were investigated in an electrolyte consisting of a 0.1 M Tetrabutylammoniumhexafluorophosphate (Bu4NPF6) in acetonitrile by cyclic voltammetry. These electrochemical data of the polymers obtained from their cyclic voltammetry are summarized in Table 2. Although all polymers are consisted of 2-diisopropylamino-1,3,5-triazine unit but no obvious peaks related to s-triazine ring are observed in anodic region during cyclic voltammetry measurements. As shown in Fig. 9 that these derived polymers are only electroactive in anodic region and p-doping process is sensitive to the substitution on the phenylene-ethynylene unit. For P1, there are no peaks in the interval of 0 ~ 2.5 V, Which reveals higher stability in oxidative region. Comparing with P3, P2 is easily p-dopable due to the coplanar 1,4-diethynyl-2,5-bis(dialkoxy)benzene unit in polymer main chain [19].
Conclusions
In summary, novel π-conjugated polymers containing 2-diisopropylamino-1,3,5-triazine unit were synthesized by Sonagashira or Suzuki coupling reaction. These polymers depicted good solubility in common organic solvents such as chloroform, THF, and toluene. The investigation showed that polymers exhibited good thermal stability with high decomposition temperature. From the optical measurement, it was found that these polymers showed the tunable emission spectra and exhibited the satisfying quantum yields through the structural modification of the main chain. Comparing to polymers containing 1,4-diethynyl-2,5-bis(dialkoxy)benzene unit in main chain, 9,9-dioctylfluorene-containing polymer displayed better acidochromic property and linear relationship between absorbance and concentration with the concentration of CF3COOH from 5.384 × 10−4 to 26.92 × 10−4 mol/L. The electrochemical behavior of the polymers depicted p-doping and certain hole-transporting properties.
References
Moroni M, Le Moigne J, Luzzati S (1994) Rigid rod conjugated polymers for nonlinear optics: 1.Characterization and linear optical properties of poly(aryleneethynylene) derivatives. Macromolecules 27:562–571
Moroni M, Le Moigne J, Pham T et al (1997) Rigid rod conjugated polymers for nonlinear optics. 3. Intramolecular H bond effects on poly(phenyleneethynylene) chains. Macromolecules 30:1964–1972
Swager TM, Gil CJ, Wrighton MS (1995) Fluorescence studies of poly(p-phenyleneethynylene)s: The effect of anthracene substitution. J Phys Chem 99:4886–4893
Ng S-C, Lu H-F, Chan HS et al (2001) Novel efficient blue fluorescent polymers comprising alternating phenylene pyridine repeat units: Their syntheses, characterization, and optical properties. Macromolecules 34:6895–6903
Detert H, Stalmach U, Sugiono E (2004) Acidochromism of the luminescence of bis (4-pyridylethenyl) arenes. Synth Met 147:227–231
Hennebicq E, Pourtois G, Scholes GD et al (2005) Exciton migration in rigid-rod conjugated polymers: an improved förster model. J Am Chem Soc 127:4744–4762
Tanase T, Goto E, Begum RA et al (2004) Reactive rigid-rod organometallic polymers involving linear triplatinum units connected by π-conjugated bisisocyanides. Organometallics 23:5975–5988
Leoni P, Marchetti L, Mohapatra SK et al (2006) Covalent rigid-rod organometallic polymers with alternating transition metal clusters and conjugated spacers in the main chain. Organometallics 25:4226–4230
Chérioux F, Audebert P, Maillotte H et al (1999) Synthesis and characterisation of an octupolar polymer and new molecular octupoles with off-resonant third order optical nonlinearities. Chem Commun 2083–2084
Inomata H, Goushi K, Masuko T et al (2004) High-efficiency organic electrophosphorescent diodes using 1, 3, 5-triazine electron transport materials. Chem Mater 16:1285–1291
Kim SW, Shim SC, Jung B-J et al (2002) Synthesis and properties of new electroluminescent polymers possessing both hole and electron-transporting units in the main chain. Polymer 43:4297–4305
Shetty AS, Liu EB, Lachicotte RJ et al (1999) X-ray crystal structures and photophysical properties of new conjugated oligoquinolines. Chem Mater 11:2292–2295
Yamamoto T, Sugiyama K, Kanbara T et al (1998) Preparation and properties of π-conjugated poly(benzimidazole-4,7-diyl)s. Macromol Chem Phys 199:1807–1813
Watanabe J, Harkness BR, Sone M et al (1994) Rigid-rod polyesters with flexible side chains. 4. Thermotropic behavior and phase structures in polyesters based on 1,4-dialkyl esters of pyromellitic acid and 4,4′-biphenol. Macromolecules 27:507–512
Li H, Powell DR, Hayashi RK et al (1998) Poly ((2,5-dialkoxy-p-phenylene) ethynylene-p-phenyleneethynylene)s and their model compounds. Macromolecules 31:52–58
Ranger M, Rondeau D, Leclerc M (1997) New well-defined poly(2,7-fluorene) derivatives: Photoluminescence and base doping. Macromolecules 30:7686–7691
Yamamoto T, Z-h Z, Kanbara T et al (1996) π-conjugated donor-acceptor copolymers constituted of π-excessive and π-deficient arylene units. Optical and electrochemical properties in relation to ct structure of the polymer. J Am Chem Soc 118:10389–10399
Yamamoto T, Nurulla I, Hayashi H et al (1999) π-conjugated polymers containing thiophene-1,1-dioxide-2, 5-diyl unit in the main chain. Synth Met 107:137–141
Zou L, Fu Y, Yan X et al (2008) Linear π-conjugated polymers containing 2,4,6-tris(thiophen-2-yl)-1,3,5-triazine unit: Synthesis and optical properties. J Polym Sci A Polym Chem 46:702–712
Wu Z, Xiong Y, Zou J et al (2008) High-triplet-energy poly(9,9′-bis(2-ethylhexyl)-3,6- fluorene) as host for blue and green phosphorescent complexes. Adv Mater 20:2359–2364
Chou CH, Hsu SL, Dinakaran K et al (2005) Synthesis and characterization of luminescent polyfluorenes incorporating side-chain-tethered polyhedral oligomeric silsesquioxane units. Macromolecules 38:745–751
Zhang B, Qin C, Ding J et al (2010) High-performance all-polymer white-light-emitting diodes using polyfluorene containing phosphonate groups as an efficient electron-injection layer. Adv Funct Mater 20:2951–2957
Zhong C, Liu S, Huang F et al (2011) Highly efficient electron injection from indium tin oxide/cross-linkable amino-functionalized polyfluorene interface in inverted organic light emitting devices. Chem Mater 23:4870–4876
Fischer CS, Baier MC, Mecking S (2013) Enhanced brightness emission-tuned nanoparticles from heterodifunctional polyfluorene building blocks. J Am Chem Soc 135:1148–1154
Knaapila M, Monkman AP (2013) Methods for controlling structure and photophysical properties in polyfluorene solutions and gels. Adv Mater 25:1090–1108
Ding L, Lu Z, Egbe DA et al (2004) Structure-morphology-electroluminescence relationship for hybrid conjugated polymers. Macromolecules 37:10031–10035
Mamtimin X, Matsidik R, Nurulla I (2010) New soluble rigid rod copolymers comprising alternating 2-amino-pyrimidine and phenylene repeat units: Syntheses, characterization, optical and electrochemical properties. Polymer 51:437–446
Mamtimin X, Tuerxun T, Aikebaierjiang A et al (2010) Synthesis, characterization, and protonation of poly (2-N, N-dimethylamino-4, 6-bis (2-furan)-pyrimidine). Fibers Polym 11:158–163
Michinobu T, Okoshi K, Osako H et al (2008) Band-gap tuning of carbazole-containing donor–acceptor type conjugated polymers by acceptor moieties and π-spacer groups. Polymer 49:192–199
Wen P, Kim Y, Chun H et al (2013) Syntheses and characterizations of cardo polyimides based on new spirobifluorene diamine monomer. Mater Chem Phys 139:923–930
Yang K, Xu M-J, Li B (2013) Synthesis of n-ethyl triazine–piperazine copolymer and flame retardancy and water resistance of intumescent flame retardant polypropylene. Polym Degrad Stab 98:1397–1406
Acknowledgments
The authors gratefully acknowledge the support from Natural Science Foundation of Xinjiang University and National Natural Science Foundation of China (No. BS150233, 21164012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdurashid, A., Ahat, B., Mirzehmet, A. et al. New soluble π-conjugated polymers containing 2-diisopropylamino-1,3,5-triazine unit: synthesis, characterization and optical properties. J Polym Res 23, 92 (2016). https://doi.org/10.1007/s10965-016-0929-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-0929-1