Abstract
An efficient, one-pot quantitative procedure for the preparation of benzo[a]pyrano[2,3-c]phenazine derivatives from four-component condensation reaction of 2-hydroxynaphthalene-1,4-dione, benzene-1,2-diamine, aromatic aldehydes, and malononitrile in the presence of [(EtO)3Si(CH2)3N+H3][CH3COO−] as basic ionic liquid as catalyst in homogenous solution under solvent-free conditions at 90 °C is described. Simple procedure, high yields, short reaction times, and an environmentally benign method are advantages of this protocol. The [(EtO)3Si(CH2)3N+H3][CH3COO−] can be recovered and reused several times without loss of its activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The main feature of the catalysis science in this century is protection of environment and human health by omission of hazardous materials and use safe non-volatile ionic liquids [1]. Ionic liquids are interesting due to high polarity [2], negligible volatility [3], high thermal stability [4], and tunable structures which have been extensively developed to promote of various organic reactions [5]. The properties of ionic liquids differ by change in their cation/anion parts and despite the influence of the anion constituent on the properties of ionic liquids, their cationic parts are mainly based on guanidine [6], amines [7], and heterocyclic salts of pyridinium [8], thiazolium [9], imidazolium [10], or triazolium rings (Fig. 1) [11]. Bronsted acid and protic Ionic liquids (PILs) are among the best green alternatives for corrosive acid catalysts/solvents in chemical transformations [12].
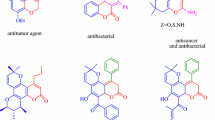
In order to make possible economic savings and pollution prevention, multi-component domino reactions (MDRs), have considerable potential for the synthesis of complex heterocyclic molecules and for drug design and drug discovery, arising from minimization of time, waste, energy, and cost [13]. Phenazine based compounds are nitrogen-containing heterocycles that are present in natural products and synthetic materials showing a variety of biological functions [14], including antibiotic [15], antimicrobial [16], antimalarial [17] and antiparasitic activities [18]. By virtue of their DNA intercalating ability, they exhibit anticancer activity in leukemia and solid tumors [19]. For example, pyridophenazinediones [20] and pyridazinophenazinedione [21] derivatives are known for their antitumor activities (Fig. 2).
In continuation of our research on the synthesis and applications of homogenous and heterogeneous catalysts in organic chemistry [22, 23], here, we use the [(EtO)3Si(CH2)3N+H3][CH3COO−] as basic ionic liquid, and reusable catalyst for the one-pot synthesis of benzo[a]pyrano[2,3-c]phenazine derivatives by four-component condensation of 2-hydroxynaphthalene-1,4-dione, benzene-1,2-diamine, malononitrile and arylaldehydes at 90 °C under solvent-free conditions (Scheme 1).
2 Experimental
2.1 Materials and methods
All reagents were purchased from Merck or Aldrich and used without any further purification. The purities of the starting materials used in the our research were 2-hydroxy-1,4-naphthoquinone (98%), benzene-1,2-diamine (98%), malononitrile (99%), (3-aminopropyl)triethoxysilane (98%), and aromatic aldehydes (98–99%). All yields refer to isolated products after purification. Nuclear magnetic resonance (1HNMR) and Carbon-13 nuclear magnetic resonance (13CNMR) spectra were recorded using a Bruker Advance DPX 300 MHz instrument. The spectra were provided in DMSO-d6 as deuterated solvent. Fourier Transform Infrared (FT-IR) spectra were recorded using a JASCO FT-IR 460 Plus spectrophotometer. Melting points were determined in open capillaries using a BUCHI 510 melting point apparatus. Thin layer chromatography (TLC) was performed on silica‐gel Poly Gram SIL G/UV 254 plates.
2.2 Preparation of [(EtO)3Si(CH2)3N+H3][CH3COO−]
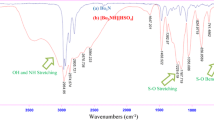
According to the literature [22], APTES ((3-aminopropyl)triethoxysilane) (10 mmol) was placed in a two necked flask equipped with a reflux condenser and a dropping funnel. The flask was placed in an ice bath. Under vigorous stirring with a magnetic stirring bar, acetic acid (10 mmol) was added dropwise to the flask in about 60 min. Stirring was continued for 24 h at room temperature, to obtain a viscous yellow liquid. The ionic liquid was prepared. The stable ionic liquid sample could be stored at room temperature for over 6 month. The ionic liquid was identified by FT-IR, 1HNMR and 13CNMR spectra [22].
2.3 General Procedure for the Direct Synthesis of Benzo[a]pyrano[2,3-c]phenazine (5A–O) Derivatives Using [(EtO)3Si(CH2)3N+H3][CH3COO−] as Basic Ionic Liquid Catalyst
[(EtO)3Si(CH2)3N+H3][CH3COO−] (30 mol%) was added to a mixture of 2-hydroxynaphthalene-1,4-dione (1 mmol) and benzene-1,2-diamine (1 mmol) at 90 °C. After 5 min an orange solid of benzo[a]phenazine was formed. Then, aryl aldehydes (1 mmol), and malononitrile (1 mmol), were added to this obtained mixture. The progress of the reaction was monitored by TLC (petroleum benzene/ethyl acetate: 1/3). After completion of the reaction, ethanol (3 mL) was added to the reaction mixture and warmed mixture. Then, mixture was cooled, the ionic liquid as catalyst is dissolved in ethanol. The ethanol containing the catalyst is separated and then the resulting solid product was subsequently recrystallized from hot ethanol to give the pure solid. The synthesis of all the known products were approved by the comparison of their melting point, FT-IR, 1HNMR and 13CNMR spectra with those of authentic literature samples. The new products were identified by FT-IR, 1HNMR and 13CNMR spectra. The new unknown products are characterized as follows:
2.4 Experimental Characterization Data and Spectra of the New Compounds
3-Amino-1-(2-hydroxy-3-methoxyphenyl)-1H-benzo[a]pyrano[2,3-c]phenazine-2-carbonitrile (5E): Time: 55 min, Yield (%): 89%, M.p. (°C) (Observed): 267–269; IR (KBr) (νmax/cm−1): 3440, 3374, 3316, 3199, 3069, 2169, 1663, 1630, 1594, 1513, 1461, 1386, 1346, 1293, 1248, 1221, 1174, 1159, 1140, 1110, 1052, 953, 855, 826, 794, 771, 749, 735, 724, 656, 640, 621, 583, 554, 527, 476, 448, 413; 1HNMR (300 MHz, DMSO-d6): δH (ppm) 3.69 (3H, s, CH3), 5.69 (1H, s, CH), 6.62 (1H, t, J = 7.2 Hz, Ar–H), 6.69 (1H, d, J = 6.3 Hz, Ar–H), 6.80 (1H, d, J = 6.3 Hz, Ar–H), 7.22 (2H, s, NH2), 7.87–7.98 (4H, m, Ar–H), 8.04–8.07 (1H, m, Ar–H), 8.18–8.21 (1H, m, Ar–H), 8.40 (1H, d, J = 7.8 Hz, Ar–H), 8.88 (1H, s, OH), 9.13 (1H, d, J = 7.5 Hz, Ar–H), 13CNMR (75 MHz, DMSO-d6): δC (ppm) 33.0, 56.0, 57.5, 110.4, 113.8, 119.0, 120.9, 121.9, 122.5, 125.2, 126.1, 128.9, 129.2, 130.1, 130.4, 130.8, 131.1, 131.7, 140.1, 140.3, 141.3, 141.6, 144.5, 147.1, 148.2, 160.6.

3-Amino-1-(2,5-dihydroxyphenyl)-1H-benzo[a]pyrano[2,3-c]phenazine-2-carbonitrile (5H): Time: 95 min, Yield (%): 92%, M.p. (°C) (Observed): > 280; IR (KBr) (νmax/cm−1): 3425, 3338, 3059, 2940, 2821, 2750, 2719, 2598, 2191, 1662, 1631, 1594, 1526, 1484, 1461, 1404, 1348, 1325, 1292, 1222, 1139, 1084, 1052, 1004, 916, 854, 762, 723, 698, 641, 618, 583, 554, 528, 503, 450; 1HNMR (300 MHz, DMSO-d6): δH (ppm) 6.07 (1H, s, CH), 6.37 (1H, s, Ar–H), 7.22 (2H, s, NH2), 7.37 (1H, d, J = 6.6 Hz, Ar–H), 8.16 (3H, s, Ar–H), 8.33 (6H, s, Ar–H), 8.87 (1H, s, OH), 9.08 (1H, s, OH); 13CNMR (75 MHz, DMSO-d6): δC (ppm) 33.0, 57.5, 110.4, 113.8, 119.0, 120.9, 121.9, 122.5, 125.2, 126.1, 128.9, 129.5, 129.2, 130.1, 130.4, 130.8, 131.1, 131.7, 140.1, 140.3, 141.3, 141.6, 144.5, 147.1, 148.2, 160.6.

3-Amino-1-(naphthalen-1-yl)-1H-benzo[a]pyrano[2,3-c]phenazine-2-carbonitrile (5L): Time: 35 min, Yield (%): 95%, M.p. (°C) (Observed): > 280; IR (KBr) (νmax/cm−1): 3465, 3356, 3171, 3087, 3054, 3039, 2184, 1659, 1621, 1590, 1495, 1471, 1398, 1383, 1328, 1291, 1262, 1222, 1162, 1106, 1053, 1022, 950, 860, 779, 754, 667, 637, 610, 545, 449, 424, 404; 1HNMR (300 MHz, DMSO-d6): δH (ppm) 6.33 (1H, s, CH), 7.22–7.30 (2H, t, m, Ar–H), 7.34 (2H, s, NH2), 7.55–7.69 (3H, m, Ar–H), 7.77–7.83 (2H, s, Ar–H), 7.85–7.95 (2H, m, Ar–H), 7.97–8.02 (1H, m, Ar–H), 8.05–8.07 (1H, m, Ar–H), 8.22 (1H, d, J = 8.1 Hz, Ar–H), 8.49 (1H, d, J = 7.2, Ar–H), 8.89 (1H, d, J = 8.84 Hz, Ar–H), 9.22 (1H, d, J = 7.8 Hz, Ar–H); 13CNMR (75 MHz, DMSO-d6): δC (ppm) 35.6, 55.9, 82.8, 112.1, 114.5, 122.5, 122.7, 125.2, 125.4, 126.1, 126.2, 128.9, 129.0, 129.2, 129.5, 130.2, 130.4, 130.9, 131.0, 132.3, 136.1, 140.1, 140.2, 141.4, 142.0, 144.0, 146.6, 148.2, 152.6, 158.1.

2.5 Optimization of the Reaction Conditions for Prepation of Phenazine Derivatives
To evaluate the activity of the [(EtO)3Si(CH2)3N+H3][CH3COO−] as ionic liquid, at first, the one-pot and four-component reaction of 2-hydroxynaphthalene-1,4-dione (1 mmol), benzene-1,2-diamine (1 mmol), benzaldehyde (1 mmol), and malononitrile (1 mmol) as a model reaction was selected. The model was examined in different solvents such as H2O, EtOH, DMF, toluene, and solvent-free under various temperatures conditions in the presence of [(EtO)3Si(CH2)3N+H3][CH3COO−] (30 mol%) as catalyst (Table 1). The best result was obtained at 90 °C under solvent-free conditions (Table 1, Entry 12).
Next, the model reaction was tested at different amount of [(EtO)3Si(CH2)3N+H3][CH3COO−] (10, 20, 30, 40, 50 mol%) at 90 °C under solvent-free conditions. The best results were obtained at 90 °C under solvent-free conditions in the presence of 30 mol% of [(EtO)3Si(CH2)3N+H3][CH3COO−] as catalyst (Fig. 3).
After optimization of the reaction conditions, to investigate the efficiency and the scope of the procedure, numerous benzo[a]pyrano[2,3-c]phenazine derivatives were produced by the one-pot four-component condensation reaction between aromatic aldehydes with 2-hydroxynaphthalene-1,4-dione, benzene-1,2-diamine, and malononitrile using basic ionic liquid [(EtO)3Si(CH2)3N+H3][CH3COO−] at 90 °C under solvent-free reaction conditions. The results are displayed in Table 2. The effect of substituents on the aromatic ring was assessed strong in terms of yields under these reaction conditions. Both categories of aromatic aldehydes counting electron-releasing and electron-withdrawing substituents on their aromatic ring gained the appropriate products in high to excellent yields in short reaction time. The reaction times of aromatic aldehydes having electron withdrawing groups was rather faster than that of electron donating groups.
According to the literature [23,24,25], The proposed mechanism for product formation is shown in Scheme 2. First, benzene-1,2-diamine (1) condenses with 2-hydroxynaphthalene-1,4-dione (2) and provides 6H-benzo[a]phenazin-5-one (A) [26]. The reaction of intermediate (A) with aryl aldehydes (3) in the presence of basic ionic liquids as catalysts under Knoevenagel condensation cause to form intermediate (B), which reacts in the presence of ionic liquids as catalysts with malononitrile (4) to form Michael adduct intermediate (C), then the intermediate (C) undergo the cyclization, and 1,3-hydrogen shift cause to prepare finally the desired products (Scheme 2).
Reusability of the catalysts is important in green environmental chemistry. For this purpose, we studied, the reaction of 2-hydroxynaphthalene-1,4-dione (1 mmol) and benzene-1,2-diamine (1 mmol), benzaldehyde (1 mmol) and malononitrile (1 mmol) as a model under solvent-free conditions at 90 °C. After completion of reactions, ionic liquid as catalyst was soluble in EtOH and could be recovered and reused conveniently after removing ethanol under vacuum at 70 °C for 2 h. The recovered catalyst was reused for at least five runs without any loss of its activity (Fig. 4).
We also compared this method for preparation of benzo[a]pyrano[2,3-c]phenazine derivatives with various catalysts in literature (Table 3). We observed that our reported method, using [(EtO)3Si(CH2)3N+H3][CH3COO−] as the catalyst, is simple, effective, applicable and comparable with many catalytic systems for the synthesis of benzo[a]pyrano[2,3-c]phenazine derivatives (Table 3, Entry 10).
3 Conclusions
The four-component and one-pot synthesis of benzo[a]pyrano[2,3-c]phenazine derivatives was achieved in a simple, and green manner conditions in the presence of ionic liquid, [(EtO)3Si(CH2)3N+H3][CH3COO−], as catalyst. The phenazine derivatives were obtained in excellent yields. Recyclability of ionic liquid, easy work-up, and uses environment-friendly reaction conditions are advantages of this effective MCR protocol.
Data Availability
The data that support the findings of this study are available in the supplementary material of this article.
References
Calvo-Flores, F.G.: Sustainable chemistry metrics. Chemsuschem 2, 905–919 (2009). https://doi.org/10.1002/cssc.200900128
Wakai, C., Oleinikova, A., Weingärtner, H.: Reply to “Comment on ‘how polar are ionic liquids? determination of the static dielectric constant of an imidazolium-based ionic liquid by microwave spectroscopy.’” J. Phys. Chem. B 110, 5824 (2006). https://doi.org/10.1021/jp0601973
Chen, Y., Han, X., Liu, Z., Li, Y., Sun, H., Wang, H., Wang, J.: Thermal decomposition and volatility of ionic liquids: factors, evaluation and strategies. J. Mol. Liq. 366, 120336 (2022). https://doi.org/10.1016/j.molliq.2022.120336
Ghorbani, M., Simone, M.I.: Developing new inexpensive room-temperature ionic liquids with high thermal stability and a greener synthetic profile. ACS Omega 5, 12637–12648 (2020). https://doi.org/10.1021/acsomega.9b04091
Niedermaier, I., Kolbeck, C., Taccardi, N., Schulz, P.S., Li, J., Drewello, T., Wasserscheid, P., Steinrück, H.P., Maier, F.: Organic reactions in ionic liquids studied by in situ XPS. ChemPhysChem 13, 1725–1735 (2012). https://doi.org/10.1002/cphc.201100965
Gao, Y., Arritt, S.W., Twamley, B., Shreeve, J.M.: Guanidinium-based ionic liquids. Inorg. Chem.. Chem. 44, 1704–1712 (2005). https://doi.org/10.1021/ic048513k
Kirchhecker, S., Esposito, D.: Amino acid based ionic liquids: a green and sustainable perspective. Curr. Opin. Green Sustain. Chem. 2, 28–33 (2016). https://doi.org/10.1016/j.cogsc.2016.09.001
Yunus, N.M., Mutalib, M.I.A., Man, Z., Bustam, M.A., Murugesan, T.: Solubility of CO2 in pyridinium based ionic liquids. Chem. Eng. J. 189–190, 94–100 (2012). https://doi.org/10.1016/j.cej.2012.02.033
Chen, X., Liu, G., Yuan, S., Asumana, C., Wang, W., Yu, G.: Extractive desulfurization of fuel oils with thiazolium-based ionic liquids. Sep. Sci. Technol. 47, 819–826 (2012). https://doi.org/10.1080/01496395.2011.637281
Fredlake, C.P., Crosthwaite, J.M., Hert, D.G., Aki, S.N.V.K., Brennecke, J.F.: Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 49, 954–964 (2004). https://doi.org/10.1021/je034261a
Sanghi, S., Willett, E., Versek, C., Tuominen, M., Coughlin, E.B.: Physicochemical properties of 1,2,3-triazolium ionic liquids. RSC Adv. 2, 848–853 (2012). https://doi.org/10.1039/c1ra00286d
Greaves, T.L., Drummond, C.J.: ChemInform abstract: protic ionic liquids: properties and applications. ChemInform 39, 206–237 (2008). https://doi.org/10.1002/chin.200818249
Bienaymé, H., Hulme, C., Oddon, G., Schmitt, P.: Maximizing synthetic efficiency: multi-component transformations lead the way. Chem. A Eur. J. 6, 3321–3329 (2000). https://doi.org/10.1002/1521-3765(20000915)6:18%3c3321::AID-CHEM3321%3e3.0.CO;2-A
Laursen, J.B., Nielsen, J.: Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chem. Rev. 104, 1663–1685 (2004). https://doi.org/10.1021/cr020473j
Borrero, N.V., Bai, F., Perez, C., Duong, B.Q., Rocca, J.R., Jin, S., Huigens, R.W.: Phenazine antibiotic inspired discovery of potent bromophenazine antibacterial agents against Staphylococcus aureus and Staphylococcus epidermidis. Org. Biomol. Chem.Biomol. Chem. 12, 881–886 (2014). https://doi.org/10.1039/c3ob42416b
Hu, L., Chen, X., Han, L., Zhao, L., Miao, C., Huang, X., Chen, Y., Li, P., Li, Y.: Two new phenazine metabolites with antimicrobial activities from soil-derived Streptomyces species. J. Antibiot.Antibiot. 72, 574–577 (2019). https://doi.org/10.1038/s41429-019-0163-2
Verma, K., Tailor, Y.K., Khandelwal, S., Agarwal, M., Rushell, E., Kumari, Y., Awasthi, K., Kumar, M.: An efficient and environmentally sustainable domino protocol for the synthesis of structurally diverse spiroannulated pyrimidophenazines using erbium doped TiO2 nanoparticles as a recyclable and reusable heterogeneous acid catalyst. RSC Adv. 8, 30430–30440 (2018). https://doi.org/10.1039/c8ra04919j
Guttenberger, N., Blankenfeldt, W., Breinbauer, R.: Recent developments in the isolation, biological function, biosynthesis, and synthesis of phenazine natural products. Bioorg. Med. Chem.. Med. Chem. 25, 6149–6166 (2017). https://doi.org/10.1016/j.bmc.2017.01.002
Low, Z.Y., Yip, A.J.W., Lal, S.K.: Repositioning ivermectin for covid-19 treatment: molecular mechanisms of action against SARS-CoV-2 replication. Biochim. Biophys. Acta Mol. Basis Dis. 1868, 166294 (2022). https://doi.org/10.1016/j.bbadis.2021.166294
Rhee, H.K., Yoo, J.H., Lee, E., Kwon, Y.J., Seo, H.R., Lee, Y.S., Choo, H.Y.P.: Synthesis and cytotoxicity of 2-phenylquinazolin-4(3H)-one derivatives. Eur. J. Med. Chem. 46, 3900–3908 (2011). https://doi.org/10.1016/j.ejmech.2011.05.061
Le-Nhat-Thuy, G., Dang Thi, T.A., Nguyen Thi, Q.G., Hoang Thi, P., Nguyen, T.A., Nguyen, H.T., Nguyen Thi, T.H., Nguyen, H.S., Nguyen, T.: Van: synthesis and biological evaluation of novel benzo[a]pyridazino[3,4-c]phenazine derivatives. Bioorg. Med. Chem. Lett.. Med. Chem. Lett. 43, 128054 (2021). https://doi.org/10.1016/j.bmcl.2021.128054
Shirzaei, F., Shaterian, H.R.: [(EtO)3Si(CH2)3NH3+][CH3COO−] as a novel basic ionic liquid catalyzed green synthesis of new 2-(phenylsulfonyl)-1H-benzo[a]pyrano[2,3-c]phenazin-3-amine derivatives. J. Mol. Struct.Struct. 1256, 132558 (2022). https://doi.org/10.1016/j.molstruc.2022.132558
Shirzaei, F., Shaterian, H.R.: Basic ionic liquid, 2-hydroxyethylammonium formate, catalyzed one-pot synthesis of novel 2-(phenylsulfonyl)-1H-benzo[a]pyrano[2,3-c]phenazin-3-amine derivatives. Res. Chem. Intermed.Intermed. 48, 751–770 (2022). https://doi.org/10.1007/s11164-021-04627-z
Shaterian, H.R., Moradi, F., Mohammadnia, M.: Nano copper(II) oxide catalyzed four-component synthesis of functionalized benzo[a]pyrano[2,3-c]phenazine derivatives. Comptes Rendus Chim. 15, 1055–1059 (2012). https://doi.org/10.1016/j.crci.2012.09.012
Shaterian, H.R., Mohammadnia, M.: Mild basic ionic liquid catalyzed four component synthesis of functionalized benzo[a]pyrano[2,3-c]phenazine derivatives. J. Mol. Liq. 177, 162–166 (2013). https://doi.org/10.1016/j.molliq.2012.11.006
Shaabani, A., Ghadari, R., Arabieh, M.: Synthesis of a new library of pyrano-phenazine derivatives via a novel three-component protocol. Helv. Chim. Acta. Chim. Acta 97, 228–236 (2014). https://doi.org/10.1002/hlca.201300006
Tabibian, M., Mohebat, R., Tabatabaee, M.: A novel one-pot and rapid synthesis of polyfunctionalized benzo[a]pyrimido[5′,4′:5,6]pyrido[2,3-c]phenazine derivatives under microwave irradiation. Turkish J. Chem. 42, 1008–1017 (2018). https://doi.org/10.3906/kim-1710-13
Naeimi, H., Zarabi, M.F.: Multisulfonate hyperbranched polyglycerol functionalized graphene oxide as an efficient reusable catalyst for green synthesis of benzo[a]pyrano-[2,3-c]phenazines under solvent-free conditions. RSC Adv. 9, 7400–7410 (2019). https://doi.org/10.1039/C8RA10180A
Hasaninejad, A., Firoozi, S.: One-pot, sequential four-component synthesis of benzo[c]pyrano[3,2-a]phenazine, bis-benzo[c]pyrano[3,2-a]phenazine and oxospiro benzo[c]pyrano[3,2-a]phenazine derivatives using 1,4-diazabicyclo[2.2.2]octane (DABCO) as an efficient and reusable solid bas. Mol. Divers. 17, 499–513 (2013). https://doi.org/10.1007/s11030-013-9446-x
Yazdani-elah-abadi, A., Razeghi, M., Shams, N.: Fulvic acid: an efficient and green catalyst for the one-pot four-component domino synthesis of benzo[a]phenazine annulated heterocycles in aqueous medium. Org. Prep. Proced. Int.Proced. Int. 52, 48–55 (2020). https://doi.org/10.1080/00304948.2019.1697608
Marsh, K.N., Boxall, J.A., Lichtenthaler, R.: Room temperature ionic liquids and their mixtures—a review. Fluid Phase Equilib.Equilib. 219, 93–98 (2004). https://doi.org/10.1016/j.fluid.2004.02.003
Dashteh, M., Safaiee, M., Baghery, S., Zolfigol, M.A.: Application of cobalt phthalocyanine as a nanostructured catalyst in the synthesis of biological henna-based compounds. Appl. Organomet. Chem.Organomet. Chem. 33, 1–14 (2019). https://doi.org/10.1002/aoc.4690
Safaei-Ghomi, J., Kareem Abbas, A., Shahpiri, M.: Synthesis of imidazoles promoted by H3PW12O40-amino-functionalized CdFe12O19@SiO2 nanocomposite. Nanocomposites 6, 149–157 (2020). https://doi.org/10.1080/20550324.2020.1858246
Ghorbani, A., Masoud, C., Lotfi, M.: Synthesis and characterization of spinel FeAl2O4 (hercynite) magnetic nanoparticles and their application in multicomponent reactions. Res. Chem. Intermed.Intermed. 4, 5705–5723 (2019). https://doi.org/10.1007/s11164-019-03930-0
Nikoorazm, M., Khanmoradi, M.: Application of Cu(II)-Guanine complexes anchored on SBA-15 and MCM-41 as efficient nanocatalysts for one-pot, four-component domino synthesis of phenazine derivatives and investigation of their antimicrobial behavior. Catal. Lett.. Lett. 150, 2823–2840 (2020). https://doi.org/10.1007/s10562-020-03185-0
Funding
We are thankful to the University of Sistan and Baluchestan Research Council for the partial support of this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FS, and HRS.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirzaei, F., Shaterian, H.R. [(EtO)3Si(CH2)3N+H3][CH3COO−] as Basic Ionic Liquid Catalyst Promoted Green Synthesis of Benzo[a]pyrano[2,3-c]phenazine Derivatives in Homogenous Solution. J Solution Chem 53, 328–340 (2024). https://doi.org/10.1007/s10953-023-01332-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01332-w