Abstract
To separate the azeotrope of 2-methyl-2-butanol and water, n-heptanol was selected as the extractant. Liquid–liquid equilibrium (LLE) data for the ternary system of 2-methyl-2-butanol + water + n-heptanol were measured at 303.15 K, 313.15 K and 323.15 K under 101.3 kPa. The quality of the experimental LLE data was verified by the Hand equation, the Othmer–Tobias equation, and the mass balance between the feed and the conjugated phases. The results show that the correlation coefficients (R2) of the equations are close to 1, the relative error of mass balance is less than 0.7%. The selectivity (S) was used to represent the ability of n-heptanol to extract 2-methyl-2-butanol from water, which is much greater than 133. The experimental data show that S decreases with the mass fraction of 2-methyl-2-butanol in aqueous phase, and increases with equilibrium temperature. The NRTL and UNIQUAC models were used to regress the experimental LLE data, and the corresponding binary interaction parameters were obtained. The root-mean square deviations (RMSDs) indicate that the UNIQUAC model performs better than the NRTL model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
2-Methyl-2-butanol is an important raw material in production of many chemical products, such as: tert-amylbenzene, tert-amyl anthraquinone, tert-amyl peroxide, pesticides of triazolone, color film developers, and pharmaceuticals [1, 2]. It can also be used as solvent, plasticizer, mineral flotation agent, and additive of petroleum products [3]. In the production and application of 2-methyl-2-butanol, the azeotropic mixture of 2-methyl-2-butanol + water is often produced [4]. The azeotrope cannot be separated by ordinary distillation. Therefore, development of a feasible process for separation of the azeotrope of 2-methyl-2-butanol + water is of great significance.
Salting out can be used to separate the azeotropic system. Also, many researchers have investigated the influence of salts, such as potassium nitrate, sodium nitrate [5], sodium chloride [6], potassium chloride [7] and lithium chloride [8], on the LLE behavior of 2-methyl-2-butanol + water system. But salting out leads to corrosion of equipment, obstruction of rectifying column and reboiler, and high energy consumption [9]. Liquid–liquid extraction is an effective alternative to separate azeotropic mixtures, which has the advantages of large processing capacity, low energy consumption and high efficiency [10, 11]. Selection of extractants is the foundation of a liquid–liquid extraction process. An economically viable solvent should be cheap, have low toxicity, a moderate boiling point, and be easily recycled. Nowadays, ionic liquids are becoming research hotspots of novel extractants because of simple recycle [12,13,14]. But traditional organic extractants are widely used in industry for their economical price. LLE data are essential for selection of extractants, design of extraction equipment, and optimization of the process [15,16,17]. So far, only few works investigated LLE data concerning the systems of 2-methyl-2-butanol + water + extractants. However, the reported extractants, such as diethylene glycol monohexyl ether [18], diethylene glycol monobutyl ether [19], 2-butyloxyethanol [20], o-xylene [21], have the demerits of high boiling point, high price, narrow two-phase zones, and high toxicity. In our previous work, n-octanol [22] and ethylbenzene [23] were used to extract 2-methyl-2-butanol from water. n-Heptanol is comparable to n-octanol in solubility with 2-methyl-2-butanol and water. It has the virtues of ease of separation from 2-methyl-2-butanol, low toxicity (LD50 oral-rat-female-5500 mg/kg) [24], moderate viscosity, and reasonable price. Moreover n-heptanol boils at lower temperature than n-octanol, and requires lower temperatures in subsequent separation. What’s more, the distribution coefficient and selectivity of n-heptanol predicted by COSMO-UNIFAC model [25, 26] using Aspen plus V11 [27] at 303.15 K are much greater than 1. Thus, it is a potential extractant candidate for separating 2-methyl-2-butanol from water. Up to now, no LLE data of the ternary system 2-methyl-2-butanol + water + n-heptanol were reported in literature. In this work, the LLE data of the ternary system were measured at 303.15 K, 313.15 K and 323.15 K under 101.3 kPa.
2 Experimental
2.1 Materials
2-Methyl-2-butanol, n-heptanol and water were used in the experiments. Detailed information of the chemicals is given in Table 1. Water was self-made by bi-distillation with the electrical conductivity less than 0.0562 µS·cm–1. All the other chemicals were used without further purification. The purity of the reagents was determined by gas chromatography (GC).
2.2 Experimental Apparatus and Procedures
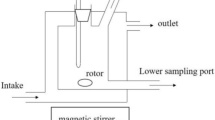
The liquid–liquid equilibrium experiments were carried out in a 50 mL glass vessel reported in literature [28]. The temperature of the system was controlled by a thermostatic water bath (GHC-30, Ningbo Scientz Biotechnology Co. Ltd.) with an accuracy of ± 0.01 K. The temperature of the equilibrium vessel was measured by a Pt-100 sensor connected to a digital temperature meter (AMETEKDTI-1000) with an uncertainty of 0.04 K. The atmospheric pressure was measured by a manometer with an uncertainty of 0.35 kPa.
About 30 mL of the mixture was gravimetrically prepared using an electronic balance (AR1140, Ohaus Corporation) with an accuracy of ± 0.0001 g. It was put into the liquid–liquid equilibrium cell and stirred with a magnetic stirrer for 4 h. Then, it was settled at the specified temperature for 24 h to ensure the phase equilibrium to be achieved. The samples of organic phase and aqueous phase were taken from the upper and lower sampling ports respectively with micro-syringes. The samples were analyzed by a gas chromatograph (SP-3420A, supplied by Beijing Beifen Ruili Analysis Instrument Ltd.) with a thermal conductivity detector (TCD) and a (2 m × 3 mm) column packed with GDX-401. The flow rate of the carrier gas, hydrogen, was 20 mL·min−1. The temperatures of the column, vaporization chamber, and detector were set as 443.15 K, 493.15 K, and 513.15 K, respectively. The samples were quantitatively analyzed by the area normalization method using ethanol as internal standard. The composition of each sample was determined by using the relative correction factors determined from mixtures prepared gravimetrically.
2.3 Standard uncertainty
The experimental standard uncertainty is determined according to the JCGM 100:2008 [29] as follows:
where n represents the measurement times of each sample, wk is the mass fraction of the k th measurement, \(\overline{w}\) is the average value of the measurement results of n measurements, s(wk) represents the experimental standard deviation, and u is the standard uncertainty.
Each sample was measured at least three times, and the average value was taken as the measurement result.
Taking into account the effects of other factors, such as thermal gradients, deviation from equilibrium and impurities, on the measurement, the corresponding experimental combined standard uncertainties were listed under Tables 1, 2 and 3.
3 Results and Discussion
3.1 Experimental LLE Data
The LLE data were measured at 303.15 K, 313.15 K, and 323.15 K under 101.3 kPa. The solubility data of the binary systems 2-methyl-2-butanol + water and water + n-heptanol are shown in Table 2, together with the corresponding literature data. And the experimental LLE data of the ternary system 2-methyl-2-butanol + water + n-heptanol are listed in Table 3. The ternary phase diagrams at different temperatures are presented in Figs. 1, 2 and 3. In this work, all concentrations are expressed in mass fraction. \(w_{i} ,{\mkern 1mu} w_{i}^{{\text{I}}} ,\,{\mkern 1mu} {\text{and}}\,{\mkern 1mu} w_{i}^{{{\text{II}}}}\) represent the mass fractions of component i in the feed mixture, the aqueous phase, and the organic phase, respectively, and i is the number of the component in the mixture, i = 1, 2 and 3, which denote the 2-methyl-2-butanol, water, and n-heptanol, respectively.
Ternary phase diagram for the system 2-methyl-2-butanol (1) + water (2) + n-heptanol (3) at 303.15 K under 101.3 kPa: black circle solid, experimental data; green triangle up solid, calculated data from NRTL model; red box solid, calculated data from UNIQUAC model; blue star solid, calculated data from COSMO-UNIFAC model; × , feed composition; cyan circle solid, experimental binary data for water (2) + n-heptanol (3); magenta circle solid, binary data for 2-methyl-2-butanol (1) + water (2) [21] (Color figure online)
Ternary phase diagram for the system 2-methyl-2-butanol (1) + water (2) + n-heptanol (3) at 313.15 K under 101.3 kPa: black circle solid, experimental data; green triangle up solid, calculated data from NRTL model; red box solid, calculated data from UNIQUAC model; blue star solid, calculated data from COSMO-UNIFAC model; × , feed composition; cyan circle solid, experimental binary data for water (2) + n-heptanol (3); magenta circle solid, binary data for 2-methyl-2-butanol (1) + water (2) [21] (Color figure online)
Ternary phase diagram for the system 2-methyl-2-butanol (1) + water (2) + n-heptanol (3) at 323.15 K under 101.3 kPa: black circle solid, experimental data; green triangle up solid, calculated data from NRTL model; red box solid, calculated data from UNIQUAC model; blue star solid, calculated data from COSMO-UNIFAC model; × , feed composition; cyan circle solid, experimental binary data for water (2) + n-heptanol (3); magenta circle solid, binary data for 2-methyl-2-butanol (1) + water (2) [21] (Color figure online)
To evaluate the ability of n-heptanol to extract 2-methyl-2-butanol from the mixture, distribution coefficient (D) and selectivity (S) were calculated by the following formulas:
The values of distribution coefficient (D) and selectivity (S) are listed in Table 3.
Table 2 shows that there are minor distinctions between the experimental solubility data and the literature data, which verifies the reliability of the experimental apparatus and method. It can be seen from Table 3 and Figs. 1, 2 and 3 that the system of 2-methyl-2-butanol (1) + water (2) + n-heptanol (3) has two partially miscible binary subsystems and one completely miscible region at the studied temperatures. Therefore, the ternary system exhibits Type II liquid–liquid phase behavior. It also can be seen from Figs. 1, 2 and 3 that the aqueous phase points are located at the lower left of the figure, while the organic phase points are distributed in the right of the figure, resulting in positive slopes of the tie-lines. Moreover, the contents of 2-methyl-2-butanol and n-heptanol in the aqueous phase are very low, while those in the organic phase are much higher. When the content of 2-methyl-2-butanol in aqueous phase increases, its content in organic phase increases significantly.
The quality of the experimental LLE data was verified by Othmer–Tobias equation [36] (Eq. 6) and Hand [37]) equation (Eq. 7):
where, \({a}_{1}\) and \({b}_{1}\) are the parameters of Othmer–Tobias equation; \({a}_{2}\) and \({b}_{2}\) are the parameters for Hand equation.
Table 4 shows the parameters and the corresponding regression coefficient (R2) values of the two equations obtained by regression the experimental data. The Othmer–Tobias and Hand plots are presented in Figs. 4 and 5, respectively. According to the fitting results, the values of R2 are close to 1, which indicates good consistency of the measured LLE data.
The quality of the experimental data was tested by the material balance between the feed and the two phases in equilibrium [38], which is described by the following equations:
where \(M,{\mkern 1mu} M^{{\text{I}}} {\mkern 1mu} {\text{and}}{\mkern 1mu} \,M^{{{\text{II}}}}\) represent the mass of the feed mixture, the mass of the aqueous phase, and the mass of the organic phase, respectively.
\(M^{{\text{I}}} {\mkern 1mu} {\text{and}}{\mkern 1mu} \,M^{{{\text{II}}}}\) were calculated by solving the overdetermined linear Eqs. (8–10) with the least—squares method using Matlab R2108a. The relative error of mass balance RE is expressed as follows:
The results show that RE is less than 0.7%, which verifies that the experimental data are reliable.
The trends of selectivity with the mass fraction of 2-methyl-2-butanol in the aqueous phase \(\left( {w_{1}^{{\text{I}}} } \right)\) at different temperatures are plotted in Fig. 6. To further appraise the extracting ability of n-heptanol, S of the reported extractants versus \(w_{1}^{{\text{I}}}\) at various temperatures are also presented in Fig. 6.
Selectivity (S) of different extractants versus mass fraction of 2-methyl-2-butanol in the aqueous phase \(\left( {w_{1}^{{\text{I}}} } \right)\) at different temperatures: a n-heptanol in this work: red circle solid, 303.15 K; red box solid, 313.15 K; red triangle up solid, 323.15 K. b n-octanol [22]: magenta circle solid, 303.15 K; magenta box solid, 313.15 K; magenta triangle up solid, 323.15 K. c o-xylene [21]: green circle solid, 303.2 K; green box solid, 313.2 K; green triangle up solid, 323.2 K. d ethylbenzene [23]: gray circle solid, 293.15 K; gray box solid, 303.15 K; gray triangle up solid, 313.15 K. e diethylene glycol monobutyl ether [19]: black circle solid, 293.15 K; black box solid, 303.15 K; black triangle up solid, 313.15 K. f 2-butyloxyethanol [20]: blue circle solid, 293.15 K; blue box solid, 303.15 K. g diethylene glycol monohexyl ether [18]: cyan circle solid, 293.15 K; cyan box solid, 303.15 K; cyan triangle up solid, 313.15 K (Color figure online)
It is obvious from Table 3 and Fig. 6 that 2-methyl-2-butanol is enriched effectively in the organic phase, and the values of selectivity are much greater than 133. The selectivity (S) of n-heptanol decreases with \(w_{1}^{{\text{I}}}\) and increases with the equilibrium temperature. It also can be seen from Fig. 6 that the selectivity of n-heptanol is much higher than the reported extractants except o-xylene, ethylbenzene and n-octanol. The selectivity of o-xylene and ethylbenzene is significantly greater than that of n-heptanol only when \(w_{1}^{{\text{I}}}\) is very low. With the increase of \(w_{1}^{{\text{I}}}\), the selectivity of o-xylene, ethylbenzene and n-octanol is only slightly greater than that of n-heptanol. Because n-heptanol boils higher than o-xylene and ethylbenzene, the separation of n-heptanol from 2-methyl-2-butanol is easier than that of o-xylene and ethylbenzene. Although n-heptanol boils lower than n-octanol, the large boiling point difference between n-heptanol and 2-methyl-2-butanol makes the degree of difficulty in its recovery from 2-methyl-2-butanol almost equivalent to that of n-octanol. And n-heptanol requires lower utility temperature in the separation process than n-octanol. Moreover, n-heptanol has lower toxicity (LD50 Oral-Rat-female-5500 mg·kg−1) [24] than o-xylene (LD50:1364 mg·kg−1) [39] and ethylbenzene (LD50 Oral-Rat-male and female-3500 mg·kg−1) [40]. Thus, n-heptanol is a promising solvent to extract 2-methyl-2-butanol from the mixture of 2-methyl-2-butanol + water.
3.2 Data Correlation
The mixture of 2-methyl-2-butanol and water is a partially miscible system, NRTL [41] and UNIQUAC models [42] are selected to correlate the experimental data. The corresponding binary interaction parameters for NRTL and UNIQUAC models were regressed by Aspen Plus V11 software. According to the recommended values by the software, the non-randomness parameters ɑij in NRTL model for the pairs of (2-methyl-2-butanol – water), (water – n-heptanol), and (2-methyl-2-butanol – n-heptanol) were set as 0.2, 0.2 and 0.3, respectively. The structural parameters r and q in UNIQUAC model from Aspen plus V11 [27] software database are given in Table 5.
The binary interaction parameters of NRTL and UNIQUAC are obtained by minimizing the following objective function:
where, i, j, and k are the serial numbers of components, phases and tie lines, respectively; N is the number of tie lines;\({\sigma }_{T}\) is the standard deviation of the experimental temperature; \({\sigma }_{w}\) is the standard deviation of the mass fraction; \({T}^{{\text{exp}}}\) and \({T}^{\text{cal}}\) represent the experimental and calculated temperatures, respectively; \({w}^{\text{exp}}\) and \({w}^{\text{cal}}\) are the experimental mass fraction and that calculated by the models, respectively.
The consistency between the experimental data and the calculated ones is evaluated by the following root mean square deviation (RMSD):
The regressed binary interaction parameters of NRTL and UNIQUAC models and root mean square deviations (RMSDs) are presented in Table 6. The LLE data calculated by NRTL and UNIQUAC using the regressed parameters are shown in Figs. 1, 2 and 3. For comparison, the LLE data predicted by the COSMO-UNIFAC model using Aspen plus V11 are also shown in Figs. 1, 2 and 3.
Table 6 and Figs. 1, 2 and 3 show that the LLE data predicted by NRTL and UNIQUAC models fit well with the experimental data, with the RMSDs no greater than 0.87%. NRTL model performs a little better than UNIQUAC model in describing the LLE behavior of the system 2-methyl-2-butanol (1) + water (2) + n-heptanol (3). Meanwhile, Figs. 1, 2 and 3 indicates that the COSMO-UNIFAC model predicts the LLE data for the system well and the RMSD is less than 0.83%.
4 Conclusion
The LLE data of the ternary system of 2-methyl-2-butanol + water + n-heptanol were determined at 303.15 K, 313.15 K, and 323.15 K under 101.3 kPa. The reliability of the experimental data was tested by the Hand equation, the Othmer–Tobias equation, and the mass balance between the feed and the two phases in equilibrium. The values of correlation coefficient R2 for both Hand and Othmer–Tobias equations are close to 1. The relative error of mass balance is less than 0.7%. The extraction effect of n-heptanol was described by selectivity. The experimental data show that the selectivity is much greater than 133, which indicates that n-heptanol is a promising extractant for separating 2-methyl-2-butanol from water. It can be derived from the experimental LLE data that S decreases with the mass fraction of 2-methyl-2-butanol in aqueous phase and increases with the equilibrium temperature. The NRTL and the UNIQUAC models were used to regress the experimental data. The RMSD between the experimental data and the calculated data by the NRTL model is 0.79%, and the RMSD for the UNIQUAC model is 0.87%. This work provides the fundamental liquid–liquid equilibrium data and related binary interaction parameters for simulation and design of the process separating the azeotrope of 2-methyl-2-butanol + water by extraction.
References
Zhou, Y., Chen, H.J., Zhang, R., Ding, Z.X., Tang, Q.Y., Yang, Z.Y., Sun, R.H., Fan, C.L., Sun, C.S., He, K., Bai, M.: Preparation of tert-amyl alcohol from isopentene. CN Patent 111(777), 490A (2020)
Chen, H.J., Hu, G.J., Qi, Z.J., Li, Y., Xu, X.H., Lu, X.G., Zheng, Z.: Method for preparation of tert-amyl alcohol by isoamylene hydration. CN Patent 108(017), 508A (2018)
Yao, Z.L., Zhao, Y.Z., Dun, J.T.: Method for preparation of tert-amyl alcohol. CN Patent 1(374), 284A (2002)
Fischer, K., Shulgin, I., Rarey, J., Gmehling, J.: Vapor–liquid equilibria for the system water + tert-pentanol at 4 temperatures. Fluid Phase Equilib. 120, 143–165 (1996)
El-Dossoki, F.I.: The influence of cation, anion and temperature on the liquid–liquid equilibrium of some pentanols–water system. Fluid Phase Equilib. 305, 161–168 (2011)
Gomis, V., Ruiz, F., Boluda, N., Saquete, M.D.: Liquid–liquid-solid equilibria for ternary systems water + sodium chloride + pentanols. J. Chem. Eng. Data 44, 918–920 (1999)
Boluda, N., Gomis, V., Ruiz, F., Saquete, M.D., Barnes, N.: Liquid–liquid-solid equilibria for ternary systems of water + potassium chloride + pentanols. Fluid Phase Equilib. 179, 269–276 (2001)
Gomis, V., Ruiz, F., Boluda, N., Saquete, M.D.: Liquid–liquid–solid equilibria for ternary systems water + lithium chloride + pentanols. Fluid Phase Equilib. 215, 79–83 (2004)
Jiang, M., Chen, Y., Shen, S.: Liquid–liquid equilibria for octan-2-one + dihydroxybenzene + water at different temperatures: experimental data and thermodynamic modeling. J. Chem. Eng. Data 64, 4454–4464 (2019)
Shen, S., Chen, Y., Jiang, M.: Experiments and COSMO-SAC modeling of methyl isobutyl ketone + dimethylphenols + water mixtures. J. Chem. Eng. Data 64, 3521–3534 (2019)
Xu, D., Zhang, L., Gao, J., Pratik, D., Zhao, L., Cui, Z.: Liquid–liquid equilibrium for ternary systems of ethyl acetate / isopropyl acetate + 2,2,3,3-tetrafluoro-1-propanol + water at 298.15, 318.15 K. J. Chem. Thermodyn. 106, 218–227 (2017)
Li, A., Xu, X., Zhang, L., Gao, J., Xu, D., Wang, Y.: Separation of cresol from coal tar by imidazolium-based ionic liquid [Emim] [SCN]: Interaction exploration and extraction experiment. Fuel 264, 116908 (2020)
Zhang, X., Wang, Z., Wang, K., Reyes-Labarta, J.A., Gao, J.: Liquid–liquid phase equilibrium and interaction exploration for separation of azeotrope (2,2,3,3-tetrafluoro-1-propanol + water) with two imidazolium-based ionic liquids. J. Mol. Liq. 300, 112266 (2020)
Wang, P., Yan, P., Reyes-Labarta, J.A., Gao, J., Xu, D., Zhang, L., Wang, Y.: Liquid–liquid measurement and correlation for separation of azeotrope (dimethyl carbonate and ethanol) with different imidazolium-based ionic liquids. Fluid Phase Equilib. 485, 183–189 (2019)
Cháfer, A., de la Torre, J., Lladosa, E., Montón, J.B.: Measurements and correlation at different temperatures of liquid–liquid equilibria of 2-butanol or 2-methyl-2-butanol + 1,2,3-propanetriol + water ternary systems. Fluid Phase Equilib. 377, 38–44 (2014)
Dai, F., Zhao, M., Jia, M.: Liquid–liquid equilibrium study for ternary systems of water + propargyl alcohol + solvents at 308.2 K: measurement and thermodynamic modeling. J. Chem. Thermodyn. 135, 149–154 (2019)
Männistö, M., Pokki, J.-P., Creati, A., Voisin, A., Zaitseva, A., Alopaeus, V.: Ternary and binary LLE measurements for solvent (4-methyl-2-pentanone and 2-methyl-2-butanol) + furfural + water between 298 and 401 K. J. Chem. Eng. Data 61, 903–911 (2016)
Chiou, D., Chen, L.: Liquid–liquid equilibria for the ternary system water + diethylene glycol monohexyl ether + 2-methyl-2-butanol. Fluid Phase Equilib. 218, 229–234 (2004)
Chiou, D., Chen, L.: Liquid–liquid equilibria for the ternary system water + 2-methyl-2-butanol + diethyleneglycol monobutylether. J. Chem. Eng. Data 46, 1530–1532 (2001)
Pai, Y., Chen, L.: Liquid–liquid equilibria of two binary systems: water + 1-pentanol and water + 2-methyl-2-butanol and two ternary systems: water + 1-pentanol + 2-butyloxyethanol and water + 2-methyl-2-butanol + 2-butyloxyethanol. Fluid Phase Equilib. 155, 95–105 (1999)
Zhang, X., Yang, X., Jian, J., Jian, C., He, J., Wang, H., Kong, W., Shangguan, G., Xia, M.: Liquid–liquid equilibrium for 2-methyl-2-butanol + water + o-xylene at different temperatures. J. Chem. Eng. Data 65, 5090–5095 (2020)
Zhang, X., Yang, F., Jian, J., Jian, C., Wang, H., Kong, W., Shangguan, G., Xia, M.: Liquid–liquid equilibrium for the ternary system of (2-methyl-2-butanol + water + n-octanol) at different temperatures. J. Chem. Thermodyn. 161, 106522 (2021)
Zhang, X., Jian, C., Du, J., Wang, H., Wang, H., Kong, W., Shangguan, G., Xia, M., Lu, H.: Measurements and thermodynamic modeling of liquid–liquid equilibrium data for 2-methyl-2-butanol + water + ethylbenzene. J. Chem. Eng. Data 66, 2153–2159 (2021)
ChemBlink: https://www.chemblink.com/MSDS/MSDSFiles/111-70-6_Sigma-Aldrich.pdf.
Dong, Y., Zhu, R., Gao, Y., Lei, Z.: A united chemical thermodynamic model: COSMO-UNIFAC. Ind. Eng. Chem. Res. 57, 15954–15958 (2018)
Zhu, R., Taheri, M., Zhang, J., Lei, Z.: Extension of the COSMO-UNIFAC thermodynamic model. Ind. Eng. Chem. Res. 59, 1693–1701 (2020)
Aspen Plus Software: Version 11. Aspen Technology Inc, Burlington, MA (2019)
Dai, F., Xin, K., Song, Y., Shi, M., Yu, Y., Li, Q.: Liquid–liquid equilibria for the ternary system containing 1-butanol + methoxy (methoxymethoxy) + water at temperatures of 303.15, 323.15 and 343.15 K. Fluid Phase Equilib. 409, 466–471 (2016)
JCGM: GUM 1995 with minor corrections. Evaluation of Measurement Data-Guide to the Expression of Uncertainty in Measurement 100, 2008 (2008)
Marongiu, B., Ferino, I., Monaci, R., Solinas, V., Torrazza, S.: Thermodynamic properties of aqueous non-electrolyte mixtures. alkanols + water systems. J. Mol. Liq. 28, 229–247 (1984)
Ginnings, P.M., Baum, R.: Aqueous solubilities of the isomeric pentanols. J. Am. Chem. Soc. 59, 1111–1113 (1937)
Adel, S.A., Aljimaz, S.H.F., Jasem, A.A., Mohamed, A.F.: Liquid–liquid equilibria of the ternary system water + acetic acid + 1-heptanol. J. Chem. Eng. Data 45, 301–303 (2000)
Darwish, N.A., Abdulkarim, M.A., Ashour, I., Dwaidar, A.M., Athamneh, F.S.: Liquid–liquid equilibrium for the system water + acetic acid + 1-heptanol at 278.1, 293.1, 303.1 and 313.1 K. Fluid Phase Equilib. 200, 277–285 (2002)
Richard, S., James, S., Mary, T.: Mutual solubility of water and aliphatic alcohols. J. Chem. Eng. Data 29, 287–290 (1984)
Goral, M., Wisniewska-Goclowska, B.: IUPAC-NIST solubility data series. 82. alcohols with water. Part 4. C7 alcohols with water. J. Phys. Chem. Ref. Data 36, 445–484 (2007)
Othmer, D.F., Tobias, P.E.: Liquid–liquid extraction data–Toluene and acetaldehyde systems. Ind. Eng. Chem. 34, 693–696 (1942)
Hand, D.B.: The distribution of consolute liquid between two immiscible liquids. J. Phys. Chem. 34, 1961–2000 (1930)
Marcilla, A., Ruiz, F., García, A.N.: Liquid–liquid-solid equilibria of the quaternary system water-ethanol-acetone-sodium chloride at 25 ℃. Fluid Phase Equilib. 112, 273–289 (1995)
ChemBlink: https://www.chemblink.com/MSDS/MSDSFiles/95-47-6_Sigma-Aldrich.pdf.
ChemBlink: https://www.chemblink.com/MSDS/MSDSFiles/100-41-4_Sigma-Aldrich.pdf
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14, 135–144 (1968)
Abrams, D.S., Prausnitz, J.M.: Statistical thermodynamics of liquid mixtures: a new expression for the excess gibbs energy of partly or completely miscible systems. AIChE J. 21, 116–128 (1975)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Yang, F., Jian, C. et al. Liquid–Liquid Equilibrium Study of the Ternary System 2-Methyl-2-butanol + Water + n-Heptanol at 303.15 K, 313.15 K and 323.15 K. J Solution Chem 51, 224–239 (2022). https://doi.org/10.1007/s10953-022-01144-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01144-4