Abstract
The liquid–liquid equilibrium (LLE) data for 1,3-propanediol + 1-nonanol + extractants (1,2-xylene, 1-chlorobutane, isopropyl ether and water) were measured at 298.2 K and 101.3 kPa. The separation efficiency of the extractant for the mixture of 1,3-propanediol and 1-nonanol was evaluated by the distribution constant (D) and selectivity (S). Meanwhile, the empirical equations of Bachman and Othmer-Tobias verified the consistency of the experimental data, and the squares of its linear correlation are all greater than 0.98. The thermodynamic models NRTL and UNIQUAC were used to correlate the experimental data, and different model parameters were obtained by regression. After comparing the calculated values of the model with the experimental data, it is found that the root means square deviation (RMSD%) and absolute mean deviation (AAD%) are less than 0.97% and 0.60% respectively, which indicate that both models have a reasonable correlation with the experimental data of the ternary system and can accurately predict the experimental data. Finally, the graphical user interface (GUI) was used to verify that the regression parameters have a good consistency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polypropylene terephthalate (PTT) is a polyester material synthesized by polycondensation of terephthalic acid (PTA) and 1,3-propanediol, as described in the literature [1]. PTT has excellent high stability and molding processing performance, and its molecular structure has a unique spiral structure, which makes it have the characteristics of softness, fluffiness and stain resistant. In addition, PTT fiber is easy to dye, has good drapability and strong resilience, and that is very suitable for textile and garment fabrics. At present, PTT is mainly used in clothing, carpet and other textile industries, as well as in engineering plastics and film fields, as described in the literature [2]. The market demand continues to rise, and it is expected that the market demand of PTT will reach about 1.1 million tons in the next few years.
Waste polyester PTT is difficult to degrade under natural conditions, and its recovery methods mainly include hydrolysis, alcoholysis and amination, as described in the literature [3]. 1-Nonanol could be used for alcoholysis with PTT, and its reaction product dinonyl terephthalate is an excellent environmental plasticizer, while the other product 1,3-propanediol can be recycled.
During the alcoholysis of PTT with 1-nonanol, the boiling points of the reactant 1-nonanol and the product 1,3-propanediol are close, which are 488.2 and 490.7 K respectively. It is difficult to separate them with conventional operation. Liquid–liquid extraction has the characteristics of large treatment capacity, good separation effect, high recovery rate, continuous operation and convenient automatic control, as described in the literature [4]. It is widely used in the separation of azeotropic or near boiling mixtures.
To provide data support for the industrial separation of 1,3-propanediol and 1-nonanol, four extractants were selected from a large number of preliminary experiments at 298.2 K and 101.3 kPa. 1,3-Propanediol and 1-nonanol were separated by 1,2-xylene, 1-chlorobutane, isopropyl ether and water at 298.2 K and 101.3 kPa. And, the thermodynamic models NRTL and UNIQUAC were used to simulate the experimental data of LLE to obtain the corresponding model parameters, as described in the literature [5, 6]. Finally, the graphical user interface (GUI) was used to verify the consistency of the regression parameters.
2 Experimental Section
2.1 Chemicals Used
The detailed information of experimental reagents used in the experiment of LLE is shown in Table 1. The purity of the reagents were checked and confirmed by gas chromatography (GC). All chemical reagents were used without further purification. The distilled water used in experiments was self-made.
2.2 Instruments and Measurement Methods
Under the conditions of 298.2 K and 101.3 kPa, 30 g of 1,3-propanediol/1-nonanol and equal mass extractant were added to the phase equilibrium cell (100 mL, Tianjin Pengxiang Glass Instrument Factory), and 2 g solute was added to the cell each time. The phase balance cell was placed on a magnetic stirrer (CJ78-1, Wuhan Grammo Testing Equipment Co., Ltd), and the water inlet and outlet of the cell were respectively connected with the constant temperature tank (CH-1015, Ningbo Haishu Yiheng Instrument Co., Ltd). Stir the cell violently for 2.5 h to fully mix the reagents in the cell, and then stand for 5 h to separate the upper and lower phases. The mercury meter with an accuracy of 0.1 K was used for temperature measurement. In addition, an analytical balance was used to weigh, and a pressure gauge was used to detect the system pressure. When the system pressure fluctuates, it can be maintained constant by air supply (or exhaust). The experimental device is shown in Fig. 1.
When the ternary mixture reached phase equilibrium, the upper and lower interfaces will obviously appear. Carefully pull out about 0.6 µL from each layer with a micro syringe. The Agilent GC6820 gas chromatography was used to detect the content of each component in the sample, which was calculated using the area normalization method. The chromatography was equipped with a thermal conductivity detector (TCD) and Porapak N (3 m × 3 mm) column. Hydrogen was used as the carrier gas, the flow rate was 50 mL·min−1, and the flow was kept constant. The detector and the vaporization chamber were kept at 518.2 K. The initial temperature of the chromatographic column was 403.2 K, and reached 493.2 K at a heating rate of 50 mL·min−1.
All samples were measured at least three times until their deviations were within one-thousandth, and the average values were recorded as the component content of the sample. The uncertainty calculation of the binary mixture was provided by the GUM standard. The formula is as follows:
where u represents uncertainty; n represents the number of samples; e represents mass fraction, pressure, density and temperature; i represents the ith result of measurement; \(\bar{e}\) and s(ei) represent the average of ei and measurement standard deviation, respectively.
3 Results and Discussion
3.1 Experimental LLE Data
Except for the binary solubility data of 1-nonanol—water, the binary solubility data of 1,3-propanediol—extractant (1,2-xylene, 1-chlorobutane and isopropyl ether) are not available, and the results are shown in Table 2.
LLE data of 1,3-propanediol + 1-nonanol + extractant (1,2-xylene, 1-chlorobutane, isopropyl ether and water) were measured at 298.2 K and 101.3 kPa, the results are shown in Tables 3 and 4. Take the system of 1,3-propanediol + 1-nonanol + water as an example, where \({w}_{1}^{\text{I}}\) and \({ w}_{2}^{\text{I}}\) represent the mass fractions of 1-nonanol and 1,3-propanediol in the extractant-rich phase, and \({\text{w}}_{1}^{\text{I}\text{I}}\) and \({\text{w}}_{2}^{\text{I}\text{I}}\) represent the mass fractions of 1-nonanol and 1,3-propanediol in the 1-nonanol-rich phase, respectively. Meanwhile, the triangular phase diagram of each system is shown in Fig. 2. The triangular phase diagram was drawn based on the mass fraction of each component. Obviously, the phase diagram conforms to the type of Treybal I, as described in the literature [8]. In addition, it can also be observed from the ternary liquid–liquid diagram that when the concentration of solute is from low to high, the connecting line has a large slope, which indicates that the affinity between solute and extractant is much higher than that between solute and solvent.
Variation of solute distribution constant in the extractant-rich phases mass at 298.2 K and 101.3 kPa. Experimental data: ( ) 1,2-xylene; (
) 1,2-xylene; ( ) 1-chlorobutane; (
) 1-chlorobutane; ( ) isopropyl ether; (
) isopropyl ether; ( ) water. NRTL: (
) water. NRTL: ( ) 1,2-xylene; (
) 1,2-xylene; ( ) 1-chlorobutane; (
) 1-chlorobutane; ( ) isopropyl ether; (
) isopropyl ether; ( ) water. UNIQUAC: (
) water. UNIQUAC: ( ) 1,2-xylene; (
) 1,2-xylene; ( ) 1-chlorobutane; (
) 1-chlorobutane; ( ) isopropyl ether; (
) isopropyl ether; ( ) water (Color figure online)
) water (Color figure online)
Variation of solute selectivity constant in the extractant-rich phases mass at 298.2 K and 101.3 kPa. Experimental data: ( ) 1,2-xylene; (
) 1,2-xylene; ( ) 1-chlorobutane; (
) 1-chlorobutane; ( ) isopropyl ether; (
) isopropyl ether; ( ) water. NRTL: (
) water. NRTL: ( ) 1,2-xylene; (
) 1,2-xylene; ( ) 1-chlorobutane; (
) 1-chlorobutane; ( ) isopropyl ether; (
) isopropyl ether; ( ) water. UNIQUAC: (
) water. UNIQUAC: ( ) 1,2-xylene; (
) 1,2-xylene; ( ) 1-chlorobutane; (
) 1-chlorobutane; ( ) isopropyl ether; (
) isopropyl ether; ( ) water (Color figure online)
) water (Color figure online)
The extraction capacity of different extractants was evaluated by D and S, as described in the literature [9, 10], and the formula is as follows:
where w represents the mass fraction, other parameters are consistent with the preceding description. The detailed changes of D and S of solute in extractant-rich phase are shown in Figs. 3 and 4.
As shown in Fig. 3, the D of the solute in the extractant-rich phase decreases with the increase of its content, and the value of D is greater than 3. This means that all four extractants can separate the mixture of 1,3-propanediol and 1-nonanol by liquid–liquid extraction. Figure 4 shows that the S of all extractants is greater than 10 and decreases with the increase of the content of solute in the extractant-rich phase. It can be seen from the experimental results of LLE that water in all extractants has the highest D and S, which means that water has the best separation effect on the binary mixture of 1,3-propanediol and 1-nonanol.
3.2 σ-Profile Analysis
The σ-profile, which was calculated by COSMO-SAC model, was chosen to describe the extraction performance of the six reagents because it can reflect the polarization effect of the molecular surface and determine the interaction energy between molecules, as described in the literature [11]. The σ-profiles of 1,3-propanediol, 1-nonanol, 1,2-xylene, 1-chlorobutane, isopropyl ether and water are shown in Fig. 5. The two dotted lines (σ = ± 0.0082 e·Å−2 ) indicate the critical value of hydrogen bonding ability. The middle region is non-polar and the electronegativity is zero, which the left and right regions are the donor and acceptor regions of the hydrogen bond, respectively. In the polar region, the closer σ is to the threshold, the stronger the compound’s ability to accept or provide hydrogen bonds, as described in the literature [12].
As show n in Figs. 1, 3 and 5-propanediol and 1-nonanol have the broad σ-profiles, both from − 0.016 to 0.016 e·Å−2. 1,3-Propanediol and 1-nonanol have further profile peaks (0.013 e·Å−2) in the hydrogen acceptor donor zone, which indicates that both 1,3-propanediol and 1-nonanol have strong ability to accept hydrogen bonds, while the profile peak of water at − 0.014 e·Å−2 indicates that water has strong ability to provide hydrogen bonds. Therefore, water can well extract 1,3-propanediol from 1-nonanol. Compared with 1-chlorobutane and isopropyl ether, 1,2-xylene has slightly greater ability to provide hydrogen bond and slightly higher extraction ability, except for water.
3.3 Data Reliability Verification
This paper used the empirical equation of Bachman equation and O-T equation to examine the linear of the measured results, and the formula is as follows:
where a and b are parameter values of the fitting equation y = a + bx, and the other parameters are the same as described above.
After obtaining the value of the parameters in the equation, the least square method was used to obtain the linear correlation coefficient (R2) of the experimental data. The closer R2 is to 1, the higher the fitting degree is. The related parameters of the equation are listed in Table 5, and the graphs are shown in Figs. 6 and 7. The data results show that R2 values are all greater than 0.98, which means that the data have good reliability and regularity.
3.4 Thermodynamic Modeling
The thermodynamic models NRTL and UNIQUAC are the activity coefficient mathematical models that can be associated with the phase equilibrium data, which are included in Aspen simulation software system. The experimental data was correlated with NRTL and UNIQUAC models, and the binary regression parameters and NRTL non-random parameter α of the two models were obtained by using Aspen Plus V11. Table 6 lists the parameters of the UNIQUAC model obtained from the Aspen Plus database.
The objective function (OF) was used to calculate the optimal binary interaction parameters of the above two models, as described in the literature [13], and the formula is as follows:
where M represents the number of tie lines, subscripts i, j and k represent the components, phases and tie lines, respectively. wcal and wexp represent the calculated and experimental composition of components respectively. At the same time, the correlation between the measured data and the fitted data was evaluated by the following formula:
where the parameters k, j, i, M, wcal, wexp are the same as described above.
Table 7 lists the estimated values of RMSD and AAD obtained from NRTL and UNIQUAC models, which are less than 0.97% and 0.60%, respectively. At the same time, the experimental and calculated value of the two models are compared in Figs. 2, 3 and 4. It indicates that the experimental data are well related to the thermodynamic models.
Take the relevant data of 1,3-propanediol + 1-nonanol + water system at 298.2 K and 101.3 kPa as an example, the binary interaction parameters were verified by GUI-MATLAB, as described in the literature [14,15,16,17]. The NRTL binary parameter miscibility boundary, the calculated GM/RT surface and the experimental LLE connection line of the system are shown in Figs. 8 and 9.
As can be seen from Fig. 8, only components 1 and 3 are partially miscible in the system, and other components can be completely miscible. This result is consistent with the LLE data measured in this study. As can be seen from Fig. 9, the connecting line calculated by the software and the measured data is tangent to the lower surface of GM/RT, indicating that the mixed Gibbs free energy is in the minimum state. The binary interaction parameter is the local optimal solution, and the linear relationship between the experimental data is good. Therefore, according to the binary interaction parameters obtained by fitting, the LLE data measured in the experiment is reliable.
4 Conclusion
The LLE data of ternary system of 1,3-propanediol + 1-nonanol + extractant (1,2-xylene, 1-chlorobutane, isopropyl ether and water) were measured at 298.2 K and 101.3 kPa. The ternary systems studied are Treybal’s type I. From the above experimental results, it can be concluded that the four extractants in this study can well separate 1,3-propanediol and 1-nonanol, with high distribution constant and selectivity, especially when the concentration of solute is low. RMSD and AAD are less than 0.97% and 0.60% respectively, which are in good agreement with the experimental data. The COSMO model was used to analyze the extraction effect from the molecular level. Compared with Van der Waals force, water can produce hydrogen bond with 1,3-propanediol, and its extraction effect is the best. In addition, the binary interaction parameters of NRTL and UNIQUAC models were obtained, which is conducive to the design and optimization of the separation process. Finally, the GUI-MATLAB tool were used to illustrate the reliability of experimental data and binary interaction parameters.
References
Ran, H.S., Wang, C.H., Yang, X.H., Tong, M.Y.: Application and market of 1,3-propanediol. Guangdong Chem. Ind 3 3, 29–32 (2006)
Wei, G.F., Gu, L.X., Dai, Z.B., Chang, H.: Research progress of poly (propylene terephthalate). Synth. Fiber China 34, 1–6 (2005)
Xie, Y.F.: Recycling of waste polyester. Chem. Ind. Times 19, 56–58 (2005)
Wu, X., Wu, J., Wang, S., Gao, J., Xu, D., Zhang, L., Wang, Y.: Liquid–liquid equilibrium for ternary systems (ethyl acetate/isopropyl acetate + 2,2-difluoroethanol + water) at 298.15 and 308.15 K. J. Chem. Eng. Data 66, 1399–1405 (2021)
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14, 135–144 (1968)
Abrams, D.S., Prausnitz, J.M.: Statistical thermodynamics of liquid mixtures: a new expression for the excess gibbs energy of partly or completely miscible systems. AIChE J. 21, 116–128 (1975)
Stephenson, R., Stuart, J.: Mutual binary solubilities: water–alcohols and water–esters. J. Chem. Eng. Data 31, 56–70 (1986)
Zhang, L., Liao, Z., Zhu, C., Yu, Y., Li, Q.: Determination and correlation of liquid–liquid equilibria data for ternary system isopropyl acetate + isopropanol + water at different temperatures. J. Chem. Eng. Data 64, 972–978 (2019)
Dong, S., Sun, W., Jiang, Y., Jia, B.: Liquid–liquid equilibrium study for ternary systems of (water + furfuryl alcohol + solvents) at 298.2 K: measurement and thermodynamic modelling. J. Chem. Thermodyn. 148, 106136 (2020)
Esquivel, M.M., Bernardo-Gil, M.G.: Liquid–liquid equilibria for the systems: water/1-pentanol/acetic acid and water/1-hexanol/acetic acid. Fluid Phase Equilib 62, 97–107 (1991)
Shen, S., Chen, Y., Jiang, M.: Experimental measurement, correlation and COSMO-SAC prediction of liquid–liquid equilibrium for MIPK + dimethylphenols + water mixtures. J. Chem. Thermodyn. 140, 105900 (2020)
Zhu, Z., Xu, Y., Feng, T., Wang, N., Liu, K., Fan, H., Wang, L.: Measurement and correlation of liquid–liquid equilibria of three imidazolium ionic liquids with acetone and cyclohexane. J. Mol. Liq. 298, 111947 (2020)
Yan, H., Fan, W., Sun, S., Guo, H., Han, Y., Li, Q.: Liquid–liquid equilibria data and thermodynamic modeling for the ternary system of water, 2,2,3,3-tetrafluoro-1-propanol, and different solvents. J. Chem. Eng. Data 66, 2327–2332 (2021)
Reyes-Labarta, J.A.: Graphical user interface (GUI) for the representation of GM surfaces (using the NRTL model) and curves, including tie-lines and hessian matrix. RUA. (2015)
Guo, H.Y., Cao, T.Y., Zhao, Y., Liu, H., Li, J., Li, Q.S.: Liquid–liquid equilibrium measurement and thermodynamic modeling for ternary system of water + cyclohexanone + solvents (n-propyl acetate, methyl acetate) at different temperatures. J. Chem. Thermodyn. 166, 106672 (2022)
Lin, S., Yan, H., Jiang, Y., Guo, H.Y., Zhao, Y., Han, M., Li, Q.S.: Liquid–liquid equilibrium for the ternary system of water, 2,2,2-trifluoroethanol and different solvents at 303.2 K under 101.3 kPa. J. Chem. Thermodyn. 170, 106783 (2022)
Wang, Z., Fan, W., Xu, D., He, S., Huang, H., Gao, J., Wang, Y.: Liquid–liquid-phase equilibrium for quaternary systems (n-decane + 1-tetradecene + 1-methylnaphthalene + sulfolane/dimethyl sulfoxide) for separation of 1-methylnaphthalene from FCC diesel. J. Chem. Eng. Data 66, 2803–2811 (2021)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Y., Yi, L., Geng, T. et al. Liquid–Liquid Equilibrium for Ternary Systems of 1,3-Propanediol, 1-Nonanol and Different Extractants at 298.2 K and 101.3 kPa. J Solution Chem 52, 187–200 (2023). https://doi.org/10.1007/s10953-022-01225-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01225-4




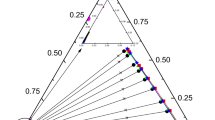


 ) experimental data; (
) experimental data; ( ) tie-line; (
) tie-line; ( ) NRTL model; (
) NRTL model; ( ) UNIQUAC model (Color figure online)
) UNIQUAC model (Color figure online)



 ) 1,2-xylene; (
) 1,2-xylene; ( ) 1-chlorobutane; (
) 1-chlorobutane; ( ) isopropyl ether; (
) isopropyl ether; ( ) water (Color figure online)
) water (Color figure online)
 ) 1,2-xylene; (
) 1,2-xylene; ( ) 1-chlorobutane; (
) 1-chlorobutane; ( ) isopropyl ether; (
) isopropyl ether; ( ) water (Color figure online)
) water (Color figure online)
