Abstract
In order to investigate the effect of dissolution of salts on the hydrogen-bonded network in liquid water, near-infrared absorption spectra of aqueous solutions of 16 salts, containing Na+ as common cation, were measured in the region where the first overtone of the –OH stretching mode of water is observed. Although the spectral variations of water resulting from dissolution of a salt is dependent on the kind of salt, principal component analysis of the observed spectra revealed that all spectral variations for the 16 salts were almost reproducible with only three components. The first component corresponds to the average of the observed spectra, while the other two components are responsible for the variations. The second component, which almost coincides with the component of the spectral variation of water from changes in temperature, was found to explain mainly the spectral changes by salts that destroy the hydrogen-bonded network. On the other hand, the third component, which includes the spectral changes at a lower wavenumber region than the second component, was found to mainly explain the spectral variation from the salts that expand the hydrogen-bonded network. These results suggest that observed spectral variations are not due to direct interaction between ions and water molecules, but due to the change of the hydrogen-bonded network because all variations produced by these 16 salts can be explained by only two components. The results suggest also that the mechanisms of destruction and expansion of the hydrogen-bonded network by the anions may be different.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The liquid structure of water, i.e. the hydrogen-bonded network in liquid water, is an important and interesting research target in relation not only to the dissolving ability of water but also to the behavior of ions and reactions in aqueous solutions. Also, most biological reactions proceed in aqueous solutions. The hydrogen-bonded network of liquid water is dynamical and is so complicated that its behavior has so far not been elucidated clearly in spite of the great number experimental and theoretical researches [1, 2].
Near-infrared (NIR) spectroscopy is one of the most effective experimental techniques for the investigation of the hydrogen-bonded network in liquid water. Since strong hydrogen-bonded –OH and weak hydrogen-bonded (or non-hydrogen-bonded) –OH are clearly distinguishable in the NIR absorption spectra of water, we can expect to get information on intermolecular interactions of water molecules in the liquid state. The NIR absorption band of liquid water around 6900 cm−1, measured at various temperatures, reveals an isosbestic point [3, 4], suggesting two components are present in liquid water. The components might be assignable to strongly hydrogen-bonded water and weakly hydrogen-bonded (or non-hydrogen-bonded) water, which causes, respectively, the lower-frequency and the higher-frequency components of the NIR absorption band of water. Observation of two components seems, however, strange because a water molecule can work both as proton donor and as proton accepter; more components due to water molecules with hydrogen bonds of various types are expected. There must be more than two components present in liquid water which are not distinguishable from the spectral variations with temperature.
In order to check whether or not more components exist in liquid water, we have observed the changes of NIR absorption spectra of water upon the dissolution of salts. There have been some reports on NIR spectra of aqueous solutions of salts [3–17]. We measured the NIR absorption spectra of aqueous solutions of various salts at various concentrations and at various temperatures in order to investigate the dependence of the spectra of water on the ion in the solutions in more detail than in the previous studies. The variations of spectra with concentration were found to depend slightly on the salts. The spectra varied also with change of temperature. We found it rather difficult to analyze the spectra systematically with the traditional method where the variations of peak positions and intensities of absorption bands are examined. Therefore, we applied principal component analysis (PCA) [18] for the precise and detailed analyses of the spectral variation of aqueous solutions with the kind and concentration of salt and the temperature. Recently, PCA was employed to analyze the observed variation of NIR spectra with the temperature [3] and on the dissolution of some salts [4, 15, 16]. In the PCA scheme, each spectrum is expressed as a point in the multidimensional space. An orthogonal linear transformation is performed for the space to find a new coordinate system where the greatest variance by the projection of the data comes to lie on the first coordinate axis, the second greatest variance on the second coordinate axis, and so on. Each coordinate axis corresponds to a principal component. From the analysis, each observed spectrum is expressed as a linear combination of principal components, where the coefficients for the components are called scores and are used for score plots. PCA abstracts common components of variations of the observed spectra, which make quantitative analyses of the variations possible.
There have been many reports on the changes in the hydrogen-bonded network of water upon the addition of ions from dissolved salts [2, 19–21]. The effects of ions have been mainly investigated by the variation of macroscopic properties of water such as salting out and salting in or viscosity. Different effects observed for different ions have been considered to originate from the different abilities of ions; some extend the hydrogen-bonded network of water, while the other ions destroy it. These abilities are often explained by the Hofmeister series [2, 22, 23] where ions are classified into kosmotropes and chaotropes that are believed, respectively, to expand or destroy the hydrogen-bond network of water. The Hofmeister series of some anions found in the literature [8, 15] are summarized in Fig. 1. Recently, however, there have been some reports where the observed spectral variations of water from the dissolution of salts are not attributable to the variation of the hydrogen-bonded network in bulk water but to direct interaction between water molecule and ions [20, 24, 25], although most other reports claim that ions in aqueous solutions can affect the hydrogen bonded network of water molecules in addition to the direct interaction [26–29]. Therefore, the elucidation of effects of ions in water is another puzzle to be solved. Here we discuss this point based on the microscopic information of intermolecular interaction in aqueous salt solutions obtained by NIR spectroscopy.
2 Experimental
2.1 Chemicals Used
Sixteen salts containing sodium ion as the common cation were used in this study. Here the effects of anions on the hydrogen-bonded network of water are discussed. All salts of reagent grade were bought from Wako Pure Chemical Industries Ltd. and were used without further purification. They were used without drying since the strictly quantitative effects of salts are not being discussed here. Some salts were bought as hydrates.
The salts and their purities were as follows: sodium fluoride (NaF, >99.0 %), sodium chloride (NaCl, >99.5 %), sodium bromide (NaBr, >99.5 %), sodium iodide (NaI, >99.5 %), sodium nitrate (NaNO3, >99.0 %), sodium carbonate (Na2CO3, >99.8 %), sodium sulfate (Na2SO4, >99.0 %), sodium acetate (CH3COONa, >98.5 %), sodium thiocyanate (NaSCN, >99.0 %), sodium hydroxide (NaOH, >97.0 %), sodium perchlorate (NaClO4·H2O, >98.0 %), sodium sulfite (Na2SO3, >97.0 %), sodium nitrite (NaNO2, >98.5 %), sodium dihydrogenphosphate (NaH2PO4·2H2O, >99.0 %), sodium thiosulfate (Na2S2O3·5H2O, >99.0 %) and sodium l-(+)-tartrate (C4H4O6Na2·2H2O, > 99.0 %).
2.2 Spectroscopic Measurements
The salts were dissolved at various concentrations in deionized water whose resistivity was larger than 3 MΩ·cm. The concentrations of salts were adjusted to 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5 % in mole fraction. NIR absorption spectra were measured with an FT-NIR spectrophotometer (Bruker, MPA, resolution: 8 cm−1, accumulation: 64 scans) using a quartz cell whose optical path-length was 1 mm. The temperature of each sample was stabilized at 20, 25, or 30 °C with the thermostat (Taitec, CTU-Mini) attached with a home-made cell holder. Solutions were prepared just before the measurements of spectra to avoid their deterioration. The observed spectra were corrected with the densities of solutions determined by weighing the solutions of a definite volume. FreeMat 4.0 (http://freemat.sourceforge.net/) was used for the PCA calculations.
3 Results and Discussion
Figure 2a, b shows the variations of NIR absorption spectra at 20 °C of (a) Na2CO3 and (b) NaClO4 aqueous solutions with changes of the concentration in the region 7400–6200 cm−1 where the first overtone of the –OH stretching mode of water is observed. Since only dilute solutions were measured in this study, the changes are not clearly observed although five spectra for the aqueous solutions with different concentrations are shown in each figure. In order to show the variations definitely, difference spectra obtained by subtracting the spectrum of pure water from the spectra of aqueous solutions are shown in Fig. 2c, d. Since Na2CO3 and NaClO4 contain a common cation, Na+, the effects of \( {\text{CO}}_{3}^{2 - } \) and \( {\text{ClO}}_{4}^{ - } \) on the hydrogen-bond network of water are being compared. Although \( {\text{CO}}_{3}^{2 - } \) in aqueous solution is partially hydrolyzed by water to form \( {\text{HCO}}_{3}^{ - } \), here for simplicity we refer to \( {\text{CO}}_{3}^{2 - } \) for the ions resulting from Na2CO3. Although the hydrolysis by water simultaneously produces OH− which might be observed in the spectra, the absorption bands caused by OH− are not discussed here because their contribution in the dilute solutions is expected to be small compared with the strong absorption bands due to water. Spectra of other salts producing OH− in aqueous solutions, such as NaOH and NaH2PO4, were treated similarly.
NIR absorption spectra of aqueous solutions of a sodium carbonate (Na2CO3) and b sodium perchlorate (NaClO4) at five different concentrations (0.1, 0.2, 0.3, 0.4, and 0.5 % in mole fraction) measured at 20 °C, NIR difference spectra of c Na2CO3 and d NaClO4 obtained by subtracting the spectrum of pure water from the observed spectra of the solutions
In the Hofmeister series \( {\text{CO}}_{3}^{2 - } \) and \( {\text{ClO}}_{4}^{ - } \) are known, respectively, as a kosmotrope and a chaotrope. By increasing the concentration of Na2CO3, the intensity of the band at the lower wavenumber side increased, while that at the higher wavenumber side decreased. The absorption at the lower wavenumber region is due to –OH which forms a stronger hydrogen bond. The variation of the spectra with the dissolution of Na2CO3 revealed that the salt expands the hydrogen-bonded network of water. On the other hand, increasing the concentration of NaClO4 caused a decrease and an increase in the absorption intensities at the lower and the higher wavenumber sides, respectively, suggesting the destruction or weakening of the hydrogen-bonded network of water. No change in the positions of the features of the difference spectra upon the concentration changes was observed for both of their aqueous solutions within the concentration range used for the present study.
Careful comparison of spectral variations in Fig. 2c, d revealed that the wavelength regions where the absorbance varied most were different for Na2CO3 and NaClO4 solutions, in addition to the opposite directions of the spectral variations. Isosbestic points for the solutions were also observed at different wavelengths. Therefore, the effects of \( {\text{CO}}_{3}^{2 - } \) and \( {\text{ClO}}_{4}^{ - } \) on the hydrogen-bonded network of water are found to be opposite but not completely reversed.
Figure 3 shows near-infrared difference spectra of aqueous solutions of the fourteen salts, obtained by subtracting the spectrum of pure water from the spectra measured at five concentrations of 0.1, 0.2, 0.3, 0.4, and 0.5 % in mole fraction. Spectra of NaOH, Na2SO3, NaF, NaH2PO4, CH3COONa, and sodium l-(+)-tartrate aqueous solutions were found to show concentration dependences similar to that of the Na2CO3 aqueous solutions; these salts expand the hydrogen-bonded network of water. On the other hand, other salts, NaNO2, NaNO3, NaSCN, Na2S2O3, NaI, NaBr, NaCl and Na2SO4 were found to affect water similarly to NaClO4; these salts destroy or weaken the hydrogen-bonded network of water. These results are consistent with the Hofmeister series which categorizes ions as kosmotropes and chaotropes [2, 22, 23].
In spite of some similarities among spectral changes for kosmotropes and among those for chaotropes, detailed investigation revealed that the concentration dependences of the NIR absorption spectra were different for each of the 16 salts. In order to analyze the dependences systematically, PCA was performed for spectra in the 7400–6200 cm−1 region of pure water and the aqueous solutions of 16 salts with five different concentrations at three different temperatures, i.e. 243 spectra were measured in all. Figure 4 shows the first four principal components (loading vectors), P1–P4, obtained by PCA. Component P1 corresponds to the average of all observed spectra, which is almost equal to the spectrum of water because the spectral changes in this study were very small as shown in Fig. 1a, b, and PCA were performed for the observed raw spectra and not the calculated difference spectra without being mean-centered. Components P2–P4, on the other hand, are responsible for the variations in the spectra. As for the contribution ratios of P1–P4 for the observed spectra shown in Table 1a, more than 99 % of the spectra can be reproduced by P1; spectral changes resulting from dissolution of salts in this study are very small. Here, the contribution ratios are cumulative contributions, which represent the ratio of square sum of scores for the principal components considered for the reproduction, against the square sum of all scores for all principal components.
In Table 1b are shown the contributions of P2–P4 to the spectral changes. About 96 % of the observed spectral changes can be reproduced only with P2, while 99.8 % of the variations can be reproduced with P2 and P3. Since the contributions of P4 to the variations are so small compared with those of P2 and P3, we only discuss in this study the spectral variations with P2 and P3. Although P4 may be responsible for the species dependence of the spectral variations, the detailed discussion of P4 and the higher components are left as a subject for the future.
The spectral variations caused by the 16 salts could almost be sufficiently explained with only three components, suggesting that the observed variations are not due to the water molecules interacting with ions directly but are caused by the change of the hydrogen-bonded network of water by ions; spectra of water molecules directly interacting with ions should be salt dependent, resulting in many more components for the spectral variations.
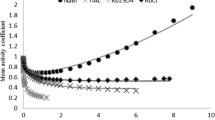
Variations of spectra for the 16 salts were analyzed in more detail with the score plot shown in Fig. 5, where score t 3 for P3 against score t 2 for P2 of each spectrum measured at 20 °C are plotted. The plot for pure water is at around (0.35, 0.03). The plots corresponding to the solutions are spread radially from the plot for pure water. For each salt, the plot was found to yield an almost linear variation from the plot for pure water in a specific direction as the concentration of the solution was increased. Since the concentrations of solutions used for measurements had a common molar fraction of anion, the distance of the plot for the solutions with the highest concentration from the plot for pure water reflects the degree of the effect for each anion.
Plots for kosmotropes and chaotropes in Fig. 5 are found to behave in quite different manners. Plots for chaotropes, which destroy the hydrogen-bonded network of water in aqueous solutions, moved to the left or to the upper left on increasing the concentration of the solutions. On the other hand, plots for kosmotropes, which expand the hydrogen-bonded network, moved downward or to the lower right. Therefore, the score t 2 for P2 mainly changed when a chaotrope was dissolved, while score t 3 for P3 mainly changed when a kosmotrope was dissolved. For example, the score t 2 mainly changed for the plots of \( {\text{ClO}}_{4}^{ - } \), while the score t 3 mainly changed for the plots of \( {\text{CO}}_{3}^{2 - } \). The score plot in Fig. 5 again suggests that the effects of chaotropes and kosmotropes on the hydrogen-bonded network of water are essentially different. They are opposite, but not perfectly reversed, as was found in Fig. 2c, d. It should be noted that Na2SO4 was the only exception among the 16 salts, because the plots for Na2SO4 moved slightly to the lower left as the concentration was increased.
In Table 2, the assignment of anions as chaotropes and kosmotropes, based on the PCA score plot in Fig. 5, is shown. Most anions are classified as has been reported in the literature [22, 23] and shown in Fig. 1. Exceptions are \( {\text{SO}}_{4}^{2 - } \) and \( {\text{S}}_{2} {\text{O}}_{3}^{2 - } \), which were categorized as kosmotropes in the literature while in this study \( {\text{S}}_{2} {\text{O}}_{3}^{2 - } \) is categorized as a chaotrope and \( {\text{SO}}_{4}^{2 - } \) was found to be almost neutral in its effect on the hydrogen bonded network. The discrepancy is presumably caused by the difference in the method of classification. The traditional Hofmeister series was determined by their ability for salting in and salting out by anions, while the effect on the hydrogen-bonded network is the basis of the classification used here. This discrepancy should be discussed in more detail in the future.
Figure 6 shows the score plots for all 243 spectra measured at three different temperatures. Three groups of plots, which are distinguished with different markers, correspond to spectral variations measured at different temperatures of 30, 25, and 20 °C, sequentially from the left. At each temperature, the relative positions of plots for salts with different concentrations are almost the same. All plots shift along the t 2 axis upon changing the temperature, shifting to the left upon the rise of temperature, which corresponds to destruction of the hydrogen-bonded network. Therefore, component P2 corresponds to the changes of the hydrogen-bonded network caused not only by the dissolution of a chaotrope but also by the rise in temperature. Upon the rise of temperature, the plots shift not only leftward but also downward although the downward shift is small. The downward shift, which corresponds to an extension of the hydrogen-bonded network, might be an artifact. Since PCA was performed for the spectra at various temperatures and at various concentrations of salts, the t 2 axis does not coincide completely with the spectral variation upon the temperature change. Component P3, whose variation is mainly induced by the dissolution of a kosmotrope, corresponds to changes of the hydrogen-bonded network different from that caused by the temperature variation.
4 Conclusions
In relation to the variation of hydrogen-bonded network of water, changes of the near-infrared absorption band of the first overtone of the –OH stretching mode of water upon the dissolution of various anions were analyzed by PCA and score plots. Score plots in Figs. 5 and 6 show how the spectra vary from the dissolution of chaotropic anions, which destroy or weaken the hydrogen-bonded network of water, resembling that resulting from the rise in temperature. On the other hand, the spectral variations upon the dissolution of kosmotropic anions, which expand or strengthen the hydrogen-bonded network of water, were found to be different from those resulting from the temperature change. Chaotropic and kosmotropic anions are found to mainly affect the P2 and P3 principal components of the spectra, respectively, although some chaotropic and kosmotropic anions also cause changes, respectively, to some extent in the P3 and P2 components.
Comparison of the loading vectors P2 and P3 in Fig. 2 reveal that only P3 includes the spectral variation in the lower wavenumber region around 6500–6200 cm−1, which corresponds to water molecules with stronger hydrogen bonds. Kosmotropic anions mainly cause variations in the P3 component, showing that they are apt to increase the hydrogen bond strength compared to that in pure water.
As mentioned above, the effects of chaotropic and kosmotropic anions on the hydrogen-bond network of water are not completely reversed as far as they are observed as variations in the near-infrared spectra. The difference in the effects might be caused by basicity in the aqueous solutions. Since most kosmotropic anions except OH− from NaOH, are anions from weak acids, they bring about hydrolysis by water to produce basic solutions. Aqueous solutions of NaOH are also basic. Therefore, the P3 component might have some relation with OH− in solution, i.e. the spectra of aqueous solutions of kosmotropic anions might be affected by their basicity, although elucidation of the effect is a subject for future study.
We have also investigated the effect of cations, which is different from that of anions. The results will be reported in another paper in the near future.
References
Murrell, J.N., Jenkins, A.D.: Properties of Liquids and Solutions, 2nd edn. Wiley, Chichester (1994)
Marcus, Y.: Effect of ions on the structure of water. Pure Appl. Chem. 82, 1889–1899 (2010)
Czarnik-Matusewicz, B., Pilorz, S.: Study of the temperature-dependent near-infrared spectra of water by two-dimensional correlation spectroscopy and principal components analysis. Vib. Spectrosc. 40, 235–245 (2006)
Gowen, A.A., Amigo, J.M., Tsenkova, R.: Characterization of hydrogen bond perturbations in aqueous systems using aquaphotomics and multivariate curve resolution-alternating least squares. Anal. Chim. Acta 759, 8–20 (2013)
Lin, J., Brown, C.W.: Novel applications of near-infrared spectroscopy of water and aqueous solutions from physical chemistry to analytical chemistry. Trends Anal. Chem. 13, 320–326 (1994)
Lin, J., Zhou, J., Brown, C.W.: Identification of electrolytes in aqueous solutions from near-IR spectra. Appl. Spectrosc. 50, 444–448 (1996)
Pegau, W.S., Gray, D., Zaneveld, J.R.V.: Absorption and attenuation of visible and near-infrared light in water: dependence on temperature and salinity. Appl. Opt. 36, 6035–6046 (1997)
Frost, V.J., Molt, K.: Analysis of aqueous solutions by near-infrared spectrometry (NIRS) III. Binary mixtures of inorganic salts in water. J. Mol. Struct. 410–411, 573–579 (1997)
Saitow, K., Kobayashi, K., Nishikawa, K.: How are hydrogen bonds perturbed in aqueous NaClO4 solutions depending on the concentration?: A near infrared study of water. J. Solution Chem. 33, 689–698 (2004)
Omar, A.F., Atan, H., Matjafri, M.Z.: NIR spectroscopic properties of aqueous acids solutions. Molecules 17, 7440–7450 (2012)
Sebe, F., Nishikawa, K., Koga, Y.: Spectrum of excess partial molar absorptivity. Part II: A near infrared spectroscopic study of aqueous Na-halides. Phys. Chem. Chem. Phys. 14, 4433–4439 (2012)
Chang, K., Jung, Y.M., Chung, H.: Two-dimensional correlation analysis to study variation of near-infrared water absorption bands in the presence of inorganic acids. J. Mol. Struct. 1069, 122–126 (2014)
Davidian, A.G., Kudrev, A.G., Myund, L.A., Khripun, M.K.: Near infrared spectral studies of aqueous solutions of metal perchlorates in groups IA, IIA, IIB, IIIA and IIIB of the periodic table. J. Near Infrared Spectrosc. 22, 27–34 (2014)
Sobinaa, E.P., Neudachinaa, L.K., Medvedevskikh, S.V., Medvedevskikh, MYu.: Effect of the nature of ions on the position of the absorption bands of water OH bonds in diffuse-reflection spectra in the near-infrared region. Russ. J. Phys. Chem. A 85, 1168–1173 (2011)
Davidian, A.G., Kudrev, A.G., Myund, L.A., Khlynova, O.S., Khripun, M.K.: Structure of aqueous electrolyte solutions estimated by near infrared spectroscopy and chemometric analysis of spectral data. Russ. J. Gen. Chem. 84, 1877–1887 (2014)
Gowena, A.A., Marini, F., Tsuchisaka, Y., DeLuca, S., Bevilacqua, M., O’Donnell, C., Downey, G., Tsenkova, R.: On the feasibility of near infrared spectroscopy to detect contaminants in water using single salt solutions as model systems. Talanta 31, 609–618 (2015)
Heiman, A., Licht, S.: Fundamental baseline variations in aqueous near-infrared analysis. Anal. Chim. Acta 394, 135–147 (1999)
Hasegawa, T.: Chemometrics in Infrared Spectroscopic Analysis. In: Tasumi, M. (ed.) Introduction to Experimental Infrared Spectroscopy, pp. 97–113. Wiley, Chichester (2015)
Ohtaki, H., Radnai, T.: Structure and dynamics of hydrated ions. Chem. Rev. 93, 1157–1204 (1993)
Bakker, H.J.: Structural dynamics of aqueous salt solutions. Chem. Rev. 108, 1456–1473 (2008)
Marcus, Y.: Effect of ions on the structure of water: structure making and breaking. Chem. Rev. 109, 1346–1370 (2009)
Zhang, Y., Cremer, P.S.: Interactions between macromolecules and ions: the Hofmeister series. Curr. Opin. Chem. Biol. 10, 658–666 (2006)
Kunz, W.: Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Interface Sci. 15, 34–39 (2010)
Omta, A.W., Kropman, M.F., Woutersen, S., Bakker, H.J.: Negligible effect of ions on the hydrogen-bond structure in liquid water. Science 301, 347–349 (2003)
Smith, J.D., Saykally, R.J., Geissler, P.L.: The effects of dissolved halide anions on hydrogen bonding in liquid water. J. Am. Chem. Soc. 129, 13847–13856 (2007)
Bruni, F., Imberti, S., Mancinelli, R., Ricci, M.A.: Aqueous solutions of divalent chlorides: ions hydration shell and water structure. J. Chem. Phys. 136, 064520 (2012)
Mancinelli, R., Botti, A., Bruni, F., Ricci, M.A., Soper, A.K.: Hydration of sodium, potassium, and chloride ions in solution and the concept of structure maker/breaker. J. Phys. Chem. B 111, 13570–13577 (2007)
Kondoh, M., Ohshima, Y., Tsubouchi, M.: Ion effects on the structure of water studied by terahertz time-domain spectroscopy. Chem. Phys. Lett. 591, 317–322 (2014)
Waluyo, I., Huang, C., Nordlund, D., Weiss, T.M., Pettersson, L.G.M., Nilsson, A.: Increased fraction of low-density structures in aqueous solutions of fluoride. J. Chem. Phys. 134, 224507 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uchida, N., Yoshimura, N. & Takayanagi, M. Variation of the Near-Infrared Spectrum of Water from Dissolved Salts. J Solution Chem 44, 2167–2178 (2015). https://doi.org/10.1007/s10953-015-0399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0399-9