Abstract
Illuminating the complex reactions of molecules in aqueous solution is one of the most important tasks of experimental studies of reaction dynamics. The oxidant diperiodatocuprate(III) (DPC) was used for the oxidation of acebutolol (ACBT) in aqueous alkaline medium, at an ionic strength of 0.30 mol·dm−3. The stoichiometry was found to be 1:2 ([ACBT]:[DPC]). The order of the reaction with respect to [DPC] was unity when [DPC] << [ACBT], while the orders with respect to [ACBT] and [OH−] were less than unity, whereas it was negative less than unity in [\( {\text{IO}}_{ 4}^{ - } \)] over the concentration range studied. The final products of oxidation were identified by GC–MS analysis. Based on the experimental observations, a plausible mechanism is proposed. The reaction constants involved in the different steps of the reaction mechanism were calculated. The activation parameters and the thermodynamic quantities were determined and discussed. Active species of diperiodatocuprate(III) was found to be monoperiodatocuprate(III), i.e. [Cu(H2IO6)(H2O)2]. An understanding of the mechanism allows the chemistry to be interpreted, understood and predicted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Acebutolol (ACBT Scheme 1) is one of the prominent groups of prescription-blockers [1, 2]. They are advantageous in treatment of hypotensive circulatory disorders, hyper kinetic heart syndrome, tremor, migraine, portal hypertension, hyperthyroidism, anxiety, psychosomatic disorders and glaucoma [3]. Acebutolol is a cardioselective, lipophilic β-adrenoreceptor blocking agent that can cause the activation of adrenergic receptors so as to produce stimulation of the sympathetic nervous system. Therefore, it is more suitable for stimulation of the sympathetic nervous system than non-cardioselective β-blockers. It is commercially available for oral admininistration [4].
An important characteristic of transition metals is that they exhibit multiple oxidation states and, at higher oxidation states, they can form stable complex compounds with suitable polydentate ligands, for example, diperiodatocuprate(III) (DPC) [5], diperiodatoargentate(III) (DPA) [6] and diperiodatonickelate(IV) (DPN) [7], which are well known oxidizing agents in buffer media with a suitable pH. Diperiodatocuprate(III) (DPC) is a flexible one-electron oxidant [8] and due to its poor solubility and stability in aqueous medium, knowledge of the involvement DPC in oxidation reactions is inadequate or scanty. Its use as an analytical reagent is now well recognized [9]. The extraordinary role of copper complexes in biological systems is explained by several studies. The ongoing fascination with copper complexes is due to their uses as antimicrobial, antiviral, anti-inflammatory, antitumor agents, etc. [10]. There are multiple equilibria between different copper(III) species when the copper(III) periodate complex is the oxidant and it would be interesting to know which of the species is the active oxidant.
There were no reports on the oxidation of ACBT by any oxidant from kinetic and mechanistic points of view in the literature. Such studies are of importance in understanding the mechanistic pathways of oxidation of ACBT and to provide an insight into the interaction of metal ions with substrates. Hence, the present investigation is aimed to elucidate the reactivity of ACBT towards DPC, to arrive at a plausible mechanism and to understand the reactive species.
2 Experimental
2.1 Chemicals and Solutions
Acebutolol (Analytical Standard, 99 % pure) was purchased from Sigma Aldrich, India. Analytical grade reagents were used in the experiments and Millipore water was used throughout the work. A stock solution was prepared by dissolving a known amount of the ACBT in Millipore water. The required concentration of ACBT was obtained from its stock solution. Copper sulfate (BDH) solution was prepared by dissolving a known amount of the sample in Millipore water.
The diperiodatocuprate(III) (DPC) was prepared [5, 11, 12] and standardized by a standard procedure [13]. Copper sulfate pentahydrate (3.54 g), potassium periodate (6.80 g), potassium persulfate (2.20 g) and potassium hydroxide (9.0 g) were added to 250 mL of water. The mixture was shaken thoroughly and heated on a hot plate. The mixture turned an intense red after 3 hours and boiling was continued for 20 min more to complete the reaction. The mixture was filtered through a sintered crucible (G4), cooled and diluted to 250 mL. The UV–vis spectrum of the copper(III) complex exhibited three absorption bands at 211, 263 and 418 nm, which are characteristic of DPC. The ionic strength was maintained by adding KNO3 (AR) solution and the pH of the medium was maintained with KOH (BDH) solution. A stock solution of \( {\text{IO}}_{ 4}^{ - } \) was prepared by dissolving an appropriate weight of KIO4 (Riedel-de-Hean) in hot water to make a clear solution and then standardized iodometrically [14], at neutral pH, maintained using phosphate buffer.
2.2 Instruments and Kinetic Measurements
The kinetic measurements were carried out on a Varian CARY 50 Bio UV–Vis spectrophotometer (Varian, Victoria-3170, Australia) connected to a rapid kinetic accessory (HI-TECH SFA-12, U.K.) attached with a Peltier accessory for temperature control. The product analysis was carried out using GC–MS (Agilent 1100 series-API 2000) mass spectrometer (ionization technique). For pH measurement an Elico pH meter model LI 120 was used.
The kinetics of oxidation of acebutolol was followed under pseudo-first order condition where, [ACBT] > [DPC] at 298 ± 0.1 K. The reaction was initiated by mixing thermally equilibrated DPC with acebutolol solution, with required concentrations of KOH, KNO3 and KIO4, and the progress of the reaction was followed spectrophotometrically at 418 nm by monitoring the decrease in the absorbance due to DPC (with molar absorption index, ɛ = 6220 ± 100 dm3·mol−1·cm−1). The interference from other species in the reaction mixture at 418 nm was negligible.
Regression analysis of the experimental data to obtain regression coefficient r and the standard deviation S, of points from the regression line, was performed with Microsoft Excel 2007.
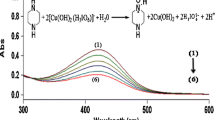
The rate constants, k obs were obtained from plots of log10 (absorbance) versus time and were reproducible within ±5 %. All the kinetic runs were followed to more than 80 % completion. During the kinetics studies a constant concentration of 1.0 × 10−4 mol·dm−3 of KIO4 was used throughout. Since periodate is present in excess, the possibility of oxidation of ACBT by periodate in alkaline medium at 298 K was tested. The progress of the reaction was followed iodometrically. However, it was found that there was no significant reaction under the experimental conditions employed compared to the DPC oxidation of ACBT. The total concentrations of periodate and OH− were calculated by considering the amount present in the DPC solution and that added. The spectral changes during the reaction are shown in Fig. 1. It is evident from the fit that the absorbance of DPC decreases at 418 nm.
3 Results and Discussion
3.1 Stoichiometry and Product Analysis
In order to find the exact number of MPC molecules required to oxidize one molecule of ACBT, the stoichiometry study has been carried spectrophotometrically with excess DPC in 0.25 mol·dm−3 KOH and at constant ionic strength of 0.3 mol·dm−3. Reaction mixtures containing different initial concentrations of the reactants were equilibrated for about 3 hr under a nitrogen atmosphere at 298 K. The unreacted DPC was estimated spectrophotometrically. The experimental results indicate that one mole of acebutolol consumes two moles of diperiodatocuprate(III) (1:2) as shown in Eq. 1:

The reaction products were identified as Cu(II), 3-(isopropylamino)propane-1,2-diol (A) and N-(3-acetyl-4-oxocyclohexa-2,5-dienylidene)butyramide (B). Product (A) was isolated from the reaction mixture by ether extraction and confirmed by GC–MS with the molecular ion peak at m/z = 135 (M+2) as shown in Fig. 2. The GC–MS spectrum (Fig. 3) showed a molecular ion peak at m/z = 221 (M+2) confirming product B. The byproduct Cu(II) was identified by its UV–vis spectrum.
3.2 Reaction Orders
The orders were calculated from the slopes of log10 k obs against log10 (concentration) plots by varying the concentrations of acebutolol, KOH and KIO4, while keeping all other conditions constant.
3.2.1 Effect of [DPC] on the Rate of Oxidation
The concentration of DPC was varied in the range of 2.0 × 10−5 to 2.0 × 10−4 mol·dm−3 keeping concentrations of all other reaction species constant. The k obs values are nearly constant (Table 1) indicating the order with respect to DPC concentration is unity. This was further confirmed by the linearity of the plots of log10 absorbance versus time (Fig. 4).
3.2.2 Effect of [ACBT] on the Rate of Oxidation
The effect of acebutolol concentration on the rate was studied in the range of 2.5 × 10−4 to 2.5 × 10−3 mol·dm−3, the concentrations of all other reaction components being kept constant. It was observed that increasing the concentration of acebutolol resulted in an increase in rate constants (Table 1). The order with respect to acebutolol concentration was obtained from the plot of log10 k obs against log10 [ACBT] and was found to be less than unity. It was confirmed by the plot of k obs against [ACBT] 0.68, which is linear, as opposed to the direct plot of k obs versus [ACBT] (Curve) as shown in Fig. 5.
Plots of [ACBT]0.68 against k obs and [ACBT] against k obs (conditions as in Table 1)
3.2.3 Effect of [OH−] and [IO −4 ] on the Rate of Oxidation
The effect of increase in concentration of alkali on the reaction was studied at constant concentrations of acebutolol, DPC and periodate at a constant ionic strength of 0.30 mol·dm−3 at 298 K. The rate constants increased with increase in alkali concentration (Table 1) and the order with respect to alkali concentration was found to be fractional, i.e., 0.42 (r ≥ 0.9891). The effect of periodate on the reaction was studied in the range of 1.0 × 10−5 to 1.0 × 10−4 mol·dm−3, keeping the concentrations of all other reactants constant. It was found that added periodate had a retarding effect on the rate of reaction (Table 1), the order with respect to periodate concentration being negative and less than unity (−0.39 (r ≥ 0.976, S ≤ 0.027)).
3.3 Effect of Ionic Strength on the Rate of Oxidation
Ionic strength was varied from 0.25 to 0.40 mol·dm−3 by varying the potassium nitrate concentration at constant concentrations of DPC, acebutolol, periodate and alkali. The k obs values were almost constant with increasing ionic strength. Hence, there was no influence of ionic strength on the rate of oxidation.
3.4 Effect of Dielectric Constant on the Rate of Oxidation
The dielectric constant of the medium, D, was varied by varying the t-butyl alcohol–water percentage. The dielectric constants of the reaction medium at various composition of t-butyl alcohol and water (v/v) were calculated as D = V 1 D 1 + V 2 D 2, where D 1 and D 2 are the dielectric constants of pure water and t-butyl alcohol, 78.5 and 10.9 respectively at 25 °C, and V 1 and V 2 are the volume fractions of water and t-butyl alcohol in the mixtures. No reaction of the solvent with the oxidant occurred under the experimental conditions. It was found that the rate constants increased with decreasing dielectric constant and the plot of log10 k obs against D−1 was linear with negative slope (r > 0.9876, S ≤ 0.0131) as shown in Fig. 6.
3.5 Effect of Added Product
The cases of initially added products, Cu(II), and 3-(isopropylamino)propane-1,2-diol, did not have any significant effect on the rate of reaction.
3.6 Rate Law
Under optimized experimental conditions, the rate law is given by:
3.7 Test for Free Radicals (Polymerization Study)
The involvement of free radicals in the reaction was examined as follows. A known quantity of acrylonitrile monomer was added to the initial reaction mixture which was kept for 2 h in an inert atmosphere. A white precipitate was formed on dilution with methanol, indicating the involvement of free radicals in the reaction [15]. The blank experiments with either DPC or acebutolol alone with acrylonitrile did not induce any polymerization under the same conditions.
3.8 Effect of Temperature
The oxidation reaction of ACBT was studied at four different temperatures 288, 298, 308 and 318 K under varying [ACBT], [OH−] and [\( {\text{IO}}_{ 4}^{ - } \)]. With increase in the temperature, the rate constants increased. The rate constant k of the slow step in Scheme 2 was obtained from the intercept of a plot of \( k_{\text{obs}}^{ - 1} \) against [ACBT]−1 at all the four temperatures (Table 2). The energy of activation for the rate determining step was obtained by the plot of log10 k against T −1 from which the other activation parameters were evaluated (Table 2).
3.9 Reaction Mechanism
Due to the versatile behavior of one-electron oxidants, the oxidation of many organic and inorganic compounds by Cu(III) species had been carried out. The literature survey reveals that the water soluble copper(III) periodate complex is reported [16] to be [Cu(HIO6)2(OH)2]7−. However, in aqueous alkaline medium and at the high pH values employed in the study, periodate is unlikely to exist as \( {\text{HIO}}_{ 6}^{ 4- } \) (as present in the complex) as is evident from its involvement in multiple equilibria, Eqs. 3–5 [17] depending on the pH of the solution, as given below.
Periodic acid exists in acid medium as H5IO6 and as \( {\text{H}}_{ 4} {\text{IO}}_{ 6}^{ - } \) at around pH = 7. Thus, under the alkaline conditions employed, the main species are expected to be \( {\text{H}}_{ 3} {\text{IO}}_{ 6}^{ 2- } \) and \( {\text{H}}_{ 2} {\text{IO}}_{ 6}^{ 3- } \). At higher concentrations, periodate also tends to dimerize [18]. However, formation of this species is negligible under conditions employed for this study. Hence, at the pH employed in this study, the soluble copper(III) periodate complex exists as diperiodatocuprate(III), [Cu(H3IO6)(H2IO6)]2−, a conclusion also supported by earlier work [18].
Lister [19] proposed three forms of copper(III) periodate in alkaline medium, viz., diperiodatocuprate(III) (DPC), monoperiodatocuprate(III) (MPC), and tetrahydroxocuprate(III). The last one is ruled out, as its equilibrium constant is 8.0 × 10−11 at 313 K. Hence, in the present study, DPC and MPC are considered as the active forms of copper(III) periodate complex. It may be expected that a lower periodate complex such as MPC is more important in the reaction than DPC. The results of increase in the reaction rate with increase in alkali concentration and decrease in rate with increase in periodate concentration (Table 1) suggest an equilibrium of the copper(III) periodate complex to form a monoperiodatocuptrate(III) (MPC) species as shown in Eqs. 6 and 7. Similar results have been well reported in literature [20].
The reaction between the diperiodatocuprate(III) complex and acebutolol in alkaline medium has a 1:2 stoichiometry (ACBT:DPC) with a first order dependence on [DPC], less than unit order in [substrate] and [alkali] and a negative fractional order in [periodate]. No effect of the added products was observed. Based on the experimental results, a mechanism as in Scheme 2 was proposed in which all the observed orders in each constituent, [oxidant], [reductant], [OH−], and [\( {\text{IO}}_{ 4}^{ - } \)] are well accommodated.
The less than unit order in [ACBT] is due to the formation of a complex (C) between the oxidant and ACBT prior to the formation of the products. K 3 is the composite equilibrium constant comprising the equilibrium to bind active species of acebutolol to MPC species to form a complex (C). Then, this complex (C) decomposes in a slow step to form 3-(isopropylamino)propane-1,2-diol (A) in addition to a free radical derived from acebutolol. Similar aromatic ether cleavage reactions have been observed in the literature [21, 22]. Thus, aromatic ether cleavage is the initial step in the oxidation of ACBT. The free-radical species formed further reacts with another molecule of MPC species in a fast step to yield N-(3-acetyl-4-oxocyclohexa-2,5-dienylidene)butyramide. The detailed mechanism for the oxidation of acebutolol by diperiodatocuprate(III) is represented as given in Scheme 2.
Since Scheme 2 is in accordance with the generally well accepted principle of non-complementary oxidations taking place in a sequence of one-electron steps, the reaction between the substrate and oxidant will afford a radical intermediate. A free radical scavenging experiment revealed such a possibility. This type of radical intermediate has also been observed in earlier work [23, 24]. Spectroscopic evidence for the complex formation between oxidant and substrate was obtained from UV–vis spectra of acebutolol (1.0 × 10−3 mol·dm−3), DPC (1.0 × 10−4 mol·dm−3), [OH−] = 0.25 mol·dm−3 and a mixture of both. Spectroscopic evidence for the complex formation between DPC and acebutolol was obtained from UV–visible spectra of ACBT and DPC and a mixture of both. A bathochromic shift of about 8 nm from 263 to 271 nm was observed [23].
According to Scheme 2, the rate law Eq. 8 is derived as:
By rearranging the above Eq. 8, Eq. 9, which is suitable for verification, is obtained.
According to Eq. 9, other conditions being constant, plots of 1/k obs against 1/[OH−] (r ≥ 0.9961, S ≤ 0.001), 1/k obs against 1/[ACBT] (r ≥ 0.9874, S ≤ 0.004), and 1/k obs against [H3IO 2-6 ] (r ≥ 0.9909, S ≤ 0.005) are linear at different temperatures (Fig. 7). The slopes and intercepts of such plots lead to the values of k, K 1, K 2 and K 3 of 3.2 × 10−2 s−1, 1.9 × 101 dm3·mol−1, 0.56 × 10−5 mol·dm−3 and 11.1 × 103 dm3·mol−1, respectively. Using these K 1, K 2, K 3, and k values, the rate constants under different experimental conditions were calculated by Eq. 8 and compared with experimental data (Table 1). The values of K 1 and K 2 are in good agreement with the literature [25]. The equilibrium constant K 1 is far greater than K 2, which may be attributed to the greater tendency of DPC to undergo hydrolysis as compared to the dissociation of hydrolyzed species in alkaline medium. All these results are interpreted satisfactorily in Scheme 2. In the same manner the values of k, K 1, K 2 and K 3 were calculated at all temperatures and are tabulated in Table 2.
The thermodynamic quantities for the different equilibrium steps in Scheme 2 can be evaluated as follows. The acebutolol, perioadate and hydroxide ion concentrations (Table 1) were varied at different temperatures. A van’t Hoff’s plot was made for variation of K 1 with temperature (log10 K 1 against 1/T (r ≥ 0.9569, S ≤ 0.007) and the values of the apparent standard state enthalpy of reaction ΔH, entropy of reaction ΔS, and Gibbs energy of reaction ΔG were calculated for the final equilibrium step. These values are given in Table 2. A comparison of the latter values (ΔH = 11.84 kJ·mol−1) with those obtained for the slow step of the reaction (ΔH # = 33.45 kJ·mol−1) shows that these values mainly refer to the rate-limiting step, supporting the fact that the reaction before the rate-determining step is fairly fast since it involves a low-activation energy [26]. In the same manner, K 2 and K 3 were calculated at different temperatures and their corresponding values of the thermodynamic quantities are given in Table 2.
The negligible effect of ionic strength on rate of reaction reveals the involvement of neutral species in the reaction as seen in Scheme 2. The effect of solvent on the reaction rate is described in detail in the literature [27, 28]. For the limiting case of a zero angle approach between two dipoles or an ion-dipole system, the plot of log10 k obs against 1/D gives a straight line, with a negative slope for a reaction having negative ion and a dipole or between two dipoles, while a positive slope is obtained for positive ion–dipole reactions. In the present investigation, the plot of log10 k obs against 1/D (Fig. 6) is linear with negative slope, which supports the involvement of negative ions as in Scheme 2. A high negative value of ΔS # (−162.8 J·K−1·mol−1) suggests that intermediate complex (C) is more ordered than the reactants [29]. The observed modest activation energy and sizeable entropy of activation supports a complex transition state in the reaction [30].
4 Conclusions
For the first time the oxidation mechanism of cardio-selective acebutolol by diperiodatocuprate(III) in aqueous alkaline medium was studied. Among various species of diperiodatocuprate(III) in alkaline medium, monoperiodatocuprate(III) [Cu(OH)2(H3IO6)−] is considered as the active species for the oxidation reaction of ACBT by DPC. The rate constant of the slow step and other equilibrium constants involved in the mechanism as well as the activation parameters of the reaction have been computed. The description of the mechanism is consistent with all the experimental evidences including kinetic, spectral and product studies.
References
Mompelat, S., Bot, B.L., Thomas, O.: Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 35, 803–814 (2009)
Vieno, N.M., Harkki, H., Tuhkanen, T., Kronberg, L.: Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environ. Sci. Technol. 41, 5077–5084 (2007)
Parfitt, K.: Martindale: The Complete Drug Reference, 33rd edn. Pharmaceutical Press, London, UK (2002)
Lima, J.J.: Relationship between beta adrenoceptor occupancy and receptor down-regulation induced by beta antagonists with intrinsic sympathomimetic activity. J. Recept. Signal Transduct. Res. 16, 357–372 (1996)
Yao, H., Zhang, M., Zeng, W., Zeng, X., Zhang, Z.: A novel chemiluminescence assay of mitoxantrone based on diperiodatocuprate(III) oxidation. Spectrochim. Acta Part A 117, 645–650 (2014)
Kumar, A., Kumar, P., Ramamurthy, P.: Kinetics of oxidation of glycine and related substrates by diperiodatoargentate(III). Polyhedron 18, 773–780 (1999)
Shettar, R.S., Nandibewoor, S.T.: Kinetic, mechanistic and spectral investigations of ruthenium(III)-catalysed oxidation of 4-hydroxycoumarin by alkaline diperiodatonickelate(IV) (stopped flow technique). J. Mol. Catal. A 234, 137–143 (2005)
Li, T., Xie, H.Y., Fu, Z.F.: Micellar electrokinetic chromatography-chemiluminescent detection of biogenic amines using N-(4-aminobutyl)-N-ethylisoluminol as derivatization reagent and trivalent copper chelate as chemiluminescence enhancer. Anal. Chim. Acta 719, 82–86 (2012)
Hu, Y., Li, G., Zhang, Z.: A flow injection chemiluminescence method for the determination of lincomycin in serum using a diperiodato-cuprate(III)–luminol system. Luminescence 26, 313–318 (2011)
Weder, J.E., Dillon, C.T., Hambley, T.W., Kennedy, B.J., Lay, P.A., Biffin, J.R., Regtop, H.L., Davies, N.M.: Copper complexes of non-steroidal anti-inflammatory drugs: an opportunity yet to be realized. Coord. Chem. Rev. 232, 95–126 (2002)
Shan, J., Wang, X., Shen, H.: Kinetics and mechanism of oxidation of N, N-dimethylethanolamine by diperiodatocuprate(iii) complex in alkaline medium. Asian J. Chem. 23, 180–182 (2011)
Shan, J., Wang, Q., Zhang, Z., J, Han: Oxidation of neopentyl glycol by diperiodatocuprate(III) in alkaline medium- a kinetic and mechanistic study. Asian J. Chem. 27, 1537–1539 (2015)
Jeffery, G.H., Bassett, J., Mendham, J., Denney, R.C.: Vogel’s Textbook of Quantitative Chemical Analysis, 5th edn. ELBS, Longman, Essex, UK (1996)
Magdum, P.A., Bagoji, A.M., Nandibewoor, S.T.: Ruthenium(III) catalysed and uncatalysed oxidative mechanisms of methylxanthine drug theophylline by copper(III) periodate complex in alkali media: a comparative approach. J. Phys. Org. Chem. 28, 743–754 (2015)
Jagdeesh, R.V.: Puttaswamy,: Ru(III), Os(VIII), Pd(II) and Pt(IV) catalysed oxidation of glycyl–glycine by sodium N-chloro-p-toluenesulfonamide: comparative mechanistic aspects and kinetic modeling. J. Phys. Org. Chem. 21, 844–858 (2008)
Hu, Y., Li, Gongke: A novel flow injection chemiluminescence method for the determination of ractopamine in urine by using trivalent copper. Anal. Methods 5, 678–683 (2013)
Bailar, J.C., Emeleus, H.J., Nyholm, S.R., Trotman-Dickenson, A.F.: Comprehensive Inorganic Chemistry, vol. 2. Pergamon Press, Oxford (1975)
Abbar, J.C., Malode, S.J., Nandibewoor, S.T.: Osmium(VIII) catalyzed and uncatalyzed oxidation of a hemorheologic drug Pentoxifylline by alkaline copper(III) periodate complex: a comparative kinetic and mechanistic approach. Polyhedron 29, 2875–2883 (2010)
Lister, M.W.: The stability of some complexes of trivalent copper. Can. J. Chem. 31, 638–652 (1953)
Hosamani, R.R., Shetti, N.P., Nandibewoor, S.T.: A kinetic and mechanistic study on the oxidation of l-cystine by alkaline diperiodatocuprate(III): a free radical intervention. Kinet. Catal. 50, 530–539 (2009)
Lohmann, W., Karst, U.: Electrochemistry meets enzymes: instrumental on-line simulation of oxidative and conjugative metabolism reactions of toremifene. Anal. Bioanal. Chem. 394, 1341–1348 (2009)
Madsen, K.G., Olsen, J., Skonberg, C., Hansen, S.H., Jurva, U.: Development and evaluation of an electrochemical method for studying reactive phase-I metabolites: correlation to in vitro drug metabolism. Chem. Res. Toxicol. 20, 821–831 (2007)
Jaky, M., Szeverenyi, Z., Simandi, L.I.: Formation of manganate(V) in oxidations by permanganate ion in strongly alkaline solutions. Inorg. Chim. Acta 186, 33–37 (1991)
Chougale, R.B., Hiremath, G.A., Nandibewoor, S.T.: Kinetics and mechanism of oxidation of l-alanine by alkaline permanganate. Pol. J. Chem. 71, 1471–1478 (1997)
Hiremath, D.C., Kiran, T.S., Nandibewoor, S.T.: Oxidation of vanillin by diperiodatocuprate(III) in aqueous alkaline medium: a kinetic and mechanistic study by stopped flow technique. Int. J. Chem. Kinet. 39, 236–244 (2007)
Bilehal, D.C., Kulkarni, R.M., Nandibewoor, S.T.: Kinetics and mechanistic study of the ruthenium(III) catalyzed oxidative deamination and decarboxylation of l-valine by alkaline permanganate. Can. J. Chem. 79, 1926–1933 (2001)
Laidler, K.J.: Chemical Kinetics, 3rd edn., 7th impression, Pearson Education Inc., New Delhi (2012)
Amis, E.S.: Solvents Effect on Reaction Rates and Mechanisms. Academic Press, New York (1966)
Weissberger, A.: Investigations of Rates and Mechanism of Reactions in Techniques of Chemistry, vol. 4. Wiley, New York (1974)
Farokhi, S.A., Nandibewoor, S.T.: Kinetic, mechanistic and spectral studies for the oxidation of sulfanilic acid by alkaline hexacyanoferrate(III). Tetrahedron 59, 7595–7602 (2003)
Acknowledgments
Atmanand M. Bagoji thanks Karnatak University, Dharwad for the award of UGC-UPE fellowship. S.T. Nandibewoor thanks the UGC, New Delhi for the award of BSR faculty fellowship (F.NO.18-1/2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bagoji, A.M., Magdum, P.A. & Nandibewoor, S.T. Oxidation of Acebutolol by Copper(III) Periodate Complex in Aqueous Alkaline Medium: A Kinetic and Mechanistic Approach. J Solution Chem 45, 1715–1728 (2016). https://doi.org/10.1007/s10953-016-0539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0539-x