Abstract
Bi2Sr2Ca1Cu2O8+σ (Bi-2212) superconducting thin films were prepared using the sol–gel method on a LaAlO3 (LAO) single crystal substrate. The influences of oxygen content in the process of heat treatment on the orientation degree and superconducting performance of the Bi-2212 films are discussed, and the results show that changing the oxygen content in a mixed atmosphere during the high-temperature heat treatment process could control the growth orientation of the Bi-2212 films. When the oxygen content was in a range from 0.5 to 2.0%, with an increase in oxygen content, the c-axis growth orientation degree of the thin film presented a linear growth trend, but if the oxygen content was too high in the atmosphere, it could lead to the generation of impure phases. Further study found that both Tc and Jc of the film increased with an increase in the c-axis orientation degree. Among different oxygen contents, when the oxygen content in the atmosphere was 2.0%, the prepared sample had good biaxial texture, compact surface structure, and a regular atomic arrangement; the corresponding c-axis orientation degree could reach 97%, and the values of Tc and Jc were 85.3 K and 4.46 × 105 A/cm2, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The preparation of large-scale and high-performance Bi-2212 thin film is the basis and prerequisite for the integration of superconducting electronic devices. No matter if the Bi-2212 thin film is applied in a strong or weak current field, it is required that the Bi-2212 thin film should have good superconducting performance [1,2,3,4]. The sol–gel method is generally considered to be the most effective method for preparing low-cost and high-performance thin films, and it shows great advantages in the preparation of super-large-scale thin films. In addition, Bi-2212 thin film has strong anisotropy [5,6,7,8]. Therefore, it is of great significance to develop a method for the preparation of Bi-2212 thin film with good growth orientation and high critical current density for the large-scale industrial development of Bi-2212 thin film in the future [9,10,11,12,13].
The purpose of this paper is to investigate the influences of the orientation degree of Bi-2212 film on the critical current density. The Bi-2212 film was prepared using the sol–gel method, and the regulation effect of oxygen content in the heat treatment process on the orientation degree of the Bi-2212 film was discussed. Furthermore, the influence rules of the film orientation degree on the critical transition temperature and critical current density were analyzed. The results of this work can provide a reference for the preparation of high-quality Bi-2212 thin films.
2 Experimental
Bi(OAC)3 (GR, 99.999%) was obtained from Alfa Aesar (China) Chemicals Co. Ltd. Sr(OAC)2·1/2H2O (AR, 99%), Cu(OAC)2·H2O (AR, 98%), Ca(OAC)2·H2O(AR, 98%), and CH3OH(AR) were supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). CH2 = CH-COOH (AR) was obtained from Tianjin Yongsheng Fine Chemical Co., Ltd.
In this study, Bi(CH3COO)3, Sr(CH3COO)2·1/2H2O, Ca(CH3COO)2·H2O, and Cu(CH3COO)2·H2O were used as the starting materials. CH2 = CH-COOH was used as a chemical modifier, and MeOH was used as a solvent to prepare Bi-2212 sol. First, according to the molar ratio of metal ions (i.e., Bi3+:Sr2+:Ca2+:Cu2+ = 2:2:1:2), the masses of the starting materials were calculated and weighed. (Bi)Ac3, Sr(Ac)2, Ca(Ac)2, and Cu(Ac)2 were dissolved into AA and MeOH, respectively, in a stoichiometric quantity of (Bi)Ac3:AA:MeOH = 1:10:20, Sr(Ac)2:AA:MeOH = 1:12:20, Ca(Ac)2:AA:MeOH = 1:12:20 and Cu(Ac)2:AA:MeOH = 1:3:60 at room temperature to prepare Bi-solution, Sr-solution, Ca-solution, and Cu-solution, respectively. The four acetates were each dissolved in the mixed solution of methanol and acrylic acid to obtain four solutions. Then, the solutions were each stirred until the acetates were fully dissolved. Finally, the four solutions were mixed together and stirred until the mixture became blue and transparent. After standing for 48 h, the Bi-2212 sol with stable performance was obtained.
Bi-2212 gel film was prepared on a LAO (00 l) single crystal substrate using the sol–gel dip-coating (SGDC) method. The film sample was processed into an “H-shape” microbridge with a width of 100 μm and a length of 2 mm (as shown in Fig. 1), according to the method reported in literature [14]. Then, the Bi-2212 gel film was dried at 80 °C in a drying oven for 10 min. The dried Bi-2212 gel film was heat-treated in a horizontal quartz tube furnace under a specific atmosphere. To be specific, the Bi-2212 gel film was first heated from room temperature to 420 °C under a N2 atmosphere with a certain humidity; then, it was heated to 550 °C and held for 40 min under a dry O2 atmosphere to provide excess O2 for the formation of intermediate phases at this stage; after that, the film was heated to 815 °C under a mixed atmosphere of N2 and O2 with a certain humidity and held for 80 min; afterward, the film was cooled to 550 °C in the oven and oxygenated with a large flow of O2 for 60 min; finally, the film was cooled to room temperature in the oven. During the heat treatment process, the humidity was controlled by making the gas in the heat treatment process pass through a water bath of 35 °C. To study the influences of oxygen content on the superconductivity of the material in the phase formation process, a N2 + O2 mixed atmosphere with a certain humidity was used in the heat treatment at 815 °C, and the oxygen content was set as 0.5%, 1.0%, 1.5%, 2.0%, and 2.5%. The corresponding samples were labeled as BW-1, BW-2, BW-3, BW-4, and BW-5, respectively.
The current–voltage (I-V) curves were measured using the four-lead method [15], as shown in Fig. 1. The current and voltage were each introduced to the two sides of the “H-shape” sample, and the processed sample was put into a Versalab integrated physical property measurement system (Quantum Design Company, USA) to measure the value of Tc. The I–V curves were measured at 70 K.
Structure and phase analysis was performed on the thin films by θ-2θ scanning of X-ray diffraction (XRD, 7000S-type). The in-plane and out-of-plane textures were characterized by Phi scanning and Omega rocking curve, respectively, using a SmartLab X-ray diffractometer. The surface morphology was observed using a Merlin Compact field-emission scanning electron microscope (FESEM). The microstructure was observed using a JEM-ARM200CF NEOARM Cs-corrected transmission electron microscope (TEM, JEOL, Japan).
3 Results and Discussion
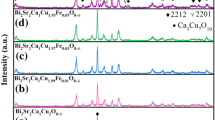
Figure 2a shows XRD patterns of Bi-2212 superconducting thin films prepared with different oxygen contents. As can be seen, when the oxygen content in the high-temperature heat treatment atmosphere was 0.5%, in addition to the c-axis characteristic diffraction peaks of (002), (006), (0010), and (0012) of the Bi-2212 phase, the diffraction peaks of (002) and (006) of the Bi-2201 phase and the non-c-axis peaks of the Bi-2212 phase also appeared. With an increase in oxygen content, the diffraction peak of the Bi-2212 (001) crystal plane gradually intensified, but the diffraction peaks of the Bi-2201 phase and the non-c-axis peaks of the Bi-2212 phase are still present, showing a gradually decreasing intensity. When the oxygen content was 2.0%, the sample had the highest diffraction peak intensity of Bi-2212(00 l) and the best c-axis orientation. When the oxygen content was further increased to 2.5%, the diffraction peak intensity of the (001) crystal plane became weak, and the impure phase of SrCu2O3 appeared. These results indicate that, when the oxygen content was in the range of 0.5–2.0%, with an increase in oxygen content, the crystalline degree of the Bi-2212 phase was higher and higher and that the epitaxial growth orientation was also better and better. According to the Jade 6 software, the lattice parameter c values of Bi-2212 thin films prepared under different oxygen content were analyzed and calculated as 30.72 Å, 30.77 Å, 30.78 Å, 30.81 Å, and 30.75 Å, respectively.
To quantitatively calculate the preferred orientation degree of thin-film growth, we calculated the volume fraction of c-axis grains in the film sample (α(001), and the calculation formula is as follows [16]:
where \({I}_{(hkl)}\) is the relative intensity of the diffraction peak corresponding to the (hkl) crystal plane in the measured XRD pattern of the thin film sample, and \({I}_{(hkl)}^{*}\) is the relative intensity of the diffraction peak corresponding to the (hkl) crystal plane in the powder diffraction pattern of Bi-2212 in the standard ASTM database. The calculated values of \({\alpha }_{(00l)}\) of the thin film samples prepared with different oxygen contents are 82%, 86%, 91%, 97%, and 93%, respectively, indicating that the orientation degree of the thin film first increases and then decreases with an increase in oxygen content, as shown in Fig. 2b. Among all of the samples, BW-4 has a better orientation degree than the other films prepared under other oxygen content conditions.
Figure 3a shows the relationship curves (R-T curve) between the film resistance and temperature for Bi-2212 superconducting thin films prepared with different oxygen contents. As can be seen, a superconducting transition occurs for all of the Bi-2212 superconducting thin films prepared in the oxygen content range of 0.5 ~ 2.5%, and the critical transition temperature (Tc) first increases and then decreases with an increase in oxygen content. Among the Bi-2212 superconducting thin films, BW-4 had the highest critical transition temperature of 85.3 K and the lowest superconducting transition temperature width (ΔTc) of 3.2 K. When the oxygen content was increased to 2.5%, Tc decreased to 79.8 K, and ΔTc increased to 7 K. These results indicate that different oxygen contents can affect the superconductivity of the films. Among the different oxygen contents, when the oxygen content was 2%, the purity of Bi-2212 phase was the highest, and its superconductivity was the best. To more clearly analyze the influences of the orientation degree on Tc and ΔTc values, the relationship curves between Tc (ΔTc) and α(001) are plotted in Fig. 3b. As can be seen, the Tc value increases gradually while the ΔTc value decreases with an increase in orientation degree. The Bi-2212 film with the best comprehensive superconducting performance can be obtained by heat treatment when the oxygen content is 2.0%.
Changing oxygen content during high-temperature heat treatment can not only affect the superconducting performance of the films but also change the critical current density. Figure 4a shows the V–I curves of thin films prepared with different oxygen contents at 70 K. Based on this, Eq. (2) can be used to calculate the critical current density:
where \(w\) is the width of the microbridge film, \(t\) is the thickness of the film, and Ic is the inflection point of each curve in Fig. 3. From Eq. (2), the Jc values of the samples can be calculated. Table 1 provides the Jc values, thickness, superconducting performance parameters, and α (00 l) of the samples. According to the data in Table 1, the variation curves of Jc with respect to α(00 l) can be plotted, as shown in Fig. 4b. It can be seen that the critical current density Jc of the film increases linearly with an increase in α(00 l). This indicates that the growth orientation of the film can affect the critical current density; the higher the orientation degree, the higher the critical current density.
Figure 5 shows the surface morphologies of the Bi-2212 films obtained at 815 °C with different oxygen contents of 0.5%, 2.0%, and 2.5%. By comparison, it can be found that there are many acicular a-axis grains on the surface of the BW-1 and BW-5 film samples and that the amount of the rod-like grains on the surface of BW-5 is less than that on the surface of BW-1. In contrast, the surface layer of the BW-4 film sample is compact, and few a-axis grains can be observed on the surface; instead, island-like Bi-2212 grains grow epitaxially along the c-axis orientation and show a lamellar structure. These results are consistent with the results of the above XRD analysis. The EDS analysis data of BW-1, BW-4, and BW-5 film samples are shown in Table 2. It is found that the atomic ratio of the four metal elements is Bi:Sr:Ca:Cu≈2:2:1:2, indicating that the material with the lamellar structure is Bi-2212 phase.
The Omega rocking curve and Phi scanning curve of the BW-4 sample with a high orientation degree were further analyzed. Figure 6a shows the Omega scanning pattern of the BW-4 sample, and its half-height width (FWHM∆Omega) is equal to 0.30°, indicating that the Bi-2212 thin film has a good out-plane texture. The (115) crystal plane of the Bi-2212 film was selected for a Phi scanning test, and the curve is shown in Fig. 6b. The angle between the (115) and (00 l) crystal planes is 58.21°. After the sample was tilted by 58.21°, it was rotated by 360° around the c-axis, and four diffraction peaks separated by 90° appeared in the Phi scanning pattern. The average half-height width (FWHM∆Phi) of the Phi scanning pattern of BW-4 is about 0.95°, indicating that the Bi-2212 film has a good in-plane texture.
The BW-4 sample was also observed and analyzed using a Cs-corrected transmission electron microscope with atomic resolution, and the image is shown in Fig. 7. As can be seen, the thin film grows epitaxially along the c-axis orientation, and the atoms are arranged periodically along the c-axis in the order of BiO–SrO–CuO2–Ca–CuO2–SrO–BiO, indicating perfect crystal growth orientation of the sample.
4 Conclusions
In this paper, a method of regulating the c-axis growth orientation of Bi-2212 thin film that was prepared using the sol–gel method was studied. It was found that the growth orientation of the thin film could be controlled by changing the oxygen content in the atmosphere during the heat treatment process. The main research conclusions are as follows:
-
(1)
When the oxygen content in the high-temperature heat treatment atmosphere was in the range of 0.5–1.5%, with an increase in oxygen content, the diffraction peak intensity of the (00 l) crystal plane of the Bi-2212 thin films was gradually enhanced, and the c-axis orientation degree was increased. Excess oxygen would lead to the formation of a SrCu2O3 impure phase. When the oxygen content was 2.0%, the prepared Bi-2212 thin film had a good c-axis orientation degree.
-
(2)
The orientation degree of the film had an influence on the superconducting properties of Tc and Jc. The higher the orientation degree, the higher the values of Tc and Jc of the film. When the c-axis orientation degree reached 97%, Tc and Jc of the film were 85.3 K and 4.46 × 105 A/cm2, respectively.
-
(3)
The Bi-2212 thin film prepared in high-temperature heat treatment atmosphere with 2.0% oxygen content had good biaxial texture, and its surface was compact. The growth orientation of the film was mainly along the c-axis, and the grains on the surface of the film presented an island-like growth mode.
References
Endo, K., Arisawa, S., Kaneko, T.: Characterization by X-ray diffraction of non c-axis epitaxial Bi2Sr2CaCu2O8+δ thin films. IEEE Trans. Appl. Supercond. 26, 1–1 (2016)
Nane, O., Abukay, D.: The effects of the post-annealing time on the growth mechanism of Bi2Sr2Ca1Cu2O8+ thin films produced on MgO (100) single crystal substrates by pulsed laser deposition (PLD). Ceram. Int. 42, 5778–5784 (2016)
Wei, R., Li, Z., Hu L.: P-type transparent conductivity in high temperature superconducting Bi-2212 thin films. Appl. Phys. Lett. 112, 251109 (2018)
Matsushita, T., Kiuchi, M., Yasuda, T.: Thickness dependence of pinning properties in Bi-2212 superconductor. Supercond. Sci. Tech. 18, 1348 (2005)
Lu, X., Miao, X., Sun, D.: Crystalline characteristics and superconducting properties of Bi-2212 thin films prepared by the Pechini sol–gel method: the effect of different substrates on the film growth. J. Appl. Crystallogr. 48, 100–108 (2015)
Yamada, Y., Okamoto, T.: Preparation of (11n) oriented Bi2Sr2CaCu2O8+x thin films by solution methods using NdGaO3 (100) substrates. J. Phys. Conf. Ser. 1054, 012009 (2018)
Yu, X., Yang, H., Yang, Q.: The preparation of c-axis epitaxial Bi2212 films on STO (100) by sol–gel method. Appl. Surf. Sci. 258, 38–43 (2011)
Zalga, A., Beganskiene, A., Kareiva, A.: Sol-Gel synthesis and superconducting properties of Bi-2212 high-Tc superconductors. Pol. J. Chem. 81, 1547–1553 (2007)
Tla, B., Cz, B., Sg, A.: The influence of critical current density of Bi-2212 superconductors by defects after Yb-doping. Physica C 519, 24–27 (2015)
Haraguchi, T., Takayama, S., Kiuchi, M.: Influence of anisotropy and pinning centers on critical current properties in Bi-2212 superconductors. Physica C 445, 123–127 (2006)
Ni, B., Kiuchi, M., Otabe, E.: Enhancement of critical current density and flux pinning in Bi-2212 thick films due to MgO addition. IEEE Trans. Appl. Supercond. 13, 3695–3698 (2003)
Jiang, J., Miao, H., Huang, Y.: Reduction of gas bubbles and improved critical current density in Bi-2212 round wire by swaging”. IEEE Trans. Appl. Supercond. 23, 1–6 (2013)
Baorong.: Relationship between MgO particles addition and critical current density in Bi-2212 thick film grown on oxidized Ni substrate. Physica C. 372, 1868–1871 (2002)
Liu, X., Zhao, G.: One step preparation of photosensitive Bi2Sr2CaCu2O8+x films and their fine patterns by a photosensitive sol–gel method. Supercond. Sci. Tech. 31, 125017 (2018)
Kosse, I., Prokhorov, Y., Khokhlov, A.: Measurements of the magnetic field and temperature dependences of the critical current in YBCO films and procedures for an appropriate theoretical model selection. Supercond. Sci. Tech. 21, 075015 (2008)
Zhao, G., Lei, L.: Effect of copper content in precursor solution on the superconducting properties of YBCO films derived from low-fluorine solution. Physica C 468, 2317–2321 (2008)
Funding
This work was supported by the Major Science and Technology Projects of Shaanxi Province (No. 2020zdzx04-04–02). This work was in part supported by the Youth Innovation Team of Shaanxi Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, M., Zhao, G., Ren, P. et al. Regulation of the Growth Orientation of Bi-2212 Thin Film and the Corresponding Influences on Critical Current Density. J Supercond Nov Magn 35, 33–39 (2022). https://doi.org/10.1007/s10948-021-05998-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-021-05998-5