Abstract
Zirconium is an important element in the nuclear fuel cycle. Thermodynamic data and models to reliably predict Zr–OH system behavior in various conditions including high ionic strengths are required and currently are unavailable. Most available experimental data are rather old, obtained using inadequate methodologies, and provide equilibrium constant values that differ by many orders of magnitude. Previous reviews have recommended values based on available data. These reviews used all of the available data, including poor quality data, in a global fit to determine these values. This has resulted in recommended thermodynamic models with a large number of polynuclear species and a number of mononuclear species with values of thermodynamic constants for the solubility product of ZrO2(am) and Zr–OH hydrolysis constants that are many orders of magnitude different from those for the reliable analogous Hf reactions. In this critical review, we have evaluated the quality of the available data, selected only those data that are of high quality, and reinterpreted all of the high quality data using SIT and Pitzer models for applications to high ionic strength solutions. Herein for 25 °C we (1) present formation constant values for ZrOH3+, \( {\text{Zr}}\left( {\text{OH}} \right)_{2}^{2 + } \), Zr(OH)4(aq), \( {\text{Zr}}\left( {\text{OH}} \right)_{5}^{ - } \), and \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \), and the solubility product for ZrO2(am) which are consistent with the Hf system, (2) report a revised value for the formation constant of \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \), (3) show that several hypothetical polynuclear species (\( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9}^{3 + } \), Zr4(OH) +15 , and Zr4(OH)16(aq)) proposed in previous reviews are not needed, and (4) show that polynuclear species (\( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \)) are not important in a very extensive H+ concentration range (0.1–10−15.4 mol·kg−1). Our review has also resulted in SIT and Pitzer ion-interaction parameters applicable to as high ionic strength solutions as 5.6 mol·kg−1 in NaCl, 2.11 mol·kg−1 in CaCl2, and 23.5 mol·kg−1 in NaOH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zirconium is an important element in the nuclear fuel cycle. In 2005 the thermodynamic data for Zr were reviewed by the Nuclear Energy Agency (NEA) (Brown et al. [1]). The NEA publications are highly regarded and are based on critical reviews of the available data. The accuracy of the values based on any critical review depends on (1) the extent and quality of the experimental data available for the review, and (2) the proper selection of publications for such reviews. In the case of Zr, the quality of the available data was extremely poor, and the NEA’s recommended ZrO2(am) solubility product value and Zr hydrolysis constants in the environmental range of pH values provide predicted Zr solubilities that are unexpectedly up to six orders of magnitude higher than many other amorphous dioxides of tetravalent ions of several elements (e.g., U, Np, Pu, Sn, Hf) [2,3,4].
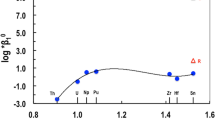
The ionic radii of Zr and Hf are almost identical, and they are excellent chemical analogs of each other. For this reason, it is not surprising that the reported values of equilibrium constants for the formation of Zr and Hf complexes with Cl−, F−, \( {\text{NO}}_{3}^{ - } \), \( {\text{SO}}_{4}^{2 - } \) and \( {\text{CO}}_{3}^{2 - } \) are essentially identical (Table 1).Footnote 1 The reported equilibrium constant values for the MO2(cr) solubility product and the formation of MOH3+ for Zr and Hf are also similar (Table 1). However, the reported Zr [1] as compared to Hf [4] values for (1) MO2(am) solubility product is approximately four orders of magnitude lower, (2) the formation constant of \( {\text{M}}\left( {\text{OH}} \right)_{6}^{2 - } \) is approximately four orders of magnitude higher, and (3) the formation constant of M(OH)4(aq) is approximately nine orders of magnitude higher. The observed large differences in the predicted solubilities and values for equilibrium constants for the formation of mononuclear complexes of Zr and Hf are not expected, especially considering that the values for Cl−, F−, \( {\text{NO}}_{3}^{ - } \), \( {\text{SO}}_{4}^{2 - } \), and \( {\text{CO}}_{3}^{2 - } \) complexes are very similar (Table 1). Two of the main reasons for such differences are (1) the literature data available to Brown et al. [1] for review were of poor quality and highly contradictory, and (2) Brown et al. [1] made an inappropriate choice to globally fit all available data, including data of poor quality, to develop a Zr–OH system model. These aspects are further discussed in detail below.
Equilibrium constant values for a large number of Zr–OH species including [ZrOH3+, \( {\text{Zr}}\left( {\text{OH}} \right)_{2}^{2 + } \), \( {\text{Zr}}\left( {\text{OH}} \right)_{3}^{ + } \), Zr(OH)4(aq), \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \), \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{6}^{2 + } \), \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{7}^{ + } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{5}^{7 + } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{7}^{5 + } \), \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \)] are reported by various authors [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. Brown et al. [1] summarized these extensive available data for the Zr–OH system and concluded that the reported values for both the monomeric and poly-nuclear hydrolysis constants are highly contradictory. For examples, for an overall reaction \( \left[ {m{\text{Zr}}^{4 + } + q{\text{H}}_{2} {\text{O}} \rightleftharpoons {\text{Zr}}_{m} \left( {\text{OH}} \right)_{q}^{4m - q} + q{\text{H}}^{ + } } \right] \) they report that at the same or similar ionic strength the disagreements among the values reported by different authors amount to approximately (1) four orders of magnitude in the formation of \( {\text{Zr}}\left( {\text{OH}} \right)_{2}^{2 + } \) and \( {\text{Zr}}\left( {\text{OH}} \right)_{3}^{ + } \), (2) five orders for Zr(OH)4(aq), (3) five orders for \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \), and (4) ten orders for \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \). Brown et al. [1] further report that by re-analyses of literature data they were able to derive acceptable values only for Zr(OH)3+ and \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \).
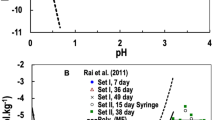
The main reasons for disagreement in most cases are poor experimental, analytical, or interpretational techniques involving relatively high ionic strength solutions. Brown et al. [1] were correct in their assessment that the quality of the available data is extremely poor. Therefore, it is surprising that they proceeded to use these data to develop a comprehensive Zr–OH model. In their desire to develop such a model they used most available data including ZrO2(am)/Zr(OH)4(am)Footnote 2 solubility data that varied widely in the observed concentrations as a function of pH/pC +H (e.g., see partial data sets plotted in Fig. 1 along with data from newer articles), and they fitted equilibrium constant values for several species. One cannot hope to develop a final model based on poor quality and such divergent sets of data. Conversely, models can be developed to explain all data (whether good or not) if a sufficient number of species is included. In order to fit these divergent data they proposed several additional polynuclear species [(Zr4((OH)16(aq), \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{15}^{ + } \), \( {\text{Zr}_3}\left( {\text{OH}} \right)_{9}^{3 + } \)], which are not observed or reported by any other researcher, in addition to mononuclear species [Zr(OH)4(aq), \( {\text{Zr}}\left( {\text{OH}} \right)_{3}^{ + } \), ZrOH3+]. Both of these sets of species provide the same corresponding slope dependence of Zr concentrations as a function of pH for the ZrO2(am) solubility data and thus are redundant. These polynuclear species are an artifact of fitting divergent, poor quality data, and contrary to expectation are dominant in relatively dilute Zr solutions and are not expected to form from component monomeric species based on molecular modeling calculations (these aspects are discussed in detail later). We believe Brown et al. [1] recognized these short-comings, because in the preface of the book they state that “due to complexity and contradictory nature of currently available hydrolysis data, … at least in the low pH region, the data selected in this review will be susceptible to amendments in the future.” In fact Brown in collaboration with Ekberg [21]Footnote 3 revisited the Zr review and stated that Zr4((OH)16(aq) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{15}^{ + } \) species were used by Brown et al. [1] to describe solubility data from several references where the measured solubility was overestimated due to the presence of colloids. Consequently Brown and Ekberg [21] deleted the Zr4((OH)16(aq) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{15}^{ + } \) species from the model they recommended. It is surprising, then, that they still included the \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9}^{3 + } \) species in their model, the species based on global fits to all data by Brown et al. [1] as discussed above, and basically accepted Brown et al.’s [1] Zr–OH model. The result is that the values of the solubility product for ZrO2(am) and the formation constants Zr(OH)4(aq) and \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \) are still similar to the values reported in Brown et al. [1] and are still quite different than those for the analogous Hf reactions (Table 1).
ZrO2(am) solubility as a function of pH/pCH+ reported by (1) 66BIL/BRA [6] and 61KOV/BAG [27] for dilute solutions, (2) 2004EKB/KAL [26] and 2006SAS/KOB [23] for 1.0 mol·dm−3 NaClO4, and (3) 2008ALT/NEC] [24] for 1.0 mol·dm−3 NaCl. Solubility of HfO2(am) reported by 2001RAI/XIA [4] for dilute solutions is plotted for camparison. A reference line of slope − 3 is plotted. (Note: Not all available data are plotted. ZrO2(am) solubility data in 1.0 mol·dm−3 NaClO4 from Bilinski et al. [6] and data in 0.2, 1.0, 2.0 mol·dm−3 NaNO3 from Veyland [17] are similar to the dilute data from [6] and are not plotted here)
Some of the newer experimental data that have become available [22, 23] since the review by Brown et al. [1] also do not allow definitive assignments of Zr–OH system equilibrium constants. More recent ZrO2(am) solubility data as a function of concentrations of different electrolytes (NaCl, CaCl2, NaOH, and NaClO4) reported by Altmaier et al. [24] are extensive and of excellent quality. However the main objective of their study was to investigate the ternary complexes of Zr (Zr–Ca–OH) and they primarily accepted Brown et al.’s [1] thermodynamic model for the Zr–OH system to interpret their data, resulting in the logarithm of ZrO2(am) solubility product value (− 60.3 ± 0.2) that is very similar to the value (− 59.2 ± 0.1) reported in [1]. Therefore, there is a need to reinterpret these data along with other reliable data to develop a reliable model for the Zr–OH system.
It is clear from the above discussion that there is a need to re-evaluate the available data, discard the studies that are unreliable, select those studies that are well executed and use those for reinterpretations to develop a comprehensive thermodynamic model that is consistent with all of the available reliable experimental data. In addition, thermodynamic models (such as, Pitzer’s) for the Zr–OH system applicable to predicting Zr behavior in high ionic strength solutions are needed and currently are unavailable. There are sufficient good quality experimental data available for ionic strength solutions up to as high as 23.5 molal (e.g., [24,25,26]) that need to be reinterpreted, and thus it should be possible to develop such a model.
The objective of this study, therefore, is to develop a reliable thermodynamic model for the Zr–OH system based on critical review and reinterpretations of the existing high quality experimental data. We will accomplish this goal by:
-
discarding the polynuclear species (such as Zr4((OH)16(aq), \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{15}^{ + } \) and \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9}^{3 + } \)), the main basis for calculated solubility product values and thereby the Zr–OH constants, that were used by Brown et al. [1]) and Brown and Ekberg [21] to fit the discordant solubility data, which (1) cannot be differentiated from and do show the same pH dependenceFootnote 4 as mononuclear Zr(OH)4(aq), \( {\text{Zr}}\left( {\text{OH}} \right)_{3}^{ + } \) and ZrOH3+, respectively, (2) unexpectedly appear dominant at lower Zr concentrations than the mononuclear species (discussed later), and 3) are not expected to be more stable than the mononuclear species, based on computational molecular simulation calculations (discussed later),
-
classifying available solubility data based on salient characteristics of an ideal solubility study and selecting those studies for reinterpretation that best meet these criteria,
-
reinterpreting the data using both the SIT and Pitzer models,
-
taking advantage of the reliable data available for the Hf–OH system.
These steps will provide more reliable equilibrium constant values for the mononuclear Zr–OH species and a reliable value for the solubility product of ZrO2(am) in addition to developing parameters for use in models (such as Pitzer) applicable to high ionic strength solutions.
2 General Aspects of Zr–OH System Data
It is known [22] that Zr colloids, which are stable for several months, are formed within a few minutes after the solubility limit is exceeded. Therefore, it is possible that the results are adversely impacted by the presence of colloids in potentiometric studies or solubility studies conducted from the over-saturation direction, especially if fine-pore sized filters were not used in solubility studies to separate the solids from solutions.
The ZrO2(am) solubility studies conducted by Veyland [17] and Bilinski et al. [6] in several different ionic strength solutions were conducted from the over-saturation direction (Table 2), and they did not filter the suspensions through fine pore-sized filters to separate the solids from solutions. It turns out that these are the very studies Brown et al. [1] used to determine the solubility product of ZrO2(am) and to develop part of their Zr hydrolysis model, especially the equilibrium constant values for the formation of (Zr(OH)4(aq), Zr4((OH)16(aq), \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{15}^{ + } \) and \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9}^{3 + } \)). A fit to the solubility data from these studies, along with the concentrations of different species based on the Brown et al. [1] model, are shown in Fig. 2. Based on the facts that (1) these solubility studies [6, 17] were conducted from the over-saturation direction and are most likely adversely impacted by the presence of colloids, resulting in higher aqueous Zr concentrations than we expect to be in equilibrium with ZrO2(am), and (2) Zr and Hf are chemical analogs of each other (Table 1) and thus ZrO2(am) solubility should be similar to HfO2(am) solubility (we will later show this to be the case), but is several orders of magnitude higher (Fig. 3), we can therefore conclude that the data reported in Bilinski et al. [6] and Veyland [17] are adversely impacted by the presence of colloids and/or poor detection limits and that the ZrO2(am) solubility product value and the Zr–OH hydrolysis constants calculated by Brown et al. [1] from studies that included these data cannot be correct.
Comparison of ZrO2(am) solubility in dilute solutions (66BIL/BRA) [6] to predictions based on the thermodynamic data reported in [1]. The solid black line is the total predicted concentration and the other lines represent concentrations of different species as identified. The hydrolyses species [\( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \)] not shown in the graph fall outside the boundaries of the graph. (Note: Although additional ZrO2(am) solubility data in 1.0 mol·dm−3 NaClO4 [6], and in 0.2, 1.0, and 2.0 mol·dm−3 NaNO3 [17] are available and were used by [1] to develop their thermodynamic model, and are similar to the experimental data plotted in this figure; these data are not plotted here. Readers interested in graphs of these data see [1]. It should also be mentioned that these data do not represent realistic solubility of ZrO2(am), see text for details)
As mentioned earlier, there are several orders of magnitude disagreements in the values reported for the formation of polynuclear species (\( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{7}^{ + } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{5}^{7 + } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{7}^{5 + } \) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \)). We cannot know for certain the exact reasons for these disagreements, but surmise that it may be due in part to the fact that the potentiometric (the method used to develop most of the data) studies involved higher initial Zr concentrations than can be maintained in equilibrium with ZrO2(am). For examples, (1) Veyland [17] used log10 [Zr] concentrations ranging from − 2.39 to − 2.09 at pH values of 1.368 and 1.314 for titrations, and (2) Ekberg et al. [26] used log10 [Zr] concentrations of − 1.54 for a pH titration range of 0.829–1.420 in one molar constant ionic strength solutions. On the other hand (1) Rai et al. [4], using the analogy to Hf, expected the log10 [Zr] concentration in dilute solutions in equilibrium with ZrO2(am) at pH = 1.37 to be about − 3.25, (2) Ekberg et al. [26] report log10 [Zr] = − 4.02 at pC +H of 0.92 in equilibrium with ZrO2(am) in one molar ionic strength solutions, (3) Kovalenko and Bogdasarov [27] report log10 [Zr] = − 3.94 at pH of 1.54 in equilibrium with ZrO2(am), and (4) Altmaier et al. [24] report log10 [Zr] = − 3.58 at pC +H of 1.34 in equilibrium with ZrO2(am) in 0.51 mol·kg−1 NaCl solutions, thereby showing that the Zr solutions used in potentiometric studies are over-saturated with respect to ZrO2(am) solubility and casting serious doubt on the accuracy of the equilibrium constants reported in [17, 26] for polynuclear species based on potentiometric titrations.
HfO2(am) data at pH values > 1.3 [4] can all be interpreted with mononuclear species without including any polynuclear species. In the relatively low pH/pC +H region, ZrO2(am) solubility data [23, 26, 27] with the exception of data from [6, 17, 22]Footnote 5 discussed above, as well as HfO2(am) solubility data show about three orders of magnitude decrease in Zr/Hf concentration with a unit increase in pH/pC +H (slope of − 3 as a function of increase in pH) (Fig. 1). This fact suggests that polynuclear species such as (\( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{5}^{7 + } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{7}^{5 + } \), \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \)) proposed previously [10, 16, 18, 19] are not dominant in these solutions because the dominant presence of these species would have required far less than three orders of magnitude decrease in Zr concentrations with each unit increase in pH/pC +H (ranging from − 5 to –8 orders of magnitude) (Table 3). These observations suggest that(1) Zr solubility behavior is consistent with Hf behavior, (2) MOH3+ is the dominant species in this relatively low pH/pC +H region, and (3) polynuclear species are not important. These facts suggest that a reliable Zr–OH system model applicable to a wide range in pH/pC +H values, certainly for the environmental range in pH values [28] for disposal of nuclear wastes, can be developed without the inclusion of uncertain polynuclear species, for which reliable data currently do not exist.
Our general approach to developing an improved Zr–OH model consists of (1) developing criteria for selecting reliable solubility data for interpretation, (2) using these criteria to classify existing studies, (3) selecting the study/studies that best meet these criteria, (4) using SIT and Pitzer models to reinterpret the data from the selected studies.
3 Developing Criteria for Selecting Reliable Solubility Data for Re-evaluation
In order to have any hope of developing a reliable thermodynamic model, it is imperative that the data selected for these analyses are of high quality. An ideal solubility study of metal oxides or hydroxides that are not redox sensitive should involve: (1) dilute solutions, (2) analytical methods with low detection limits, (3) effective methods (such as filtration through membrane filters) to separate solids/colloids from solutions, (4) approaching equilibrium from both the over- and under-saturation directions and if not from both directions then at least from the under-saturation direction, and (5) reliable pH values or concentrations of hydrogen or hydroxide ions. In addition, ideal solubility data should exhibit (1) a systematic change in metal ion concentration as a function of pH or hydrogen ion concentration and (2) behavior that is consistent with observed accurate behavior for its chemical analog. A brief elaboration of some of the important aspects of ideal solubility studies is given below along with the classification of the available ZrO2(am) literature data using these ideal solubility study criteria.
A large number of studies involving ZrO2(am) were conducted using relatively high ionic strength solutions. Several potential problems result from the use of relatively high ionic strength solutions. (1) The pH electrode-measured values are neither true pH values nor accurate values of hydrogen ion concentrations but must be corrected by empirical factors. (2) Detection limits are relatively high because the solutions generally have to be diluted before they can be analyzed. (3) Extrapolations of the data to zero ionic strength can be a problematic, especially for highly charged species. In the past, studies were conducted at constant relatively high ionic strengths in order to maintain constant activity coefficients that would aid in the interpretation of data using simple slope analyses techniques. However with the availability of comprehensive computer models (e.g., INSIGHT, [29]) to interpret data, there is no longer an overriding need to conduct studies in constant high ionic strength solutions, although performing studies at constant ionic strength still retains its merits. The models now available are capable of handling variations in chemical compositions and electrolytes, and data for all samples and electrolytes can simultaneously be used as an input to these models to determine values for equilibrium constants for various reactions.
To obtain solutions for analyses, it is important that solutions are separated from colloids/solid-phase. This is most effectively accomplished by filtering the equilibrated suspensions through the finest available pore-sized filters treated by various steps such as those outlined in publications of Rai and coworkers (e.g., see [3, 30]) to remove any possibility of filters altering the actual solution concentrations. The stability of colloidal solutions is dependent on several factors including the size of colloids, pH of the suspensions, ionic strength of the solutions, and specific chemical composition of solutions. Therefore there is no a priori way to determine whether colloids will be present in a given system, and thus filtration through fine filters is the only way to be certain to obtain colloid-free solutions.
It is best to conduct solubility studies from both the over- and under-saturation directions to be certain that equilibrium is achieved. However, in the case of many amorphous oxides/hydroxides that show very rapid precipitation/dissolution kinetics, it may not be necessary to conduct extensive studies from both the over- and under-saturation directions to be sure that equilibrium is achieved. This is the case for ZrO2(am), and most studies have been conducted from the oversaturation direction. When studies are conducted from the over-saturation direction only, there is a possibility that meta-stable colloids can form (e.g., see [22, 31]Footnote 6 which can impact measured concentrations, especially if the suspensions are not filtered. If the studies are conducted from the under-saturation direction there is no guarantee that colloids will be absent (the presence of colloids is dependent on many factors, see above paragraph), but in general there is less likelihood of the presence of colloids compared to the over-saturation direction. Therefore if it is not possible to do both, the under-saturation method is preferable.
It goes without saying that accurate pH values and hydrogen or hydroxide ion concentrations are required for accurate determination of equilibrium constants of reactions involving metal oxides/hydroxides. The values of dilute solutions can be accurately measured with ion selective hydrogen electrodes. Hydrogen/hydroxide ion concentrations of solutions containing relatively concentrated acids or bases can be set at the molality of the acid or base used. Measuring hydrogen ion concentrations of relatively concentrated solutions with low H+/OH− activities is not straightforward and requires steps such as calibrating pH electrodes through modified Gran titration methods (e.g., see [32]).
4 Selection of Studies for Determining Solubility Product of ZrO2(am) and Zr4+ Hydrolyses Constants
Spectroscopic methods (e.g., UV Vis, TRLFS) are not applicable to analyze Zr-hydrolyses species; therefore solubility studies remain a robust methodology to obtain values for solubility product and hydrolyses constants involving Zr. Thus it is desirable that the solubility studies for re-analyses are selected with care and that the selected studies are the best among the available data, especially considering that there are many orders of magnitude variability in ZrO2(am) solubility reported by many authors (Fig. 1). To accomplish this goal we outlined selection criteria above for an ideal solubility study. Using these criteria, the reported ZrO2(am) solubility studies [6, 17, 22, 23, 25,26,27] are classified as to their desirable characteristics (Table 2). This classification shows that the studies reported by Bilinski et al. [6], Veyland [17], and Cho et al. [22] have the least desirable characteristics, consistent with the conclusions drawn above. In addition to the least desirable characteristics of ZrO2(am) solubility studies [6, 17, 22] (Table 2), the ZrO2(am) solubility reported by these authors is several orders of magnitude higher than any of the hydrous oxides of tetravalent ions, and more importantly than HfO2(am) [4]. Therefore, the solubility product and hydrolysis constant values for Zr reactions based on these data [1] cannot possibly be correct. Based on these selection criteria, the ZrO2(am) solubility studies conducted by Kovalenko and Bogdasarov [27] and Altmaier et al. [24] meet more of these ideal study criteria than any other studies. We disagree with Brown and Ekberg [21] that Kovalenko and Bogdasarov’s [27] data are adversely affected by the presence of colloids and on the contrary will later show that these data are reliable in spite of the fact that Kovalenko and Bogdasarov did not use fine pore-sized-membrane filters to separate solutions from solids. We will discuss the ZrO2(am) solubility data reported in [23, 25,26,27] in more detail in the following sections.
5 Development of Zr–OH System Thermodynamic Data
The selected ZrO2(am) solubility data are interpreted using both the Pitzer (NONLINT) and SIT (NONLINT-SIT) models. These models use coupled nonlinear least squares and a chemical equilibrium program based on minimization of Gibbs energy. A general description of the NONLINT-SIT code is given in Rand et al. [33]. The NONLINT-SIT is an extended version of GMIN, NONLIN and NONLINT codes described in Felmy [34] and Sterner et al. [29]. In these programs, the actual quantity minimized is the standard deviation (σ), defined as in (Eq. 1) where N is the total number of data points and f(x) is given by the phase equilibrium condition (Eq. 2), where ΔGsolid is the iteratively-fitted dimensionless Gibbs energy (ΔfGm/RT) of the solid and ΔGsolution is the dimensionless Gibbs free energy calculated from the experimental aqueous phase data and the associated ion-interaction parameters (or equivalently the chemical potential of the second phase). In the simplest case of ZrO2(am) solubility
reaction [ZrO2(am) + 3H+ ⇌ ZrOH3+ + H2O] in the low pH region involving ZrOH3+ species, for example, this condition gives (Eq. 3) where \( {{\Delta_{\text{f}} G_{\text{m}}^{0} } \mathord{\left/ {\vphantom {{\Delta_{\text{f}} G_{\text{m}}^{0} } {RT}}} \right. \kern-0pt} {RT}} \) is the dimensionless standard molar Gibbs energies of formation of the subscripted species, the quantities in brackets are concentrationsFootnote 7 in mol·kg−1, and γ i refers to the activity coefficient of species i. The activity coefficients in the NONLINT-SIT model are calculated using SIT ion-interaction parameters and in the NONLINT using Pitzer ion-interaction parameters.
The equilibrium constant value for the formation of ZrOH3+ and the \( \Delta_{\text{f}} G_{\text{m}}^{0} \) of Zr4+ and ZrOH3+ reported in Brown et al. [1] are reasonable and are accepted in this review. With the exception of the value for \( \left( {{{\Delta_{\text{f}} G_{\text{m}}^{0} } \mathord{\left/ {\vphantom {{\Delta_{\text{f}} G_{\text{m}}^{0} } {RT}}} \right. \kern-0pt} {RT}}} \right)\left( {{\text{ZrO}}_{2} \left( {\text{am}} \right)} \right) \), the values of all of the variables in Eq. 3 are known from standard values reported in the literature and specific experimental solubility data reported in a given publication. Therefore, NONLINT and NONLINT-SIT can be used to calculate the value for \( \left( {{{\Delta_{\text{f}} G_{\text{m}}^{0} } \mathord{\left/ {\vphantom {{\Delta_{\text{f}} G_{\text{m}}^{0} } {RT}}} \right. \kern-0pt} {RT}}} \right)\left( {{\text{ZrO}}_{2} \left( {\text{am}} \right)} \right) \) and thus the solubility product for ZrO2(am).
Equation 3 is an example of a very simple specific case involving only one Zr species (ZrOH3+). In reality any number of Zr aqueous species can be included in NONLINT and NONLINT-SIT for calculations.
5.1 Solubility Product of ZrO2(am)
Aqueous elemental concentrations of each and every sample reported by Kovalenko and Bagdasarov [27] along with the \( \left( {{{\Delta_{\text{f}} G_{\text{m}}^{0} } \mathord{\left/ {\vphantom {{\Delta_{\text{f}} G_{\text{m}}^{0} } {RT}}} \right. \kern-0pt} {RT}}} \right) \) values for Zr4+ and ZrOH3+, based on Brown et al. [1], and with the appropriate SIT ion-interaction parameters (Table 4) were used to fit the \( \left( {{{\Delta_{\text{f}} G_{\text{m}}^{0} } \mathord{\left/ {\vphantom {{\Delta_{\text{f}} G_{\text{m}}^{0} } {RT}}} \right. \kern-0pt} {RT}}} \right)\left( {{\text{ZrO}}_{2} \left( {\text{am}} \right)} \right) \) value.Footnote 8 These data provide log10 K0 of − 56.19 ± 0.03 for the ZrO2(am) solubility product (Eq. 4) when a log10 K0 value of 14.32 ± 0.22 for the formation of ZrOH3+ (Eq. 5) from Brown et al. [1] is included in the analyses. This simple model, that does not include any polynuclear or other monomeric species, such as Zr(OH) 2+2 and Zr(OH) +3 , provides close agreement between the predicted (this model) and observed [27] Zr concentrations in equilibrium with ZrO2(am) (Fig. 4).
Aqueous Zr concentrations (61KOV/BAG, [27]) from ZrO2(am) suspensions equilibrated from the under-saturation direction. Lines represent predicted concentrations based on the SIT model thermodynamic data for Zr-OH mononuclear complexes (Tables 4, 7) that included \( \log_{10} K_{\text{SP}}^{0} \) = − 56.19 for ZrO2(am)
Altmaier et al. [24] is the best study for ZrO2(am) solubility, based on our ideal solubility study criteria (Table 2), that can be used to develop/verify the solubility product of ZrO2(am). Briefly, the study was conducted from the under-saturation direction, membrane filters were used to separate solids form solutions, reliable methods were used to quantify H+ concentrations, and the data are consistent with the Hf data. Altmaier et al. [24] present extensive data on the solubility of ZrO2(am) in relatively low pC +H (< 3) as a function pC +H and NaCl concentrations (0.51, 1.02, and 3.20 mol·kg−1) or CaCl2 concentrations (0.20 and 1.02 mol·kg−1). Because the solubility was measured in relatively concentrated electrolytes, their Zr detection limits are rather high. However, a large number of data points in each electrolyte are above the detection limit and can be used for meaningful reinterpretation of their data. We used both the SIT and Pitzer models to reinterpret these data. The plots of log10 [Zr] concentrations as a function of increase in pC +H in all cases show a slope of − 3, indicating that the dominant aqueous Zr concentration in this low pC +H region is ZrOH3+, consistent with the Kovalenko and Bagdasarov [27] data discussed above. The interpretation of these data with SIT and Pitzer models requires values for ion-interaction parameters for [H+, Cl−; Na+, Cl−; Ca2+, Cl−; Zr4+, Cl−; ZrOH3+, Cl−] as well as other data discussed below. Reliable ε (kg·mol−1) values for these parameters for the SIT model are available based on previous publications [1, 24] and are listed in Table 4. Reliable values for binary and ternary Pitzer parameters for the (H, Na, Ca)–Cl systems are available based on previous publications [35]. It was assumed that the binary parameters for [Zr4+, Cl−; ZrOH3+, Cl−] are the same as for the parameters for the corresponding Hf system [4], a very reasonable assumption. The specific Pitzer ion-interaction parameters used in this study are all listed in Table 5. To re-analyze the entire extensive ZrO2(am) solubility data [24] in NaCl and CaCl2 electrolytes in the acidic region, we included (1) the specific chemical composition of each and every sample where the measured Zr concentrations are above the detection limit, (2) the equilibrium constant value for the formation of ZrOH3+ reported in Brown et al. [1], and (3) appropriate values for the ion-interaction parameters for a given model as described above. These modeling inputs were used to fit a solubility product value based on the SIT or Pitzer model. The log10 K0 for the ZrO2(am) solubility product (Eq. 4) we thus calculated was − 56.19 ± 0.58 based on the SIT model and − 56.19 ± 0.51 based on the Pitzer model. These average values are identical to the value we calculated based on low ionic strength data of Kovalenko and Bagdasarov [27] but with a bit larger uncertainties. The facts that I1) values based on Kovalenko and Bagdasarov [27] and extensive data of Altmaier et al. [24] are identical, and (2) there are close agreements between the experimental and predicted Zr concentration as functions of pC +H , NaCl, and CaCl2 concentrations based on both the SIT and Pitzer models (Figs. 5, 6) attest to the reliability of the solubility product determined in this study.
Aqueous Zr concentrations, as a function of pC +H values of \( { \lesssim } \) 3.5 and NaCl concentrations, from ZrO2(am) suspensions equilibrated from the under-saturation direction (Altmaier et al. [24]). Lines represent predicted concentrations based on the SIT and Pitzer models using the thermodynamic data developed in this study (Tables 4, 5, and 7) that included \( \log_{10} K_{\text{SP}}^{0} \) = − 56.19 for ZrO2(am)
Aqueous Zr concentrations, as a function of pC +H values of \( { \lesssim } \) 3.5 and CaCl2 concentrations, from ZrO2(am) suspensions equilibrated from the under-saturation direction (Altmaier et al. [24]). Lines represent predicted concentrations based on the SIT and Pitzer models using the thermodynamic data developed in this study (Tables 4, 5, and 7) that included \( \log_{10} K_{\text{SP}}^{0} \) = − 56.19 for ZrO2(am)
The log10 K0 of − 56.19 ± 0.58 (Eq. 4) determined in this study from the data reported in Kovalenko and Bagdasarov [27] and extensive data reported in Altmaier et al. [24] is similar to the value (− 56. 94 ± 0.32) reported by Sasaki et al. [23], based on estimated data, and a value (− 57.2) reported by Latimer [36], based on calorimetric data and estimated entropies of ions and solids. These results are also consistent with the available HfO2(am) solubility data (Fig. 1) and the thermodynamic data for Hf in that (1) the log10 of the solubility product (− 55.1 ± 0.7) of HfO2(am) [4] compares very favorably with the log10 of the solubility product (− 56.19 ± 0.58) we calculate for ZrO2(am), and (2) no additional species other than MOH3+ are required to adequately fit the MO2(am) solubility data for Zr (this study) or Hf [4] in the relatively low pH regions.
Ekberg et al. [26] reported the solubility of ZrO2(am) in 1.0 mol·kg−1 (H, Na)ClO4 solutions. These data for pC +H \( { \lesssim } \) 2, which show a systematic decrease in Zr concentrations with the increase in pC +H , were interpreted using the NONLINT-SIT model. For these modeling calculations it was assumed that the reported pC +H values are correct. The model that included elemental concentrations of various species reported by Ekberg et al. [26], values for Zr4+ and ZrOH3+ species as discussed above, and values of appropriate SIT ion interaction parameters in kg·mol−1 [ε(H+, \( {\text{ClO}}_{4}^{ - } \)) = 0.14, ε(Zr4+, \( {\text{ClO}}_{4}^{ - } \)) = 0.89, ε(Na+, OH−) = 0.04, ε(Na+, \( {\text{ClO}}_{4}^{ - } \)) = 0.01, ε(ZrOH3+, \( {\text{ClO}}_{4}^{ - } \)) = 0.57] as reported in Brown et al. [1] were used to calculate log10 K0 = − (59.18 ± 0.23) for the reaction in (Eq. 4). There is close agreement between the experimental and predicted Zr concentrations (Fig. 7). Two main conclusions that can be drawn from this modeling exercise are (1) Ekberg et al.’s [26] data can be fitted very well without the inclusion of any other Zr–OH species other than ZrOH3+, consistent with the reinterpretation of data reported in [24, 27], and (2) the ZrO2(am) solubility product calculated from the Ekberg et al. [26] study is approximately three orders of magnitude lower than that based on [24, 27]. If the solid phases in both studies were identical and the H+ concentration reported in Ekberg et al. [26] were reliable, then the solubility product values would have been expected to be identical and the experimental Zr concentrations in the Ekberg et al.’s study [26] should have been higher by about three orders of magnitude (as can be calculated from Eq. 6 and the solubility product value based on [27]). The exact reasons for the lower solubility product value based on Ekberg et al.’s [26] data than on Kovalenko and Bagdasarov [27] are not known. We can perhaps rule out the presence of colloids in either study as the source of error because both studies show approximately three orders of magnitude decrease in Zr concentrations with a unit increase in pH/pC +H , consistent with the Hf data [4] where samples were filtered through fine pore-sized filters to eliminate colloids.Footnote 9
However there are several experimental variables in Ekberg et al. [26] study that cast doubt on the reliability of the aqueous Zr concentrations and pC +H values they report, possibly resulting in the observed differences in calculated solubility product values. These variables include: (1) Kovalenko and Bagdasarov [27] and Altmaier et al. [24] used a freshly prepared ZrO2(am) whereas Ekberg et et al. [26] purchased a dry solid phase and this solid phase may be more ordered (i.e. featuring larger particle size) and exhibit lower solubility. (2) Ekberg et al. [26] determined H+ concentration through titration of the equilibrated samples, and it is known [32] that the observed pH meter readings in relatively concentrated electrolytes such as 1.0 mol·kg−1 (H, Na)ClO4 used by the authors provide neither reliable pH values nor reliable H+ concentrations, and in fact in most cases the observed pH meter values indicate the presence of higher concentrations of H+ than are actually present in the samples [32]. Thus it is likely that Ekberg et al. [26] overestimated the H+ concentration, which would result in lower observed solubility. (3) In order to minimize Zr adsorption onto the autopipette Ekberg et al. [26] washed the autopipette used to withdraw equilibrated samples for analyses with alkaline solutionFootnote 10; however this washing could undoubtedly raise the pH and lower the Zr concentration of the withdrawn solution.
Aqueous Zr concentrations (Ekberg et al. [26]) from ZrO2(am) suspensions equilibrated from the under-saturation direction. Lines represent predicted concentrations based on the SIT model thermodynamic data for Zr-OH mononuclear complexes (Tables 4, 7) that also included \( \log_{10} K_{\text{SP}}^{0} \) value of − 59.18 for ZrO2(s). (Note: There are large uncertainties in the experimental data and thus the solubility product value is not reliable (see text for details). The main point of this figure is that the data can be well interpreted using only one hydrolysis species (ZrOH3+))
Sasaki et al. [23] conducted ZrO2(am) solubility studies from the over-saturation direction and in constant ionic strength solutions of 0.10, 0.51, and 1.05 mol·kg−1 NaClO4. There are several concerns in the experimental data they report. There is a large amount of scatter in the data (Fig. 8a). The reported pC +H values obtained by just filling the pH electrode with concentrated electrolytes cannot provide correct H+ concentrations, as shown by Rai et al. [32]. Although the Zr concentrations as a function of pC +H and ionic strength appear to be similar, approximately two orders of magnitude higher concentrations in 1.05 mol·kg−1 ionic strength solutions as compared to 0.10 mol·kg−1 are expected (calculations based on Eq. 6). The number of data points for ionic strengths other than 1.05 mol·kg−1 is very limited. Despite the fact that they filtered their samples through 3 kDa nominal molecular weight limit membrane filters, they report that their observed Zr concentrations are adversely impacted (higher) due to the presence of colloids. By estimating the quantity of colloids Sasaki et al. [23] report an estimated log10 solubility product value (Eq. 3) of − 56.94 ± 0.32, a value that is close to the value we calculate based on [24, 27]. However if we assume that the aqueous Zr concentrations and pC +H values Sasaki et al. [23] report are correct, then the log10 solubility product value (Eq. 3) that we calculate with NONLINT-SIT from their 1.05 mol·kg−1 NaClO4 data, the only data that are very extensive, is − 54.59 ± 0.60. This model provides reasonable agreement between the predicted and experimental concentrations Sasaki et al. [23] report for 1.05 mol·kg−1 ionic strength solutions (Fig. 8b). Because of the uncertainties outlined above, we are unable to recommend or determine an accurate solubility product value for ZrO2(am) from Sasaki et al.’s [23] data.
Aqueous Zr concentrations from ZrO2(s) suspensions equilibrated from the over-saturation direction (Sasaki et al. [23]). a Solubility in NaClO4 solutions of different concentrations. b Lines represent predicted concentrations for 1.05 mol·kg−1 NaClO4 solubility data based on the SIT model thermodynamic data for Zr-OH mononuclear complexes (Tables 4, 7) that also included \( \log_{10} K_{\text{SP}}^{0} \) of − 54.59 for ZrO2(s). (Note: Uncertainties in the experimental data including the presence of colloids as mentioned by Sasaki et al. [23] makes the solubility product value unreliable for ZrO2(am) (see text for details). In fact Sasaki et al. [23] report \( \log_{10} K_{\text{SP}}^{0} \) of − 56.94 ± 0.32 after they discounted the colloid contribution, a value close to the value (− 56.19) we recommend for ZrO2(am). Although there is a large amount of scatter in the data, the main point of this figure is that the data can be reasonably well interpreted using only one hydrolysis species (ZrOH3+))
In conclusion, the log10 K0 value for the reaction in Eq. 4 that we recommend based on the data reported by Kovalenko and Bagdasarov [27] and Altmaier et al. [24] is − 56.19 ± 0.58 based on both the SIT and Pitzer models. These values provide orders of magnitude lower predicted ZrO2(am) solubility compared to that predicted based on data in Brown et al. [1] (e.g., compare Fig. 4 to Fig. 2).
5.2 \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \) Formation Constant
Although we have provided convincing evidence that ZrOH3+ is the dominant species in the relatively low pH region (~ − 0.32 to 4.0), it is of interest to determine whether the equilibrium constant for the formation of \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \) proposed by Brown et al. [1] is consistent with the re-analyses of Kovalenko and Bagdasaro’s [27] and Altmaier et al.’s [24] data. The log10 K values for the formation of \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \) (Eq. 7) reported in the literature vary over four orders of magnitude [1]. For example, log10 K values ranging from − 0.17 to − 0.24 for nitrate media concentrations ranging from 0.1 to 1.0 mol·dm−3 are reported by Nazarenko and Mandzhgaladze [11], whereas log10 K of − 4.48 in H2SO4
Media concentrations ranging from 0.5 to 2 mol·dm−3 are reported by Chekmarev et al. [7]. Because of the large variability in reported log10 K values, Brown et al. [1] were not able to recommend values for this constant based on the specific studies aimed at determining it. However, they recommended log10 K0 = 0.98 ± 1.06 based on global fits to the data in nitrate media, which undoubtedly included data of uncertain quality because they report a scientifically unrealistically high uncertainty value for the ε((\( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \), \( {\text{NO}}_{3}^{ - } \)) = 0.16 ± 500).Footnote 11 This uncertainty would amount to approximately ± 50 orders of magnitude variability (calculated with Eq. 6 based on the SIT model) in the log10 K value for a relatively low ionic strength solution (0.1 mol·dm−3 \( {\text{NO}}_{3}^{ - } \)). Such a level of uncertainty hardly engenders confidence in the log10 K0 value Brown et al. [1] report. If we include the \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \) species with the log10 K0 value (0.98 ± 1.06) from Brown et al. [1] into the model we described above, the calculations show that \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \) instead of ZrOH3+ is the dominant species in the low pH/pC +H region of ZrO2(am) data [23, 24, 26, 27], a result inconsistent with these solubility data as well as data for Hf as shown and discussed above.
It is difficult to determine a reliable value for log10 K0 (Eq. 7) based on conflicting and uncertain quality data. However, as we discuss below, it is possible to determine upper limit value for log10 K0. If we assume that \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \) is the major species at (1) the highest pH (2.02) value in the ZrO2(am) solubility study of Kovalenko and Bagdasarov [27], where log10 [Zr] = − 5.699, then an approximate upper limit log10 K0 of − 1.5 is obtained, and (2) the highest pCH+ value (2.93) in the low pCH+ region of Altmaier et al.’s [24] ZrO2(am) solubility data in 0.51 mol·kg−1 NaCl solutions, where measured Zr concentrations (log10 [Zr] = − 7.8) are above the detection limit, then an approximate upper limit log10 K0 of − 1.74 is obtained. If, on the other hand, we make similar calculations based on the pH = 4 HfO2(am) solubility data [4], assuming chemical analogy to Zr, an approximate upper limit log10 K0 of − 2.3 is obtained. These values are consistent with ZrOH3+ rather than \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \) as the dominant species. It is interesting that Brown et al. [1] calculate log10 K0 = − 2.49 from the data reported by Davydov and Zabrodski [9], which is similar to the values we estimated above but is quite different from the value (0.98 ± 1.06) Brown et al. [1] recommend and used to fit the poor quality solubility data. An estimated log10 K0 value of − 1.3, similar, to the upper limit values calculated above, based on coordination chemistry of Zr are reported by Sasaki et al. [37]. Baes and Mesmer [38] recommend a log10 K0 value of − 1.7. Based on their recent review Brown and Ekberg [21] report a log10 K0 value of − 0.18 ± 0.17 (Eq. 7), which is inconsistent with the solubility data of Kovalenko and Bagdasarov [27] as well as extensive data of Altmaier et al. [24]. The main conclusion we can draw from this discussion is that \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \) is not important and that the log10 K0 value for the formation of this species (Eq. 7) must be \( { \lesssim } \) − 2.3.Footnote 12
5.3 Zr(OH)4(aq) Formation Constant
In the intermediate pH/pC +H value range, sparingly soluble MO2(am) solids of many elements, such as Th(IV), U(IV), Np(IV), Pu(IV), Sn(IV), and Zr(IV) [2, 3, 26, 33, 39, 40], show metal concentrations that are independent of pH/pC +H and are generally thought to be the result of equilibrium with M(OH)4(aq) species (Eq. 8). In reality however, many of the observed concentrations result from poor detection limits, the presence of un-filterable colloids, or in the case of redox sensitive elements the presence of a finite amount of a more soluble oxidation state than M(IV). Therefore, only an upper limit value for the M(OH)4(aq) formation can be reported and is necessary for practical purposes in order to calculate the upper limits of the concentrations of different elements in geological environments. These upper limit log10 K0 values for the formation of Zr(OH)4(aq) (Eq. 8) are − 5.43 [1], \( { \lesssim } \) − 4.4 [6], < − 7.4 [26], < − 7.2 [22], < − 7.4 [23], and < − 7.5 [24] (Table 6). All of these values are based on data in high ionic strength solutions in which the analytical detection limits are expected to be high, and they are also possibly adversely impacted by the presence of a finite amount of un-filterable fine colloids.Footnote 13
Therefore, the upper limit value for the reaction in Eq. 8 can be far lower than the lowest value quoted above. We have shown that Hf is an excellent analog of Zr and the log10 K0 value (Eq. 8) based on reliable Hf data [4] should be < − 10.3. In reality, the value is probably < − 11.4 (Table 6) when the actual difference in solubility product values of HfO2(am) [4] and ZrO2(am) (recommended in this study) are taken into account. However, to be conservative when calculating source term concentrations, we recommend log10 K0 < − 10.3 for the reaction in Eq. 8. The combination of reactions (Eqs. 4, 8) provide log10 K0 < 45.89 for the reaction in Eq. 9 that we recommend. This value is very similar to the value (log10 K0 < 46.17 for reaction in Eq. 9) reported by Sasaki et al. [37] based on their estimates to correct for the presence of colloids in their study.
5.4 \( {\text{Zr}}\left( {\text{OH}} \right)_{5}^{ - } \) and \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \) Formation Constants
Only two studies [25, 26] report data in the hyper-alkaline solutions that can be used to determine the complexation constants for the formation of negatively charged Zr–OH species such as \( {\text{Zr}}\left( {\text{OH}} \right)_{5}^{ - } \) and \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \). Brown et al. [1] ignored the study by Sheka and Pevzner [25], stating that their observed ZrO2(am) solubility may perhaps have been adversely influenced by the possible contamination of NaOH solutions by carbonate. Sheka and Pevzner [25] studied HfO2(am) and ZrO2(am) solubility in NaOH solutions ranging in concentrations from 1.0 to 17.6 mol·dm−3 in the case of HfO2(am) and from 1.0 to 18.4 mol·dm−3 in the case of ZrO2(am). Rai et al. [4] compared their HfO2(am) solubility, in the basic solutions (OH− reaching as high as 3.20 mol·kg−1) using carbonate-free NaOH and by filtering the solutions through membrane filters, to the Sheka and Pevzner’s [25] solubility data and reported a close agreement between them (Fig. 9). These comparisons suggest that HfO2(am) solids used in both studies have similar chemical potential and that colloids, or the possible presence of Hf–carbonate complexes in the Sheka and Pevzner [25] study do not significantly affect the observed solubility. Because Sheka and Pevzner [25] studied ZrO2(am) solubility under similar conditions as used in their solubility study for HfO2(am), it is reasonable to assume that the ZrO2(am) solubilities are reliable.
As discussed earlier, there is a concern that the aged solid hydrous oxide ZrO2(s) used by Ekberg et al. [26] in their study may have a lower chemical potential (lower solubility than the freshly prepared ZrO2(am) used by Sheka and Pevzner [25]). Comparisons of the solubility data (with Zr and NaOH molalities calculated based on data in [41])Footnote 14 reported in these publications show that the ZrO2(am) solubility reported for a few data points by Ekberg et al. [26] are up to about 1.5 log units lower at relatively lower NaOH concentrations than those reported by Sheka and Pevzner [25] (Fig. 10). However, at higher NaOH concentrations (~ 10.7 mol·kg−1), the observed solubilities are similar. In addition, some of the drawbacks (such as the possible overestimation of H+ concentration) in Ekberg et al.’s [26] relatively low pH data discussed earlier do not exist in the NaOH data. These facts suggest that the chemical potential of solid phases used by Sheka and Pevzner [25] and Ekberg et al. [26] is similar and that both of these data sets can collectively be reinterpreted.
Solubility of ZrO2(am) as a function of NaOH molalities (estimated by us using the molarities reported in the quoted references and the data in Weast [41] to convert molarity to molality). The Ekberg et al. [26] data for log10 [NaOH] mol·kg−1 < 0.0 are in 1.05 molal ionic strength made up of mixtures of NaClO4 and NaOH. Lines represent NONLINT-SIT model (Tables 4, 7) predicted concentrations using \( \log_{10} K_{\text{SP}}^{0} \) = − 56.19 for ZrO2(am), log10 K0 = 50.72 for the reaction (\( {\text{Zr}}^{ 4+ } + {\text{ 6OH}}^{ - } \rightleftharpoons {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \)) (determined in this study), and using either estimated ε(Na+, \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \)) = − 0.10 kg·mol−1 value reported in Brown et al. [1] (dotted line labeled “Zr SIT2”) or 0.068 ± 0.01 kg·mol−1 (solid line labelled “Zr SIT This study”), the value fitted by us from the experimental data reported in this figure
The HfO2(am) study [4] shows that the \( {\text{Hf}}\left( {\text{OH}} \right)_{5}^{ - } \) species is important in relatively lower NaOH concentrations (\( { \lesssim } \) 0.03 mol·kg−1) and that \( {\text{Hf}}\left( {\text{OH}} \right)_{6}^{2 - } \) is the dominant species in NaOH concentrations \( { \gtrsim } \) 0.03 mol·kg−1. Based on analogy to Hf, we would therefore expect that the dominant Zr species in the > 1.0 mol·kg−1 NaOH data [25, 26] is \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \).
Altmaier et al.’s [24] reported ZrO2(am) solubility is the only study in which the measured Zr concentrations in the relatively lower pCH+ region (\( { \lesssim } \) 14) of the hyper-alkaline region are above the detection limit. Three concerns related to these data are: (1) the meaningful data are in a very narrow range of pCH+ values, (2) the detection limits are rather poor and thus the Zr behavior in the pCH+ values of \( { \lesssim } \) 12.8 are not known with any certainty, and (3) the ZrO2(am) solubility study was conducted in NaCl solutions ranging in concentrations from 0.51 to 3.20 mol·kg−1 which results in poor detection limits and uncertaintiesFootnote 15 in developing reliable thermodynamic models from this set of data. However these are the only available data in the relatively low hyper-alkaline region (< 1.3 mol·kg−1 NaOH) where the measured Zr concentrations are meaningful and thus are useful in (1) verifying thermodynamic data for \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \) developed from the super-hyperalkaline region and (2) estimating values for the formation of \( {\text{Zr}}\left( {\text{OH}} \right)_{5}^{ - } \), the species expected to be important only in the relatively lower alkaline region.
It is generally known that interpretations using the SIT model are more accurate when used for ionic strength solutions of \( { \lesssim } \) 3.0 mol·kg−1. Whether the SIT model can be used for interpreting data obtained at ionic strengths > 3.0 mol·kg−1 is dependent on a whole host of factors such as the Δε value for a given reaction that is near zero but is not either highly positive or negative. In the case of the ZrO2(am) solubility reaction (Eq. 10) Δε = − 0.18 (based on ε(Na+, OH−) = 0.04 kg·mol−1 and ε(Na+, \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \)) = − 0.10 kg·mol−1, reported in [1]), is somewhat lower than zero. Therefore it should be possible to interpret data using the SIT model even though the studies [25, 26] were conducted in highly concentrated electrolytes. The Pitzer model is applicable to very high ionic strength solutions and Pitzer ion-interaction parameters for Hf, an analog of Zr, are available. Therefore, ZrO2(am) solubility data in hyper-alkaline solutions were interpreted using both the SIT (NONLINT-SIT) and Pitzer (NONLINT) models.
5.4.1 \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \) Formation Constant Value Using the SIT Model
To determine the equilibrium constant for the formation of \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \) (Eq. 11) using the NONLINT-SIT, we included (1) the chemical composition of every sample, (2) ε(Na+, \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \)) = − 0.10 kg·mol−1 (estimated by [1] based on data in [42])Footnote 16 and ε(Na+, OH−) = 0.04 kg·mol−1, and (3) our recommended solubility product value (log10 K0 = − 56.19 ± 0.03) of ZrO2(am) (Eq. 4). This modeling exercise provided log10 K0 = 50.72 ± 1.73 for the reaction in (Eq. 11), which shows that the predicted concentrations using the above model agree closely with the experimental data only in the relatively low NaOH concentrations (\( { \lesssim } \) 1.0 mol·kg−1) but the model over-predicts the Zr concentrations as a function of increase in NaOH concentrations of \( { \gtrsim } \) 1.0 mol·kg−1 (Fig. 10). Because the ε(Na+, Zr(OH) 2−6 ) = − 0.10 kg·mol−1 recommended by Brown et al. [1] is an estimated value only and not based on the specific data reported in Sheka and Pevzner [25] or Ekberg et al. [26], we used NONLINT-SIT to simultaneously fit the formation constant of \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \) and the value for ε(Na+, \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \)). These fits provided log10 K0 = 50.72 ± 0.24 for the reaction in (Eq. 11), ε(Na+, \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \)) = 0.068 ± 0.01Footnote 17 kg·mol−1, and a Δε = − 0.012 kg·mol−1 for (Eq. 10). These modeling parameters provide close agreement between the predicted and experimental data in the entire range of NaOH concentrations, even reaching as high as 23.5 mol·kg−1 (Fig. 10). It seems surprising that the SIT model is applicable to such high ionic strength solutions. The fact that SIT is applicable in this specific case, however, is not coincidental because (ΔZ2D − ΔεIm) is nearly zeroFootnote 18 for this reaction (Eq. 10) which, according to Eq. 6, dictates that apparent log10 K is essentially identical to log10 K0 and thus the ionic strength and ion-interaction effects cancel out in this situation. Therefore based on the SIT model, we recommend ε(Na+, \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \)) = 0.068 ± 0.01 kg·mol−1 and a log10 K0 = 50.72 ± 0.24 for the reaction in (Eq. 11). The log10 K0 value for the reaction (Eq. 11) that we recommend (50.72 ± 0.24) is over four orders of magnitude lower than the value (55.00 ± 0.70) recommended by Brown et al. [1] but is very similar to the value (51.2 ± 0.2) for the Hf reaction and lends support to the reliability of the calculated value.
5.4.2 Zr(OH) 2−6 Formation Constant Value Using the Pitzer Model
Several different modeling runs were made using the Pitzer model (NONLINT) to interpret the ZrO2(am) solubility data in NaOH solutions [25, 26] extending to as high a NaOH concentration as 23.5 mol·kg−1. The best model that described these solubility data included (1) Pitzer ion-interaction parameters (kg·mol−1) for (Na+, \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \)) of β(0) = 0.44 and β(1) = 0.7, assuming that the corresponding values for the Hf system [4] are applicable to the Zr system, Cϕ = − 0.0318, (2) a log10 K0 = − 56.19 ± 0.03 for the ZrO2(am) solubility product (Eq. 4) recommended in this study, and (3) a log10 K0 = 50.95 ± 0.35 for the formation of \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \) (Eq. 11). Using the above model, a close agreement is observed between the predicted and experimental Zr concentrations (Fig. 11).
Solubility of ZrO2(am) as a function of NaOH molalities (estimated by us using the molarities reported in the quoted references and the data in Weast [41] to convert molarity to molality). The Ekberg et al. [26] data for log10 [NaOH] mol·kg−1 < 0.0 are in 1.05 molal ionic strength made up of mixtures of NaClO4 and NaOH. Line represents predicted concentrations using the Pitzer model (Tables 5, 7) that included \( \log_{10} K_{\text{SP}}^{0} \) = − 56.19 for ZrO2(am)
5.5 \( {\text{Zr}}\left( {\text{OH}} \right)_{5}^{ - } \) Formation Constant
As mentioned earlier, Altmaier et al.’s [24] ZrO2(am) solubility data in NaCl solutions ranging in concentrations from 0.51 to 3.20 mol·kg−1 at pCH+ values ranging from ~ 12.8 to 14, are used to confirm the Zr(OH) 2−6 formation constant and estimate the value for Zr(OH) −5 . For these calculations, the chemical compositions of Altmaier et al.’s [24] samples along with the values for solubility product and formation constant for \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \), determined above and reported in Table 7, were input into the SIT (NONLINT-SIT) and Pitzer (NONLINT) models to fit the chemical potential of \( {\text{Zr}}\left( {\text{OH}} \right)_{5}^{ - } \). These calculations provided a log10 K0 value of 50.35 ± 0.23 for the formation of \( {\text{Zr}}\left( {\text{OH}} \right)_{5}^{ - } \) (Eq. 12) based on both the SIT and Pitzer models. These modeling parameters for both the SIT and Pitzer models provided predicted Zr concentrations as a function of pCH+ that agreed closely with the experimental data reported by Altmaier et al. [24] (Fig. 12). It should be mentioned that although the Zr(OH) −5 appears dominant in only a few of the lowest pCH+ samples (Fig. 12), the formation constant value (50.35 ± 0.23) that we determined for the reaction (Eq. 12) involving this species is close to the value (49.7 ± 0.2) reported (Rai et al. [4]) for the Hf reaction and thus lends credence to the reliability of this value. However, additional ZrO2(am) solubility studies in simple NaOH solutions, similar to the studies reported in [4] for the Hf system, are recommended for further verification of this value.
Aqueous Zr concentrations, as a function of pC +H values ranging from about 12.7–14.1 and NaCl concentrations ranging from 0.51 to 3.2 mol·kg−1, from ZrO2(am) suspensions equilibrated from the under-saturation direction (Altmaier et al. [24]) (only the experimental data which are above the detection limit are plotted and considered for reinterpretations). Lines represent predicted concentrations based on the SIT and Pitzer models using the thermodynamic data developed in this study (Tables 4, 5, and 7) that included \( \log_{10} K_{\text{SP}}^{0} \) = − 56.19 for ZrO2(am)
5.6 Polynuclear Species
Brown et al. [1] recommended Zr hydrolysis constant values for the formation of ZrOH3+, \( {\text{Zr}}\left( {\text{OH}} \right)_{2}^{2 + } \), Zr(OH)4(aq), \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \), \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9}^{3 + } \), \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \), \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{15}^{ + } \), and Zr4(OH)16(aq) along with the solubility product value for ZrO2(am). In almost the entire range of pH values (< 13) these data provide predicted solubilities of ZrO2(am) that are up to several orders of magnitude higher than the analogous Hf system [4]. We have reanalyzed the available data, including those presented in Brown et al. [1] and those that have become available since 2005. Our revised values for the formation of \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \), Zr(OH)4(aq), \( {\text{Zr}}\left( {\text{OH}} \right)_{5}^{ - } \) and \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \), and the ZrO2(am) solubility product are presented in Table 7. When combined with the ZrOH3+ value from Brown et al. [1], these values provide predicted ZrO2(am) solubilities that are consistent with the Hf system. We have shown that (1) the primary reason for high predicted Zr concentrations in equilibrium with ZrO2(am) in Brown et al.’s [1] model is their use of unreliable solubility studies as the basis of their model, and (2) the pH dependences of several polynuclear species, specifically (\( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9}^{3 + } \), \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{15}^{ + } \) and Zr4(OH)16(aq)), are the same as for the corresponding mononuclear species (ZrOH3+, \( {\text{Zr}}\left( {\text{OH}} \right)_{3}^{ + } \), Zr(OH)4(aq)) and thus are redundant and cannot be differentiated from the mononuclear species based on solubility data. No literature evidence is available for the existence of these polynuclear species, and Brown et al. [1] appear to have used these hypothesized species to explain divergent data sets. In addition, these species appear to be important at higher pH values than the corresponding for monomeric species (e.g., compare the relative concentrations of ZrOH3+ and \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9}^{3 + } \) in Fig. 2 based on the data in [1]), in direct contradiction to the molecular modeling results (discussed below) and the expectation that polynuclear species will form in relatively concentrated Zr solutions prevalent under acidic conditions (relatively low pH values). Therefore, we did not include these polynuclear species in our model.
The only other polynuclear species that are not a part of our model but are a part of the Brown et al. [1] model are \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \). Brown et al. [1] proposed the equilibrium constants for the formation of these species by analyses of experimental data. To evaluate the exact impact of these [\( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \)] polynuclear species, we included SIT ion-interaction parameters (ε(\( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \)), Cl− = 0.33 kg·mol−1 from [1]; ε(\( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \)), Cl− = 1.37 kg·mol−1 from [24]) and equilibrium constants for the formation of these species reported by Brown et al. [1] into our thermodynamic model (Table 7). A comparison of the experimental data reported in Kovalenko and Bagdasarov [27] and Altmaier et al. [24] with the predictions using this model (Fig. 13) shows that orders-of-magnitude higher predicted Zr concentrations with this model are completely at odds with the experimental data, suggesting that the formation constant values for \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \) are overestimated by many orders of magnitude. The reported log10 K values for the formation of (1) \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \)(Eq. 13) vary over approximately five orders of magnitude [10, 16, 19], and (2) \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \) (Eq. 14) vary over approximately ten orders of magnitude [10, 18, 19]. The log10 K0 values recommended by Brown et al. [1] for these highly charged species (Eqs. 13, 14) is very much dependent on the selected ion-interaction parameters, and the proposed values for these ion-interactions based on their analyses of literature data are rather high with an unrealistically enormous amount of uncertainty [e.g., ε(\( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \), \( {\text{NO}}_{3}^{ - } \)) = 2.26 ± 4000 kg·mol−1, ε(\( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \), \( {\text{ClO}}_{4}^{ - } \)) = 3.61 ± 1.02 kg·mol−1]. Therefore the log10 K0 value recommended by Brown et al. [1] for the formation of \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \) cannot be considered to be precise. Our analyses (Fig. 13) show that equilibrium constant values for the formation of \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \) reported in [1] are over several orders magnitude overestimated. However, we cannot provide any improved values for the reactions involving these species (Eqs. 13, 14) or the pH regions where they might be important.
Aqueous Zr concentrations from ZrO2(am) suspensions equilibrated from the under-saturation direction (Kovalenko and Bagdasarov [27] and Altmaier et al. [24]). Lines represent predicted concentrations based on the SIT thermodynamic data we recommend for the Zr–OH system and in this figure also including values for polynuclear species \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{4}^{8 + } \) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \) reported in Brown et al. [1] (Tables 4, 7). (Note: The main conclusion drawn from this figure is that when the polynuclear species reported in Brown et al. [1] are included into our model the predicted concentrations are completely at odds with the reliable experimental data and the thermodynamic model recommended in our study)
Computational molecular simulation is a useful tool for predicting the stability of hypothetical aqueous metal complexes from first principles. Following our previous methods [43,44,45,46], we performed a simple set of quantum mechanical calculations at the density functional theory level (DFT) (for details of computational methods, see Appendix) to evaluate the energy required to construct the polynuclear \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \) ion from its equivalent monomeric subunits of \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \). The thermodynamic favorability of hydrolytic polymerization of Zr is the basic concept being tested in these total energy calculations. The energy minimized structure of the \( {\text{Zr}}_{2} \left( {\text{OH}} \right)_{2}^{2 + } \) cation is shown in Fig. 14 (top). Addition of water ligands up to completion of six-fold coordination was found to be energetically favorable (i.e., 4 waters of ligation added). In addition, regarding isomerization, cis- locations for OH were found to be more favorable than trans-OH locations. Similarly, the energy minimized structure of the \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \) cation is shown in Fig. 14 (bottom). Here the lowest energy structure found, based on the assumption that the highest bridging OH bond density yields a more stable complex, is the doubly μ-hydroxy bridged square planar arrangement of Zr atoms, with an extent of water solvation and a bonding topology very similar to that found in the crystal structure of zirconyl chloride octahydrate [47]. In this cation, each Zr atom shares four bridging OH groups and seven-fold coordination is completed with three bound water ligands (unlike the four found in the crystal structure).
Graphical models for energy minimized structures of \( {\text{Zr}}\left( {\text{OH}} \right)_{2}^{2 + } \) (top) and \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8} \left( {{\text{H}}_{2} {\text{O}}} \right)_{12}^{8 + } \) (bottom) from density functional theory calculations. Large (yellow) spheres represent Zr atoms, medium (red) oxygen atoms, and small (white) hydrogen atoms (Color figure online)
We calculated the total energy difference for the formation of the tetrameric cation from its monomeric subunits as:
which yielded a highly endothermic forward reaction of more than + 300 kJ·mol−1. This large thermodynamic unfavorability suggests that the energy decrease arising from formation of μ-hydroxy bonds is not enough to overcome the large electrostatic repulsion between the like charged monomeric subunits. We performed a similar analysis to evaluate the formation energy of model trimeric species from monomers. In this case, we evaluated polymerization into both linear and trigonal trimeric species, respectively, as:
and
which yielded forward reaction energies of + 470 and + 595 kJ·mol−1. The most stable \( {\text{Zr}}\left( {\text{OH}} \right)_{3}^{ + } \) monomer configuration is the fac isomer, whereas stable linear and trigonal trimer configurations involve substantial bridging O and OH bond formation and excess water ligand dissociation (Fig. 15). Similar to the tetramer formation energy, the trimer formation energies are both large endothermic values that suggest that the increased electrostatic repulsion of the highly charged Zr cation outweighs the stabilizing effect of bridging bond formation. Indeed, between the two trimer configurations, the more open linear configuration is computed to be 123 kJ·mol−1 more stable than the compact trigonal configuration after correcting for the loss of a hydration water necessary to convert from linear to trigonal.
Graphical models for energy minimized structures of \( {\text{Zr}}\left( {\text{OH}} \right)_{3}^{ + } \) (top), linear \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9} \left( {{\text{H}}_{2} {\text{O}}} \right)_{7}^{3 + } \) (middle), and trigonal \( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9} \left( {{\text{H}}_{2} {\text{O}}} \right)_{6}^{3 + } \) (bottom) from density functional theory calculations. Large (yellow) spheres represent Zr atoms, medium (red) oxygen atoms, and small (white) hydrogen atoms (Color figure online)
A caveat to these modeling-based conclusions is that the change in solvation energy and counter ion effects has not been included in the calculations, which because the total solvation cavity volume decreases upon polymerization should make an energy contribution that favors tetramer formation. But it is anticipated that this contribution alone would not be enough to negate the large endothermicity found from the consideration of bonding energies and electrostatics. The calculations thus suggest that polynuclear Zr species, such as the one examined but perhaps also others, would be unlikely to form from its component monomeric species under dilute conditions, consistent with their known formation in very concentrated and acidic solutions [38].
We conclude that our model based on mononuclear complexes provides reliable Zr solubility predictions over a large range of H+ concentrations (0.1–10−15.4 mol·kg−1), consistent with the Hf behavior.
5.7 \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) Formation Constant
Altmaier et al. [24] report extensive data on ZrO2(am) solubility as a function of pCH+ (varying from approximately 9–12, depending on CaCl2 concentrations) and CaCl2 concentrations (0.1, 0.2, 0.51, 1.02, and 2.11 mol·kg−1). The orders of magnitude increase in Zr concentrations that occurred with the increase in Ca concentrations at a fixed pH value in the alkaline region (Fig. 16) were interpreted as resulting from the formation of ternary Ca–Zr–OH complexes. They noted that the solubility data in 0.2, 0.51, 1.02, and 2.11 mol·kg−1 CaCl2 solutions can be well explained by the formation of \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) and the fact that the inclusion of \( {\text{Ca}}_{2} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 + } \) into the model slightly improved the fit to the 0.1 mol·kg−1 CaCl2 solutions. They interpreted these data with the SIT model using the ZrO2(am) solubility product and formation constant value for \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \) that are similar to the ones reported in Brown et al. [1] which are quite different than the values recommended here. Therefore, these data need to be reinterpreted so that they are consistent with the model recommended in this study. We reinterpreted these data using both the SIT and Pitzer models. The important variables to reinterpret these data are (1) model specific ion-interaction parameters for the CaCl2 system (Tables 4, 5), (2) the ZrO2(am) solubility product value, (3) the equilibrium constant value for the formation of \( {\text{Zr}}\left( {\text{OH}} \right)_{5}^{ - } \), and (4) the model specific equilibrium constant value for the formation of \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 - } \) (Table 7). The SIT model was used to simultaneously fit ε(\( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \), Cl−) = 0.51 kg·mol−1 and log10 K0 = 57.00 ± 0.22 for the formation of \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) (Eq. 18)Footnote 19 from the entire CaCl2 data set. Similar calculations using the Pitzer model provided Pitzer ion-interaction values (kg·mol−1) for (\( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \), Cl−) of (β(0) = 1.0633, β(1) = 15.5, Cϕ = 0.0995) and log10 K0 = 57.47 ± 0.2 for the formation of \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) (Eq. 18). A close agreement is observed between the experimental and predicted concentrations using either the SIT or the Pitzer model (Fig. 16).
Aqueous Zr concentrations, as a function of pC +H values ranging from about 9.5–12.2 and CaCl2 concentrations ranging from 0.1 to 2.11 mol·kg−1, from ZrO2(am) suspensions equilibrated from the under-saturation direction (Altmaier et al. [24]) (only the experimental data which are above the detection limit are plotted and considered for reinterpretations). Lines represent predicted concentrations based on the SIT (top graph) and Pitzer (bottom graph) models using the thermodynamic data developed in this study (Tables 4, 5, 7) that included \( \log_{10} K_{\text{SP}}^{0} \) = − 56.19 for ZrO2(am)
Altmaier et al. [24] also report somewhat limited data, compared to CaCl2 electrolytes discussed above, on ZrO2(am) solubility as a function of pCH+ and Ca(ClO4)2 concentrations of 0.2 and 1.0 mol·dm−3. The reinterpretation of these data using the SIT and the Pitzer models with ion-interaction parameters listed in Tables 4 and 5 that included reasonable (\( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \), \( {\text{ClO}}_{4}^{ - } \)) ion-interactions values (kg·mol−1) of (ε = 0.89 ± 0.12 for the SIT model from Altmaier et al. [24]; and β(0) = 1.675; β(1) = 16.01; Cϕ = 0.0995) for the Pitzer model provided the same average equilibrium constant values for the formation of \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) (Eq. 18) as based on the CaCl2 discussed above. However, these data indicate a larger average log10 standard deviation (0.51) compared to the CaCl2 data. The graphical display of these data is not included in this manuscript. Because the data in Ca(ClO4)2 are somewhat limited, we have chosen to recommend the values for the reaction in Eq. 18 based on CaCl2 system data.
Change history
08 June 2018
In the original publication of the article, there are errors in this document that pertain to a species that is reported as Zr2(OH) 2+2 . There is no such species; in each instance this species should have been reported as Zr(OH) 2+2 . Specifically this erroneous species, Zr2(OH) 2+2 , appears once in the heading of Sect. 5.2 on p. 873 and at nine different places including Eq. 7 in Sect. 5.2 on pp. 873 and 874, once in Sect. 5.6 on p. 880, once in the third equation of Table 7 on p. 881, and twice in Sect. 5.6 on p. 883. Wherever it occurs in the document it should be replaced with Zr(OH) 2+2
Notes
We do not necessarily believe that the reported reactions or their equilibrium constant values (especially involving carbonate) reported in Table 1 are accurate. However, the important point we want to make here is that when the experimental data are obtained using similar techniques and the data are interpreted using a similar theory, the reported equilibrium constant values for Hf and Zr are similar, showing that they are excellent analogs of each other.
The amorphic solid phase can be represented as either hydrous oxide or hydroxide; in this manuscript we chose to represent it as ZrO2(am).
Brown and Ekberg [21], using the same method Brown et al. [1] used to interpret Zr data, reinterpreted HfO2(am) and ZrO2(am) solubility data of Larsen and Gammill [20], who reported unusually high solubilities for both Hf and Zr due most likely to the presence of unfiltered colloids and other experimental problems. Not surprisingly, the values Brown and Ekberg [21] report for the HfO2(am) solubility product and Hf–OH system are very similar to the values for the corresponding Zr reactions reported by Brown et al. [1]. These values cannot possibly be correct. Brown and Ekberg’s [21] review included publications prior to 1973. Apparently, they missed a much more recent, very comprehensive article of Rai et al. [4] dealing with the Hf-OH system and covering a wide range in H+ (0.1–10−15.3 mol·kg−1) and NaCl (ranging up to 5.6 mol·kg−1), and NaOH (ranging up to 21.7 mol·kg−1) concentrations. In the Rai et al. [4] publication a reliable thermodynamic model is presented, based on all high quality literature as well as their own experimental data.
As for example, the pH dependence of ZrO2(am) solubility reaction [4ZrO2(am) + 8H2O ⇌ Zr4((OH)16(aq)] involving Zr4((OH)16(aq) is identical to the pH dependence of [ZrO2(am) + 2H2O ⇌ Zr((OH)4(aq)] involving Zr((OH)4(aq). Similar ZrO2(am) solubility reactions involving (\( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{15}^{ + } \) and \( {\text{Zr}}\left( {\text{OH}} \right)_{3}^{ + } \)) or (\( {\text{Zr}}_{3} \left( {\text{OH}} \right)_{9}^{3 + } \) and ZrOH3+) can be written to show that the pH dependence of each set of species is identical.
Data reported in these studies are fraught with the possible presence of colloids, poor detection limits, and other uncertainties, making these studies unsuitable for use in developing a reliable Zr–OH model.
All of the calculations performed in this document involve (either originally reported or calculated by us) concentrations of species in mol·kg−1, even though the original authors may have reported concentrations in mol·dm−3.
Because Kovalenko and Bagdasarov [27] conducted their study in very dilute solutions, both the NONLINT-SIT and NONLINT can be used to accurately interpret the data and they will provide very similar fitted values.
Here ΔZ2 is the sum of the valence squared of ions weighted by the stoichiometric coefficients of products minus the reactants, Im is the ionic strength in mol·kg−1, D = {0.509(Im)1/2}/{1 + 1.5(Im)1/2} at 25 °C, and Δε is the difference between the sum of ion interaction parameters (kg·mol−1) weighted by the stoichiometric coefficients of the products minus the reactants.
We understand Ekberg et al.’s [26] rationale in attempting to minimize the potential adsorption of aqueous Zr by autopipette. However, we are very surprised that they chose to do this by treating the autopipette with an alkaline solution, because the solubility in the acidic region decreases approximately three orders of magnitude with a unit increase in pH, and treating the autopipette with an alkaline solution will have the effect of increasing the pH, thereby decreasing the equilibrated aqueous Zr concentration. It is essential to minimize adsorption and changes in pH value of equilibrated samples during the process of preparing samples for analyses. Rai [30] reported that the untreated filters he was using to filter aqueous Pu(IV) suspensions increased the pH values of the samples significantly enough that PuO2(am) precipitated on the filter surfaces. As a result, to avoid adsorption of metals and potential changes in pH during sample processing, Rai and coworkers (e.g., see [3, 30]) have demonstrated that these problems can be mitigated by equilibrating the filters and filtration equipment with pH solutions (without the presence of the metal of interest) adjusted to the exact pH value of the sample to be processed and then passing a portion of the sample through the equipment and discarding this filtrate before collecting the sample for analyses.
This uncertainty is so high one wonders whether it might be a typo; however there are similarly high uncertainties in several ion interaction parameters reported by these authors.
Equivalently log10 K0 of \( { \lesssim } \) 25.7 for the reaction \( {\text{Zr}}^{ 4+ } + 2 {\text{OH}}^{ - } \rightleftharpoons {\text{Zr}} \left( {\text{OH}} \right)_{2}^{2 + } \).
Bilinski et al. [6], Ekberg et al. [26], and Cho et al. [22] did not filter their samples through membrane filters to eliminate colloids. Sasaki et al. [23] specifically note that their results are affected by the presence of colloids even though they filtered their samples through fine membrane filters.
Zr and NaOH concentrations reported by the authors in molarity units were converted to molality units by using the NaOH data reported in Weast [41] for these conversions. The data in Weast [41] are for NaOH molarities ranging from 0.125 to 14.295. Therefore the converted values for NaOH molality of < 14.295 are based on interpolations and are much more reliable than the values for NaOH molality of > 14.295 based on extrapolations.
Poor detection limits and uncertainties in these data could have been avoided had the studies been conducted in the absence of NaCl solutions.
Based on analogy to known interaction coefficients of doubly charged anionic species with alkali ions reported in Lemire et al. [42].
Assuming that the entire uncertainty in the equilibrium constant for the formation of \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{- } \) results from the uncertainty in the ε(Na+, \( {\text{Zr}}\left( {\text{OH}} \right)_{6}^{- } \)) value.
For example ΔZ2D − ΔεIm = − 0.022 at 23 molal ionic strength.
For these highly charged species, the equilibrium constant value for the formation of \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) and the values for the ion-interaction parameters are interdependent and thus it is not possible to determine unique values for these parameters. The values for the formation constant of \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) determined in this study are obtained by using/fitting reasonable values of the SIT and Pitzer ion-interaction parameters that provide close agreement with the ZrO2(am) solubility data in all CaCl2 solutions. It is possible to fit these data using both \( {\text{Ca}}_{2} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 + } \) (log10 K0 of ~ − 27.1 ± 0.3 determined from SIT or Pitzer modeling of data using reasonable values of ion-interaction parameters (kg·mol−1) for \( {\text{Ca}}_{2} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 + } \) with Cl− of ε = 0.1, and β(0) = 0.3159, β(1) = 1.614, Cϕ = − 0.00034) and \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \). However, the inclusion of \( {\text{Ca}}_{2} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 + } \) in modeling only marginally improved the fit to the ZrO2(am) solubility data in the lowest concentration of CaCl2 solutions investigated (0.1 mol·kg−1) and these species are not dominant in most of the samples. The overall standard deviations in fitting all of these data with either \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) or both \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) and \( {\text{Ca}}_{2} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 + } \) are identical. In order to obtain a reliable equilibrium constant value for the formation of \( {\text{Ca}}_{2} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{2 + } \), ZrO2(am) solubility studies need to be conducted at CaCl2 concentrations < 0.1 mol·kg−1. For these reasons we recommend using only \( {\text{Ca}}_{3} {\text{Zr}}\left( {\text{OH}} \right)_{6}^{4 + } \) to explain ZrO2(am) solubility data in the presence of Ca.
References
Brown, P.L., Curti, E., Grambow, B.: Chemical Thermodynamics of Zirconium. Chemical Thermodynamics, vol. 8. Elsevier B. V., Amsterdam (2005)
Guillaumont, R., Fanghänel, Th, Fuger, J., Grenthe, I., Neck, V., Palmer, D.A., Rand, M.H.: Update on the Chemical Thermodynamics of Uranium, Plutonium, Americium, and Technetium. Chemical Thermodynamics, vol. 5. Elsevier B.V, Amsterdam (2003)
Rai, D., Yui, M., Schaef, H.R., Kitamura, A.: Thermodynamic model for SnO2(cr) and SnO2(am) solubility in the aqueous Na+–H+–OH−–Cl−–H2O system. J. Solution Chem. 40, 1155–1172 (2011)
Rai, D., Xia, Y., Hess, N.J., Strachan, D.M., McGrail, B.P.: Hydroxo and chloro complexes/ion-interactions of Hf4+ and the solubility product of HfO2(am). J. Solution Chem. 30, 949–967 (2001)
Shannon, R.D.: Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Cryst. A32, 751–767 (1976)
Bilinski, H., Branica, M., Sillén, L.G.: Precipitation and hydrolysis of metallic ions II. Studies on the solubility of zirconium hydroxide in dilute solutions and in 1 M NaClO4. Acta Chem. Scand. 20, 853–861 (1966)
Chekmarev, A.M., Chibrikin, V.V., Yagodin, V.G.: Study of the hydrolysis of Zr4+ and Hf4+ ions in sulfate solutions by the method of paper chromatography. Radiokhimiya 17, 165–168 (1975)
Connick, R.E., McVey, W.H.: The aqueous chemistry of zirconium. J. Am. Chem. Soc. 71, 3182–3191 (1949)
Davydov, Y.P., Zabrodskii, V.N.: Hydrolysis of zirconium(IV) with the formation of mono- and polynuclear hydroxy complexes in solutions. Vestsi Akad. Navuk BSSR, Ser. Khim. Navuk. 2, 3–8 (1987)
Ekberg, C., Brown, P.L., Comarmond, M.J., Albinsson, Y.: On the hydrolysis of tetravalent metal ions. Mater. Res. Soc. Symp. Proc. 663, 1091–1100 (2001)
Nazarenko, V.A., Mandzhgaladze, O.V.: Determination of the formation constants of hydroxo-complexes of zirconium by the method of competing reactions. Russ. J. Inorg. Chem. 14, 639–643 (1969)
Norén, B.: The hydrolysis of Zr4+ and Hf4+. Acta Chem. Scand. 27, 1369–1384 (1973)
Paramonova, V.I., Sergeev, A.N.: The application of ion-exchange in the investigation of the substance in solution—V. The study of the process of complex formation by zirconium in nitric acid. Zh. Neorg. Khim. 3, 215–221 (1958)
Peshkova, V.M., Mel’chakova, N.V., Zhemchuzhin, S.G.: Complex formation in the benzoyl–acetone [1-phenylbutane-1,3-dione]-zirconium–benzene–water system and the hydrolysis of zirconium ions. Russ. J. Inorg. Chem. 6, 630–634 (1961)
Solovkin, A.S., Ivantsov, A.I.: Hydrolysis constants of the Zr4+ ion in perchlorate media. Russ. J. Inorg. Chem. 11, 1013–1016 (1966)
Tribalat, S., Schriver, L.: Polymérisation du zirconium(IV) en solution acide. Bull. Soc. Chim. Fr. 9, 2012–2014 (1975)
Veyland, A.: Propriétés Thermodynamiques, Cinétiques et Structurales de Complexes Simples et Mixtes du Zirconium(IV) avec les Ions Hydroxyle et Carbonate. University of Reims Champagne-Ardenne, Reims (1999)
Zielen, A.J.: The Hydrolytic Polymerization of Zirconium, in Radiation Laboratory. University of California, Berkeley (1953)
Zielen, A.J., Connic, R.E.: The hydrolytic polymerization of zirconium in perchloric acid solutions. J. Am. Chem. Soc. 78, 5785–5792 (1956)
Larsen, E.M., Gamhill, A.M.: Electrometric titration of zerconium and hafnium solutions. J. Am. Chem. Soc. 72, 3615–3619 (1950)
Brown, P.L., Ekberg, C.: Hydrolysis of Metal Ions. Wiley-VCH Verlag GmbH and Co. KGaA, Weinheim (2016)
Cho, H.-R., Walther, C., Rothe, J., Neck, V., Denecke, M.A., Dardenne, K., Fanghänel, Th: Combined LIBD and XAFS investigation of the formation and structure of Zr(IV) colloids. Anal. Bioanal. Chem. 383, 28–40 (2005)
Sasaki, T., Kobayashi, T., Takagi, I., Moriama, H.: Solubility measurement of zirconium(IV) hydrous oxide. Radiochim. Acta 94, 489–494 (2006)
Altmaier, M., Neck, V., Fanghänel, Th.: Solubility of Zr(IV), Th(IV) and Pu(IV) hydrous oxides in CaCl2 solutions and the formation of ternary Ca–M(IV)–OH complexes. Radiochim. Acta 96, 541–550 (2008)
Sheka, I.A., Pevzner, T.V.: Solubility of zirconium and hafnium hydroxides in sodium hydroxide solutions. Russ. J. Inorg. Chem. 5, 1119–1121 (1960)
Ekberg, C., Källvenius, G., Albinsson, Y., Brown, P.L.: Studies on the hydrolytic behavior of zirconium(IV). J. Solution Chem. 33, 47–79 (2004)
Kovalenko, P.N., Bagdasarov, K.N.: The solubility of zirconium hydroxide. Russ. J. Inorg. Chem. 6, 272–275 (1961)
Becking, L.B., Kaplan, I.R., Moore, D.: Limits of the natural environment in terms of pH and oxidation–reduction potentials. J. Geol. 68, 243–284 (1960)
Sterner, S.M., Felmy, A.R., Rustad, J.R., Pitzer, K.S.: Thermodynamic Analysis of Aqueous Solutions Using INSIGHT. Pacific Northwest National Laboratory, Richland (1997)
Rai, D.: Solubility product of Pu(IV) hydrous oxide and equilibrium constants of Pu(IV)/Pu(V), Pu(IV)/Pu(VI), and Pu(V)/Pu(VI) couples. Radiochim. Acta 35, 97–108 (1984)
Rai, D., Swanson, J.L.: Properties of plutonium (IV) polymer of environmental importance. Nucl. Tech. 54, 107–112 (1981)
Rai, D., Felmy, A.R., Juracich, S.P., Rao, L.: Estimating the Hydrogen Ion Concentration in Concentrated NaCl and Na2SO4 Electrolytes. Sandia National Laboratories, Albuquerque NM (1995)
Rand, M., Fuger, J., Grenthe, I., Neck, V., Rai, D.: Chemical Thermodynamics of Thorium. Chemical Thermodynamics, vol. 11. OECD Publishing, Paris (2008)
Felmy, A.R.: GMIN: A Computerized Chemical Equilibrium Model Using a Constrained Minimization of the Gibbs Free Energy. Pacific Northwest National Laboratory, Richland (1990)
Harvie, C.E., Moller, N., Weare, J.H.: The prediction of mineral solubilities in natural waters: the Na–K–Mg–Ca–H–Cl–SO4–OH–HCO3–CO2–H2O system to high ionic strengths at 25 °C. Geochim. Cosmochim. Acta 48, 723–751 (1984)
Latimer, W.M.: The Oxidation States of the Elements and Their Potentials in Aqueous Solutions, 2nd edn. Prentice-Hall Inc., Englewood Cliffs (1952)
Sasaki, T., Kobayashi, T., Takagi, I., Moriyama, H.: Hydrolysis constant and coordination geometry of zirconium(IV). Nucl. Sci. Technol. 45, 735–739 (2008)
Baes Jr., C.F., Mesmer, R.E.: The Hydrolysis of Cations. Wiley, New York (1976)
Rai, D., Felmy, A.R., Ryan, J.L.: Uranium(IV) hydrolysis constants and solubility product of UO2·xH2O(am). Inorg. Chem. 29, 260–264 (1990)
Ryan, J.L., Rai, D.: Thorium(IV) hydrous oxide solubility. Inorg. Chem. 26, 4140–4142 (1987)
Weast, R.C.: Handbook of Chemistry and Physics, 53rd edn., 1972–1973. CRC Press, Cleveland (1972)
Lemire, R.J., Fuger, J., Nitsche, H., Potter, P., Rand, M.H., Rydberg, J., Spahiu, K., Sullivan, J.C., Ullman, W.J., Vitorge, P., Wanner, H.: Chemical Thermodynamics of Neptunium and Plutonium. Chemical Thermodynamics, vol. 4. Elsevier, Amsterdam (2001)
Rai, D., Moore, D.A., Felmy, A.R., Rosso, K.M., Bolton Jr., H.: PuPO4(cr, hyd.) solubility product and Pu3+ complexes with phosphate and ethylenediaminetetraacetic acid. J. Solution Chem. 39, 778–807 (2010)
Rai, D., Moore, D.A., Hess, N.J., Rosso, K.M., Rao, L., Heald, S.M.: Chromium(III) hydroxide solubility in the aqueous K+–H+–OH−–CO2–HCO3 −– CO3 2−–H2O system: a thermodynamic model. J. Solution Chem. 36, 1261–1285 (2007)
Rai, D., Moore, D.A., Rosso, K.M., Felmy, A.R., Bolton Jr., H.: Environmental mobility of Pu(IV) in the presence of ethtylenediaminetetraacetic acid: myth or reality. J. Solution Chem. 37, 957–986 (2008)
Rai, D., Yui, M., Moore, D.A., Lumetta, G.J., Rosso, K.M., Xia, Y., Felmy, A.R., Skomurski, F.N.: Thermodynamic model for ThO2(am) solubility in alkaline-silica solutions. J. Solution Chem. 37, 1725–1746 (2008)
Mak, T.C.W.: Refinement of the crystal structure of zirconyl chloride octahydrate. Can. J. Chem. 46, 3491–3497 (1968)
Ryabchikov, D.I., Marov, I.N., Ermakov, A.N., Belyaeva, V.K.: Stability of some inorganic and organic complex compounds of zirconium and hafnium. J. Inorg. Nucl. Chem. 26, 965–980 (1964)
Karlysheva, K.F., Chumakova, L.S., Malinko, L.A., Sheka, I.A.: Reaction of zirconium and hafnium oxide chlorides with sodium carbonate in solution. Russ. J. Inorg. Chem. 27, 1582–1585 (1982)
João, A., Bigot, S., Fromage, F.: Etude des carbonates complexes des éléments IVB II—Détermination des constants d’équilibre de formamtion des tétracarbonatozirconate (IV) et -hafnate (IV). Bull. Soc. Chim. Fr. 6, 943–947 (1987)
Rai, D., Kitamura, A., Rosso, K.M., Sasaki, T., Kobayashi, T.: Issues concerning the determination of solubility products of sparingly soluble crystalline solids: solubility of HfO2(cr). Radiochim. Acta 104, 583–592 (2016)
Kobayashi, T., Sasaki, T., Takagi, I., Moriyama, H.: Solubility of zirconium(IV) hydrous oxides. J. Nucl. Sci. Technol. 44, 90–94 (2007)
Pitzer, K.S.: Activity Coefficients in Electrolyte Solutions, 2nd edn. CRC Press Inc., Boca Raton (1991)
Becke, A.D.: A new mixing of Hartree-Fock and local density functional theories. J. Chem. Phys. 98, 1372–1377 (1993)
Binkley, J.S., Pople, J.A., Hehre, W.J.: Self-consistent molecular orbital methods. 21. Small split-valence basis sets for 1st row elements. J. Am. Chem. Soc. 102, 939–947 (1980)
Dobbs, K.D., Hehre, W.J.: Molecular-orbital theory of the properties of inorganic and organometallic compounds. 6. Extended basis sets for 2nd-row transition metals. J. Comput. Chem. 8, 880–893 (1987)
Valiev, M., Bylaska, E.J., Govind, N., Kowalski, K., Straatsma, T.P., van Dam, H.J.J., Wang, D., Nieplocha, J., Apra, E., Windus, T.L., de Jong, W.A.: NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 181, 1477–1489 (2010)
Acknowledgements
We thank Japan Atomic Energy Agency for funding this research. Kevin Rosso acknowledges support from the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division through its Geosciences Program at Pacific Northwest National Laboratory.
Author information
Authors and Affiliations
Corresponding author
Appendix: Computational Methods
Appendix: Computational Methods
Ab initio molecular orbital calculations were performed to evaluate the energy required to construct the polynuclear \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \) ion from its equivalent monomeric subunits of \( {\text{Zr}}\left( {\text{OH}} \right)_{2}^{2 + } \). To do this, total energy minimizations were performed on the \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8}^{8 + } \) ion, modeled as a doubly μ-hydroxy bridged square planar Zr tetramer, and the \( {\text{Zr}}\left( {\text{OH}} \right)_{2}^{2 + } \) ion in the gas-phase at 0 K at the density functional level of theory. Energy minimizations were performed without symmetry constraint first at the spin-restricted Hartree–Fock (RHF) level of theory as a pre-optimization step, and then using the hybrid functional B3LYP [54] starting from the RHF result. The 3-21G basis set was used for all atoms (Zr, O, H) throughout [55, 56]. Water ligands were systematically added to available inner-shell coordination sites on each Zr atom in the tetramer as well as the dihydroxo monomer as long as the computed total energy of hydration remained negative. This yielded final stoichiometries of \( {\text{Zr}}_{4} \left( {\text{OH}} \right)_{8} \left( {{\text{H}}_{2} {\text{O}}} \right)_{12}^{8 + } \) and \( {\text{Zr}}\left( {\text{OH}} \right)_{2} \left( {{\text{H}}_{2} {\text{O}}} \right)_{4}^{2 + } \), respectively. All calculations were performed using the software NWChem version 6.1.1 [57].
Rights and permissions
About this article
Cite this article
Rai, D., Kitamura, A., Altmaier, M. et al. A Thermodynamic Model for ZrO2(am) Solubility at 25 °C in the Ca2+–Na+–H+–Cl−–OH−–H2O System: A Critical Review. J Solution Chem 47, 855–891 (2018). https://doi.org/10.1007/s10953-018-0766-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-018-0766-4