Abstract
Near-infrared absorption spectra of aqueous solutions of eleven polyhydric alcohols (nine dihydric alcohols and two trihydric alcohols) at concentrations up to 20% were obtained at 20, 25, and 30 °C. Variations in the band due to O–H stretching vibration overtones of water and alcohol with changes in concentration and temperature were determined by a multivariate curve resolution-alternating least squares analysis, which identified the components of the band causing the spectral variation. The band consisted of three common components almost independent of the alcohol type. The first and the second components are attributed, respectively, to water molecules weakly hydrogen-bonded (or non hydrogen-bonded) and those strongly hydrogen-bonded with other water molecules, while the third component are due to water interacting with the alcohol and to the alcohol itself. The abundance of the first and third components decreased and increased, respectively, as the alcohol concentration increased. In contrast, the abundance of the second component increased initially and then decreased. The initial increase corresponds to the enhancement of hydrogen bonding by hydrophobic interactions. The subsequent decrease is due to an increase in water–alcohol interactions and a decrease in water concentration. The maximum increase in the abundance of the second component depended on the type of alcohol. The increase in abundance was greater for alcohols with larger alkyl groups. In contrast, the increase in abundance of the second component was smaller for alcohols with more hydric groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The structure of water, i.e., the hydrogen-bonded network in liquid water, changes upon the dissolution of other substances. Previous reports [1, 2] have described these changes by obtaining near-infrared (NIR) absorption spectra of water mixed with salts or aprotic polar organic compounds. The shape of the overtone band due to O–H stretching vibration of water around 7000 cm−1 in the NIR absorption spectrum is sensitive to changes in the hydrogen-bonded network. Principal component analysis (PCA) of spectra of aqueous solutions obtained for solutes, at different concentrations and temperatures, revealed that the spectral changes in the overtone band consisted of two components. The first component is similar to the change observed upon a temperature change for pure water. The second component is the change corresponding to formation of more strongly hydrogen-bonded water. Upon the dissolution of a solute, these two changes take place simultaneously to produce different component ratios depending on the solute. Therefore, changes in the spectrum of water appear to be dependent on the solute.
Changes in the liquid structure of water upon dissolution of a protic compound such as an alcohol are also interesting. Many studies have been performed on aqueous solutions of protic compounds. Some studies were based on NIR spectroscopy [3,4,5,6,7,8,9,10,11,12]. However, interpreting the NIR absorption spectrum of an aqueous solution of a compound with OH groups can be difficult because the absorption by the solute OH group can overlap that of water. Deconvolution is often applied to separate observed spectra into components. Partial molar extinction coefficients based on difference spectra have also been used for analyses [13, 14]. However, the spectral changes have not been analyzed thoroughly using these techniques.
In the present study, changes in the liquid structure of water upon dissolution of various polyhydric alcohols (nine dihydric alcohols and two trihydric alcohols) were investigated by obtaining NIR absorption spectra of the aqueous solutions at various concentrations and temperatures. The multivariate curve resolution-alternating least squares (MCR-ALS) method [15, 16], which is a method recently becoming widely used, was used to separate the observed overtone band due to the O–H stretching vibrations into components. Concentration dependence of the abundances of components was examined in detail to elucidate changes in the structure of water upon dissolution of the polyhydric alcohols.
2 Experimental
2.1 Chemicals
Eleven polyhydric alcohols (reagent grade) were purchased from FUJIFILM Wako Pure Chemical Co. (Wako) or Tokyo Chemical Industry Co., Ltd. (TCI), and were used without further purification. The polyhydric alcohols used are listed with abbreviations, manufacture names, and purities: ethylene glycol (EG, Wako, > 99.5%), 1,3-propanediol (1,3-PrD, Wako, > 97%), 1,2-propanediol (1,2-PrD, Wako, > 99%), 1,4-butanediol (1,4-BuD, Wako, > 98%), 1,3-butanediol (1,3-BuD, Wako, > 98%) 1,5-pentanediol (1,5-PeD, TCI, > 97%), 2-methyl-1,3-propanediol (2-Me-1,3-PrD, TCI, > 98%), 2,2-dimethyl-1,3-propanediol (2,2-dMe-1,3-PrD, TCI, > 98%), 3-methyl-1,3-butanediol (3-Me-1,3-BuD, TCI, > 98%), glycerin (GLY, Wako, > 99.5%), and 1,2,4-butanetriol (1,2,4-BuT, racemate, TCI, > 97%). Structural formulae of the alcohols are shown in Fig. 1. Since the results of analyses were systematic and consistent, impurities in the reagents did not appear to influence the qualitative effects of solutes on the experimental results.
2.2 Spectroscopy
Aqueous solutions of each polyhydric alcohol at concentrations from 2 to 20% (2% interval) molar fraction were prepared by weighing the water and polyhydric alcohol on an electronic balance (Shimadzu, AUX220). The NIR absorption spectra were obtained using an NIR spectrophotometer (Bruker, MPA, resolution: 8 cm−1, accumulation: 64 scans) with a quartz cell having an optical path length of 1 mm. The temperature of each sample was set at 20, 25, or 30 °C using a thermostat (Taitec, CTU-Mini) attached to a home-made cell holder. The thermostat was adjusted so as that the digital thermometer with the probe thermocouple in the cell holder indicated the target temperature. Spectra of aqueous solutions, pure water, and pure polyhydric alcohols were obtained. Reproducibility of the measurements was confirmed by repeating measurements twice or three times for each solute.
2.3 Analyses with MCR-ALS
With the MCR-ALS method, as with deconvolution, an absorption band composed of several overlapping components is separated into components. Conventional deconvolution often involves arbitrary assignments because the number of components and the position and shape of the absorption band of each component are chosen arbitrarily for reconstruction of the observed band. Therefore, the reliability of the results often is not high. However, the MCR-ALS method, produces substantially consistent results for the same set of spectra. Therefore, it is a method capable of separating spectra that undergo complicated changes with variations in concentration and temperature into reliable components. Note, however, that the spectral components obtained do not necessarily correspond to chemical components in the target aqueous solution. In addition, the components obtained may depend on the set of spectra used for MCR-ALS. Despite these potential limitations, the results of MCR-ALS obtained in the present study provide valuable information about changes in the structure of liquid water.
For MCR, each spectrum was separated into components under the assumption that all of the spectra observed, represented by a matrix X, could be represented by a linear combination of several common spectral components. Spectral separation was performed by creating a matrix S corresponding to component spectra and a matrix C representing the abundances of the components. The ALS technique was employed for the calculations. The flow of calculations was:
- (1)
Singular value decomposition was done for the spectra of all aqueous solutions to determine the number of components.
- (2)
The initial C matrix was estimated using evolving factor analysis (EFA) [17].
- (3)
The S matrix was calculated from X and C.
- (4)
The S matrix was normalized to a maximum magnitude of 1. Since the S and C matrices represented spectral data and abundances of the components, respectively, any negative value for an element was replaced with 0.
- (5)
The revised C matrix was determined from X and S.
- (6)
Steps (3)–(5) were repeated until the values of C and S converged.
The 7400–5500 cm−1 region of the spectra obtained was analyzed using TOOLBOX of MATLAB (R2017b) [18]. The convergence criterion was set to 0.1. The number of components was determined to be three by singular value decomposition. A slight expansion of the region by 100 cm−1 used for analysis did not affect the results very much.
3 Results and Discussion
3.1 Separation of Observed Spectra into Components
Figure 2 shows the NIR absorption spectra of water, EG, and aqueous solutions of EG at different concentrations measured at 20 °C. The absorption near 7400–6000 cm−1 is due to an overtone of the O–H stretching vibration. In the spectrum of EG, the band with a wavenumber below 6000 cm−1 is due to an overtone of the C–H stretching vibration. The assignments of the C–H overtone bands of alcohols, which contain some ambiguity, were made with reference to the literature [19,20,21]. As the concentration of EG increased, absorption due to water decreased and absorption due to EG appeared. Since absorption by the OH group of EG overlapped with that of water, estimating their contributions to the observed absorption was difficult without performing analytical processing. A similar situation was seen also in the spectra of aqueous solutions of the other polyhydric alcohols. Therefore, the MCR-ALS method was employed to separate the spectra into components.
The three components obtained using MCR-ALS analysis from the spectra of aqueous solutions of EG are shown in Fig. 3. In addition to the spectra of pure water and aqueous solutions obtained at 20 °C shown in Fig. 2, spectra obtained at 25 °C and 30 °C also were included in the analysis to identify more general components. Three components were needed to reconstruct the observed spectra. Before the MCR-ALS analysis, we also checked that the spectral variations could be reproduced satisfactorily with three components by performing PCA.
Three components obtained by MCR-ALS analysis for spectra of aqueous solutions of EG. C1, C2, and C3 are attributed, respectively, to weakly hydrogen-bonded (or non hydrogen-bonded) water (C1), water strongly hydrogen-bonded with other water molecules (C2), and water interacting with alcohol and the alcohol itself (C3)
Figure 3 shows each component spectrum normalized to a maximum absorbance of 1. Component 1 (C1), shown by a black solid line, and component 2 (C2), indicated by a solid gray line, were attributed to weakly hydrogen-bonded (or non hydrogen-bonded) water and strongly hydrogen-bonded water, respectively, from the temperature dependence of the abundance ratios of the components and the temperature dependence of the absorption spectrum of pure water [22]. Component C1 was similar to the absorption observed for pure water at temperatures from 20 to 60 °C, while C2 was similar to the absorption spectra of pure water at lower temperatures. In contrast, component 3 (C3) produced an absorption in the lower wavenumber region, believerd to be due to water interacting with EG and to EG itself.
The results of MCR-ALS analyses of the NIR absorption spectra obtained for aqueous solutions of other polyhydric alcohols are shown in Fig. 4. For each alcohol, spectra of aqueous solutions obtained at concentrations from 0 to 20% and at 20, 25, and 30 °C were analyzed. For each alcohol, the spectra were separated into three components similar to those obtained for EG. Components C1, especially, was common to all alcohol solutions, revealing that the component is due only to water. The less common C2 and C3 components were identified in all alcohol solutions, indicating again that most of the absorption observed in this region is due to water and that the observed changes are caused mainly by changes in the hydrogen bonding of water. However, detailed comparisons revealed slight differences in C2 and C3 among the alcohols in the region of 6500–5500 cm−1, where strong absorption by polyhydric alcohol was observed.
3.2 Dependence of Relative Abundance of Components on Concentration
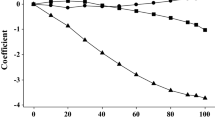
The spectra of aqueous solutions obtained at each concentration and temperature were fitted using the component spectra obtained through MCR-ALS analysis to determine how the abundance of each component varied with alcohol concentration. Results for EG are shown in Fig. 5.
The abundances of C1 and C2 are zero, respectively, at concentrations of 20% at 20 °C and 0% at 30 °C, probably due to the characteristics of the analysis method. Component separation is performed in a way that any component becomes zero at the limit of the analysis condition, suggesting that the calculated abundances do not correspond to actual values. However, this does not significantly affect the following qualitative discussions.
Raising the temperature increased the abundance of C1 corresponding to weakly hydrogen-bonded (or non hydrogen-bonded) water. This is a reasonable response as hydrogen bonds break with the increase in temperature. Since the concentration of water decreased as the alcohol concentration increased, the relative abundance of weakly hydrogen-bonded (or non hydrogen-bonded) water was expected to increase; the decrease in water concentration results in equilibrium dissociation of the hydrogen bonds of water. However, C1 decreased rapidly as the EG concentration increased. Two possible reasons for this behavior are: (1) dissociated water forms hydrogen bonds with alcohol, and (2) hydrogen bonding between water molecules is accelerated by the hydrophobic effect of the alkyl group of the alcohol.
The abundance of C3 increased with EG concentration. The temperature dependence of C3 was small. These findings suggest that EG forms a relatively strong hydrogen bond with surrounding water molecules within the concentration and temperature ranges studied; thus, almost all EG molecules were hydrogen bonded with water molecules.
The abundance of C2 increased with EG concentration when the EG concentration was low, but decreased as the EG concentration increased. The EG concentration range where C2 increased depended on temperature. Although C2 increased when the temperature was low, the concentration region where C2 increased was limited to an EG concentration of ≤ 4%. Although the increase in C2 was lower at higher temperatures, the concentration region where C2 increased included the EG concentration range up to 10%. The increase in C2 upon dissolution of EG was considered to be due to the promotion of hydrogen bonding of water by the hydrophobic effect of the alkyl chain of EG. As the EG concentration continued to increase, interactions between the OH groups of EG and water also increased, causing a decrease in C2 corresponding to hydrogen bonding between water molecules.
3.3 Dependence of Component Abundance on Alcohol Concentration
The dependence of the abundance of each component on alcohol concentration was examined in detail for aqueous solutions of 11 polyhydric alcohols. Figure 6a shows the concentration dependence of C1 abundance for the alcohols at 20 °C. To simplify the comparison, the curves showing dependence are shown with shifted baselines along the vertical axis. The results indicate that concentration dependence is similar for all alcohol mixtures, although some small differences were observed.
Figure 6b shows the concentration dependence of C3 abundances. Component C3 increased with alcohol concentration regardless of the type of alcohol. However, a detailed examination revealed that the increases were greater for solutions of GLY and 1,2,4-BuT than for the other alcohol solutions. Because GLY and 1,2,4-BuT have three OH groups, strong interactions between the alcohols and water have occurred.
Finally, the concentration dependence of the C2 abundance is shown in Fig. 6c. For the aqueous solution of GLY, no increase in C2 abundance was observed at any concentration, indicating that mixing of GLY destroys the hydrogen-bonded network of water. Because GLY has many hydroxyl groups that interact preferentially with water, it disrupts water interactions. For alcohols other than GLY, the abundance of C2 increased similar to that of the EG solution when the alcohol concentration was low. However, the degree of increase and the concentration at which C2 reached maximum abundance depended on the alcohol. Some alcohols caused only a slight increase in C2, such as EG, while other alcohols caused a large increase, such as 3-Me-1,3-BD. To investigate these differences, the maximum increase of the relative abundance of C2 for each alcohol solution was compared with the relative abundance of C2 for pure water at 20 °C is shown in Fig. 7. The results shown in Fig. 7 can be summarized as follows:
Maximum increase of the relative abundance of C2 at 20 °C for each alcohol solution compared with the relative abundance of C2 for pure water. Black bars show results for diols with OH groups at both ends of the alkyl chain. Meshed bars show results for diols and triols with an OH group at the middle of the alkyl chain. Dashed-line bars show the results for diols with OH groups at both ends of the alkyl chain containing side chains
A comparison of the results for EG, 1,3-PrD, 1,4-BuD, and 1,5-PeD showed that the increase in C2 (i.e., the structuring of water) was greater as the hydrophobic moiety increased (i.e., as the carbon chain became longer), suggesting that the structuring of water by mixing with a polyhydric alcohol is due mainly to hydrophobic effects.
A comparison of the results for 1,3-PrD with that for 1,2-PrD, and the results for 1,4-BuD with that for 1,3-BuD, showed that the increase in C2 was larger (i.e., water is more likely to be structured) when the hydroxyl group was located at a position other than the end of the alkyl chain. Because the non-terminal hydroxyl group of an alcohol is less likely to interact with water and other alcohols compared to the terminal hydroxyl groups, the structuring of water occurs more easily.
A comparison among alcohols having the same number of carbons revealed that the increase in C2 was slightly smaller with branched alcohols than with linear alcohols. Thus, the structuring of water does not proceed easily with alcohols having a branched structure because their surface areas are smaller than those of linear alcohols.
Structuring of water was promoted by alcohols with more carbon atoms. However, compared to alcohols with few carbon atoms, those with more carbons tended to cause structural destruction at high concentrations. An alcohol with many carbons having a large hydrophobic portion might not be able to maintain a stable structure of the water. Another possibility is that a rapid decrease in water concentration upon the dissolution of a large volume of alcohol with many carbon atoms more easily disrupts the structure.
Dissolution of 1,2,4-BuT improved the structuring of water slightly, although it has the same number of hydroxyl groups as GLY, presumably because the hydrophobic portion of 1,2,4-BuT is larger than that of GLY. However, the degree of structuring was small compared to that caused by diols with the same number of carbons (1,4-BuD and 1,3-BuD). Greater structural destruction of water appeared to be caused by alcohols containing a larger number of hydroxyl groups.
Structuring and structural destruction of water upon the dissolution of an alcohol has been studied conventionally by measuring molar partial heat capacity or excess molar partial volume. Only alcohols with a small number of carbons have been investigated and some contradictory conclusions have been reported. For example, based on data of molar partial heat capacity, EG was found to advance the structuring of water [23], whereas almost no effect on water structuring by EG was found using data from excess molar partial volume [24]. The influence of alcohol mixing on the structure of water is not fully understood. The technique reported here, analyzing observed NIR absorption spectra of aqueous solutions using MCR-ALS, enabled direct observation of changes in the hydrogen bonding state of water that could not be followed by obtaining measurements of heat quantity or volume of the mixtures. Therefore, this technique shows promise for more detailed exploration of the hydrogen-bonding state of aqueous solutions.
4 Conclusion
Variations in near-infrared spectra around 7400–5500 cm−1 of aqueous solutions of eleven polyhydric alcohols were analyzed by the MCR-ALS technique. The variations have been found to consist of three components regardless of the type of alcohol. The components could be attributed to (1) weakly hydrogen-bonded (or non hydrogen-bonded) water, (2) water strongly hydrogen-bonded with other water molecules, and (3) water interacting with the alcohol and the alcohol itself. The fact that the hydrogen bonding state of water in the aqueous solutions of polyhydric alcohols consists of three components is consistent with the results of our past studies [1, 2] and recently published papers on water and aqueous solutions [25, 26].
The second component was found to increase with alcohol concentration and then decrease in regions where the alcohol concentration is low. The magnitude of the maximum increase was dependent on the type of alcohol. The first increase is believed to be due to the hydrophobic effect of the alkyl group of the alcohol. The subsequent decrease is believed to be due to the decrease in water concentration and the formation of hydrogen bond between alcohol and water molecules. A larger number of hydroxyl groups in alcohol are apt to cause structural destruction of water.
References
Uchida, N., Yoshimura, N., Takayanagi, M.: Variation of the near-infrared spectrum of water from dissolved salts. J. Solution Chem. 44, 2167–2178 (2015)
Katsu, S., Yoshimura, N., Takayanagi, M.: Variation in the liquid structure of water upon dissolution of aprotic polar organic compounds as studied by near-infrared spectroscopy. J. Solution Chem. 47, 1202–1213 (2018)
Kwasniewicz, M., Czarnecki, M.A.: The effect of chain length on mid-infrared and near-infrared spectra of aliphatic 1-alcohols. Appl. Spectrosc. 72, 288–296 (2018)
Tomza, P., Wrzeszcz, W., Mazurek, S., Szostak, R., Czarnecki, M.A.: Microheterogeneity in binary mixtures of water with CH3OH and CD3OH: ATR-IR spectroscopic, chemometric and DFT studies. Spectrochim. Acta A197, 88–94 (2018)
Tomza, P., Czarnecki, M.A.: Microheterogeneity in binary mixtures of propyl alcohols with water: NIR spectroscopic, two-dimensional correlation and multivariate curve resolution study. J. Mol. Liq. 209, 115–120 (2015)
Chen, Y., Ozaki, Y., Czarnecki, M.A.: Molecular structure and hydrogen bonding in pure liquid ethylene glycol and ethylene glycol–water mixtures studied using NIR spectroscopy. Phys. Chem. Chem. Phys. 15, 18694–18701 (2013)
Czarnecki, M.A., Muszynski, A.S., Troczynska, H.: Molecular structure and hydrogen bonding in liquid cyclohexanol and cyclohexanol/water mixtures studied by FT-NIR spectroscopy and DFT calculations. J. Mol. Struct. 974, 60–67 (2010)
Haufa, K.Z., Czarnecki, M.A.: Effect of temperature and water content on the structure of 1,2-propanediol and 1,3-propanediol: Near-infrared spectroscopic study. Vib. Spectrosc. 51, 80–85 (2009)
Czarnecki, M.A., Wojtkow, D.: Effect of varying water content on the structure of butyl alcohol/water mixtures: FT-NIR two-dimensional correlation and chemometric studies. J. Mol. Struct. 883–884, 203–208 (2008)
Wojtkow, D., Czarnecki, M.A.: Two-dimensional attenuated total reflection infrared and near-infrared correlation study of the structure of butyl alcohol/water mixtures. Appl. Spectrosc. 61, 928–934 (2007)
Wojtkow, D., Czarnecki, M.A.: Effect of temperature and concentration on the structure of sec-butyl alcohol and isobutyl alcohol/water mixtures: near-infrared spectroscopic study. J. Phys. Chem. A 110, 10552–10557 (2006)
Wojtkow, D., Czarnecki, M.A.: Effect of temperature and concentration on the structure of tert-butyl alcohol/water mixtures: near-infrared spectroscopic study. J. Phys. Chem. A 109, 8218–8224 (2005)
Li, Q., Wang, N., Zhou, Q., Sun, S., Yu, Z.: Excess infrared absorption spectroscopy and its applications in the studies of hydrogen bonds in alcohol-containing binary mixtures. Appl. Spectrosc. 62, 166–170 (2008)
Koga, Y., Sebe, F., Minami, T., Otake, K., Saitow, K., Nishikawa, K.: Spectrum of excess partial molar absorptivity. I. Near infrared spectroscopic study of aqueous acetonitrile and acetone. J. Phys. Chem. B 113, 11928–11935 (2009)
de Juan, A., Tauler, R.: Chemometrics applied to unravel multicomponent processes and mixtures. Revisiting latest trends in multivariate resolution. Anal. Chim. Acta 500, 195–210 (2003)
Garrido, M., Rius, F.X., Larrechi, M.S.: Multivariate curve resolution–alternating least squares (MCR-ALS) applied to spectroscopic data from monitoring chemical reactions processes. Anal. Bioanal. Chem. 390, 2059–2066 (2008)
Keller, H.R., Massart, D.L.: Evolving factor analysis. Chemom. Intell. Lab. Syst. 12, 209–224 (1992)
Jaumota, J., Gargalloa, R., de Juana, A., Tauler, R.: A graphical user-friendly interface for MCR-ALS: a new tool for multivariate curve resolution in MATLAB. Chemom. Intell. Lab. Syst. 76, 101–110 (2005)
Beć, K.B., Futami, Y., Wójcikd, M.J., Ozaki, Y.: A spectroscopic and theoretical study in the near-infrared region of low concentration aliphatic alcohols. Phys. Chem. Chem. Phys. 18, 13666–13682 (2016)
Grabska, J., Beć, K.B., Ozaki, Y., Huck, C.W.: Temperature drift of conformational equilibria of butyl alcohols studied by near-infrared spectroscopy and fully anharmonic DFT. J. Phys. Chem. A 121, 1950–1961 (2017)
Beć, K.B., Grabska, J., Huck, C.W., Czarnecki, M.A.: Spectra–structure correlations in isotopomers of ethanol (CX3CX2OX; X = H, D): combined near-infrared and anharmonic computational study. Molecules 24, 2189 (2019)
Maeda, H., Ozaki, Y., Tanaka, M., Hayashi, N., Kojima, T.: Near infrared spectroscopy and chemometrics studies of temperature-dependent spectral variations of water: relationship between spectral changes and hydrogen bonds. J. Near Infrared Spectrosc. 3, 191–201 (1995)
George, J., Sastry, N.V.: Partial excess molar volumes, partial excess isentropic compressibilities and relative permittivities of water + ethane-1,2-diol derivative and water + 1,2-dimethoxyethane at different temperatures. Fluid Phase Equilib. 216, 307–321 (2004)
Huot, J.-Y., Battistel, E., Lumry, R., Villeneuve, G., Lavallee, J.-F., Anusiem, A., Jolicoeur, C.: A comprehensive thermodynamic investigation of water-ethylene glycol mixtures at 5, 25, and 45°C. J. Solution Chem. 17, 601–636 (1988)
Okajima, H., Ando, M., Hamaguchi, H.: Formation of “nano-ice” and density maximum anomaly of water. Bull. Chem. Soc. Jpn. 91, 991–997 (2018)
Cheng, D., Cai, W., Shao, X.: Understanding the interaction between oligopeptide and water in aqueous solution using temperature-dependent near-infrared spectroscopy. Appl. Spectrosc. 72, 1354–1361 (2018)
Funding
This study was funded only by Tokyo University of Agriculture and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katsu, S., Ito, S., Yoshimura, N. et al. Variation in Near-Infrared Spectra of Water Containing Polyhydric Alcohol. J Solution Chem 48, 1564–1575 (2019). https://doi.org/10.1007/s10953-019-00928-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-019-00928-5