Abstract
Ni0.6Cd0.2Cu0.2Fe2O4 ferrites were synthesized using sol-gel method under different sintering temperatures. XRD patterns with the Rietveld refinement indicate that samples crystallize in the cubic spinel structure. The increase of sintering temperature leads successively to the increase of lattice constant, average crystallite size, intensities of absorption bands, magnetization, and electrical conductivity of the prepared ferrites. Dielectric constants decrease with frequency and their behaviors have been investigated using the interfacial polarization theory predicted by Maxwell. The modulus analysis shows the presence of electrical relaxation phenomenon and non-Debye nature for the samples. An appropriate electrical equivalent circuit was used to analyze the Nyquist plots, and the results show that the conduction mechanism of the synthesized ferrites is mainly due to the grain boundary contribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spinel ferrites with general formula AB2O4 (where A and B are the metal cations present at tetrahedral and octahedral positions, respectively) are widely used in electronic applications such as gas sensors, information storage systems, computer memory chips, microwave devices, magnetic recording media, transducers, transformers, and other devices [1]. These numerous applications are due to the fact that these materials have excellent properties such as high initial permeability, high saturation magnetization, high resistivity, and low magnetic losses [2]. Research which has been conducted on ferrite materials showed that many parameters could affect their properties. For example, it has been revealed that the anomalous properties (i.e., microstructural, electrical, magnetic) of ferrites depend on the sintering temperature (ST) values. In this context, many studies have been presented in the literature. Reddy et al. reported the effect of ST on structural and magnetic properties of NiCuZn and MgCuZn ferrites [3]. The same study was investigated by Patil et al. for lithium chromium ferrite [4]. Kannan et al. studied the structural, magnetic, and optical properties of Ni0.5Zn0.5Fe2O4 ferrite nanoparticles at different ST [5]. Furthermore, the effect of ST of structural and magnetic properties of nanostructured Ni0.3Zn0.7Fe2O4 ferrite was examined by Rahimi et al. [6].
Along this line, we prepared in this work ferrite samples with Ni0.6Cd0.2Cu0.2Fe2O4 compositions using sol-gel method under different ST (900 °C, 1000 °C, 1100 °C, and 1200 °C). After that, their microstructural, infrared, magnetic, and impedance spectroscopic properties are successively investigated at room temperature (RT).
2 Experimental Details
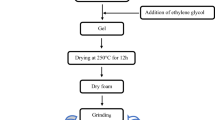
The sol-gel method was used to prepare Ni0.6Cd0.2Cu0.2Fe2O4 ferrites by using Ni(NO3)2.6H2O, Cd(NO3)2.4H2O, Cu(NO3)2.4H2O, and Fe(NO3)3.9H2O nitrates. Figure 1 shows the different synthesis steps of the samples. The free surface morphologies of samples in pellets form were analyzed by scanning electron microscopy (SEM) using Philips XL30 microscope under an accelerating voltage of 15 kV. X-ray diffractometer (Panalytical X’Pert Pro) using Ni-filtered CuKα radiation (λ = 1.5406 Å) was used to record the X-ray diffraction pattern analysis in 20° ≤ 2θ ≤ 80° angular range. The Rietveld analysis [7] using FullProf software was adopted for detailed structural parameter. FTIR spectra were performed in 400–1000 cm−1 wavenumber range with resolution of 1 cm−1 using Shimadzu Fourier Transform Infrared Spectrophotometer (FTIR-8400S). Hysteresis loops were obtained using vibrating sample magnetometer (VSM) in a maximum applied field of 10 kOe. The frequency dependences of dielectric measurements were evaluated at RT using N4L-NumetriQ (model PSM1735) connected to a computer over 100 Hz–1 MHz frequency range.
3 Results and Discussions
3.1 Microstructural Properties
Figure 2 shows the SEM micrographs for Ni0.6Cd0.2Cu0.2Fe2O4 ferrites sintering at different temperatures. The analysis of these images using ImageJ software shows average particle size of about 0.133, 0.180, 0.211, and 0.412 μm for the samples sintered at 900 °C, 1000 °C, 1100 °C, and 1200 °C, respectively. Therefore, the SEM images confirm the enlarging of particle size by increasing ST. Indeed, the tendency of particles to join together and constitute large particles with negligible porosity is evidently realized in the sample sintered at 1200 °C.
The XRD patterns with the Rietveld refinements of Ni0.6Cd0.2Cu0.2Fe2O4 ferrites at different preparation temperatures varying from 900 to 1200 °C are illustrated in Fig. 3. The XRD patterns of all compounds confirm that they belong to the cubic spinel structure. Also, it is observed from the figure that any additional peaks related to impurity phases were detected, confirming the high purity of the prepared samples. The peak indexation was performed using “X’Pert HighScore Plus” software in the cubic \( Fd\overline{3}m \) symmetry. In the Rietveld refinement procedure, we proposed the cation distribution \( {\left({Cd}_{0.2}^{2+}{Fe}_{0.4}^{3+}\right)}_A{\left[{Ni}_{0.6}^{2+}{Cu}_{0.2}^{2+}{Fe}_{1.6}^{3+}\right]}_B{O}_4^{2-} \), where the brackets () and [] contain the cations occupying the A and B sites, respectively. According to this distribution, the Cd2+ ions have preference of occupying the tetrahedral A-sites, whereas the Ni2+ and Cu2+ ions are known to occupy the octahedral B-sites; and Fe3+ ions are distributed on both the sites [8,9,10,11]. The crystalline structure of samples has been refined in \( Fd\overline{3}m \) symmetry with atomic positions taken at 8a (1/8, 1/8, 1/8) for \( {\left({Cd}_{0.2}^{2+}{Fe}_{0.4}^{3+}\right)}_A \) cations, 16d (1/2, 1/2, 1/2) for \( {\left[{Ni}_{0.6}^{2+}{Cu}_{0.2}^{2+}{Fe}_{1.6}^{3+}\right]}_B \) cations, and 32e (x, y, z) for O. A good agreement between the observed and calculated profiles was noticed in Fig. 3. The refined structural parameters are listed in Table 1 for all samples. The goodness of fit (χ2) shows small values of residual indicating excellent agreement between observed and calculated XRD data. With increasing ST, it is remarkable that both lattice constant (a) and unit cell volume (V) increase, in accordance with the results found in previous works [4, 6]. Also, the obtained oxygen coordinate values are characteristic of the spinel-type structure [12, 13]. The X-ray density was calculated according to the following equation [14]:
where M is the molecular weight of the sample, N is the Avogadro’s number, and a is the lattice constant. The estimated dx values decrease as the ST increases (see Table 1). The average crystallite size was estimated from XRD peaks using the Scherer formula as [15]:
where λ is the wavelength of X-ray, β is the corrected full-width half maxima of the XRD peaks, and θ is the Bragg angle. The D values presented in Table 1 increase with ST in good agreement with other similar results [4, 6]. The results are consistent with SEM observations; however, it is obvious that the crystallite sizes estimated from XRD peaks are smaller than the particle sizes obtained from SEM micrographs. This difference is due to the fact that the particles are composed of several crystallites, due to internal strains or defects in the structure [16].
3.2 Infrared Properties
Generally, in FTIR spectra of spinel ferrites, there are two main characteristic absorption peaks, which are related to the intrinsic vibrations of oxygen bonds with metal cations at A and B sites [17]. To detect these absorption bands, the FTIR spectra of Ni0.6Cd0.2Cu0.2Fe2O4 ferrites were carried out at RT and presented in Fig. 4. The obtained spectra confirm well the presence of the two characteristic peaks of spinel ferrites. The first band observed at the frequency (ν1) in the range of 415–420 cm−1 corresponds to the stretching vibrations of metal-oxygen at the octahedral site. While the second band observed at the frequency (ν2) in the range of 535–544 cm−1 is attributed to the vibration of metal-oxygen at the tetrahedral site. The peak positions corresponding to ν1 and ν2 frequencies are listed in Table 2 and shown in Fig. 4 for all samples. It is clear from this table that the values of ν1 and ν2 frequencies appear to increase with increasing ST. In the other hand, the difference between the band positions is related to the difference in the metal-oxygen bond distances for tetrahedral and octahedral sites (see Table 1) due to temperature effect [18]. The force constants (KT) and (KO) for the tetrahedral and octahedral sites, respectively, are calculated according to the following relation [19, 20]:
where c is the light speed, ν is the vibration frequency for tetrahedral and octahedral sites, and m is the reduced mass of the Fe3+ and O2− ions. The results presented in Table 3 show a trend increase of both KT and KO values with ST. This behavior can be attributed to the decrease of RA and RB bond lengths of cation-oxygen in A and B sites (see Fig. 5). Since the RA and RB bond lengths decrease with the increase of ST, the energy required to break short bonds is high, and this supports an increase in the force constants of tetrahedral and octahedral sites [21].
3.3 Magnetic Properties
The magnetic hysteresis (M-H) loops obtained at RT at an applied field of ± 10 kOe for Ni0.6Cd0.2Cu0.2Fe2O4 ferrites sintering at different temperatures are shown in Fig. 6. The estimated values of saturation magnetization (Ms), remanent magnetization (Mr), and coercivity (Hc) are summarized in Table 2. It is clear that Ms values were affected by the increase of ST. This can be attributed to the increase of the average crystallite size of the samples. This is in good agreement with the works reported by other authors [22, 23]. Also the decrease of Hc values could be interpreted by taking into consideration the augmentation of the average grain size with ST. In fact, the largest grains have a tendency to consist the greatest number of domain walls. The magnetization/demagnetization provoked by the domain wall movement needs less energy than that needed by domain rotation. The same as the number of walls rises with grain size, the contribution of wall movement to magnetization/demagnetization is superior to that of the domain rotation. Thus, the sample sintered at 1200 °C with bigger grains is predictable to have a low coercivity Hc comparing with the sample sintered at 900 °C.
3.4 Electrical Properties
3.4.1 Conductivity
In order to study the conduction process of the prepared ferrites, which is in general due to the electron hopping between Fe2+ and Fe3+ ions occupying the octahedral site, the variation of the electrical conductivity (σ) with frequency at RT is carried out and the results are displayed in Fig. 7. For all samples, it can be seen that the σ values have the same appearance for each ST. In fact, at the beginning, the conductivity remains constant by increasing the frequency until a certain value called hopping frequency (fh ≈ 105 Hz) which has a displacement towards high frequencies as the ST increases. This constancy could be explained by more activity of the low conductive grain boundaries in this frequency region and therefore Fe2+ ↔ Fe3+ electron hopping is less. This frequency region corresponds to the dc conductivity (σdc). For f > fh, it is observable that the conductivity increases exponentially with frequency. The conductive grains become more active in this frequency range, which promote the conduction process of the samples. To verify these observations, the conductivity data are modeled using the following Jonscher power law [24]:
where B and s are the pre-exponential and exponent factors, respectively. The modeling of experimental curves using Eq. (4) shows good agreement between the theoretical and experimental curves of the studied samples (see Fig. 7). The fitting parameters are given in Table 3. It is clear from this table that σdc and s values increase with increasing ST, due to an augmentation of the electron hopping which also rises with temperature. In the other hand, the s values are superior to 1 for all temperatures indicating that the electron hopping occurs between neighboring sites, according to the Funke criterion [25].
3.4.2 Dielectric Constants
This part is devoted to the study of dielectric properties of Ni0.6Cd0.2Cu0.2Fe2O4 ferrites, sintered at different temperatures. The frequency variations of their real and imaginary parts of permittivity as well as their loss factors at RT are presented in Fig. 8a–c. For all samples, it should be noted that the values of ε’, ε”, and tanδ are high in the low-frequency region. Then, the values of these dielectric constants decrease monotonically and become very low in the high-frequency region. Similar results have been observed in other studies [26, 27], and in most cases, the authors have interpreted the behavior of these dielectric constants by the theory of interfacial polarization predicted by Maxwell [28]. Indeed, the dielectric structure of spinel ferrites is generally divided into two parts: grains that have high conductivity and grain boundaries that act as insulators. Under the application of an electric field, the displacement of the charges in the grains is interrupted at the grain boundaries, which causes an accumulation of the charges at the interface, then the appearance of an interfacial polarization. In fact, electron exchange does not follow the applied field in the high-frequency region, resulting in a decrease in the contribution of interfacial polarization. As a result, the dielectric constant values become very low towards the high frequencies reaching values of the order of 10−1, making the samples promising candidates for the high-frequency applications [29]. In the other hand, the dielectric constant values decrease with increasing ST at low-frequency region, which is in good agreement with the rise of conductivity.
3.4.3 Modulus
The frequency variation of real part of electrical module at RT for Ni0.6Cd0.2Cu0.2Fe2O4 ferrites sintered at different temperatures is illustrated in Fig. 9a. For all the samples, the M’(f) variation can be divided into three parts: (i) at low frequencies (f < 103 Hz), the M’ values are very low, which confirms that the polarization of the electrode makes a negligible contribution to the materials [30]. (ii) At intermediate frequencies (103 Hz < f < 106 Hz), an exponential increase in M’ values was observed. (iii) At high frequencies (f > 106 Hz), the values of M’ have a tendency to saturate at a maximum asymptotic value. This may be due to the short-range mobility of charge carriers [31, 32]. Figure 9b shows the M”(f) curves at RT for the prepared samples. Well-resolved peaks are observed from these curves at characteristic frequencies (\( {f}_{M"}^{max} \)). Due to the thermal activation of the charge carriers resulting from the increase of ST, the positions of these peaks move to the higher frequency side. The M”(f) curves have been well adjusted by using the Bergman proposed Kohlrausch, Williams, and Watts (KWW) function [33]:
where \( {M}_{\mathrm{max}}^{\prime \prime } \) is the peak maxima and fmax its corresponding frequency. β is the stretching factor (0 < β < 1), which decides whether the relaxation in dielectric is Debye or non-Debye in nature [34]. The fitting parameters of M”(f) data using Eq. (5) are given in Table 5. On the one hand, the β values for all samples turned out to be less than unity, showing so a non-Debye nature of the samples, and on the other hand, they increase with increasing ST. This increase is caused by the rise of the average crystallite size with ST which also engenders a significantly drop of the number of dipoles in the grain boundary [35]. Consequently, the interaction between the dipoles in the grain boundaries decreases and, as a result, the relaxation of the dipoles becomes faster. This leads to the increase of the relaxation frequency (fmax) and the decrease of the relaxation time, τM" = 1/2πfmax (see Table 4).
Frequency dependence at RT of a real part (M') and b imaginary part (M") of electrical modulus for Ni0.6Cd0.2Cu0.2Fe2O4 ferrites sintering at different temperatures. Red solid lines represent the fit of experimental data of M"(f) using Eq. (5)
3.4.4 Impedance
Figure 10 depicts the Nyquist plots (Z″ vs. Z') at RT for Ni0.6Cd0.2Cu0.2Fe2O4 ferrites sintered at different temperatures. For each ST, only one semicircle (whose maximum and diameter decrease with increasing ST) is observed, indicating just one dominant relaxation phenomenon. In the analysis of impedance data, the Nyquist plots help us to separate the grain and grain-boundary contributions of the samples to the total electrical conductivity over wide frequency range. Indeed, in such plots, the intersection with real axis of the semicircle at low frequencies (right intersect) is ascribed to the total resistance (RT) which is equal to the sum of grain resistance (Rg) and grain boundary resistance (Rgb) [36]. On the other hand, the impedance response of grain dominates at high frequencies, so, Rg can be deduced from the left intersect of the semicircle with real axis. As shown in Fig. 10, the left intersections of the semicircles for all samples with the real axis cross the origin. These results suggest that contribution from the grain is not resolved for these samples and their conduction takes place predominantly through the grain boundary [37, 38]. This may be due to a limited frequency range used in this study (100 Hz–l MHz) or the grain boundary contribution cannot be separated from the grain contribution by the impedance spectroscopy [39]. These interpretations can be more achieved by an adjustment of the Nyquist plots by an appropriate equivalent circuit. As shown in the inset of Fig. 10, the proposed equivalent circuit is of the type of (Rgb//CPEgb) configuration [37]. Here, Rgb represents the grain boundary resistance, while CPEgb is the constant phase element for grain boundaries. The impedance response of a constant phase element (CPE) can be defined as [40, 41]:
where Q is a proportional factor, ω is the angular frequency, and α is an empirical exponent close to 1. The expressions of real (Z') and imaginary (Z″) components of impedance related to the equivalent circuit are given as [42, 43]:
The modeling of impedance spectra was performed using Zview software [44]. An excellent agreement between the experimental spectra and the calculated ones was observed (see Fig. 10). The obtained fitted parameters are listed in Table 5. It is clear from this table that Rgb values decrease when increasing ST, which indicates an increase of the conductivity. The decrease of grain boundary resistance is due to the fact that the grain boundary effect has assisted in lowering the barrier to the motion of charge carriers paving the way for increased electrical transport with rise in ST [36, 45].
4 Conclusion
Ni0.6Cd0.2Cu0.2Fe2O4 ferrite samples are prepared using sol-gel method at different ST (900 °C, 1000 °C, 1100 °C, and 1200 °C). Their RT microstructural, infrared, magnetic, and impedance spectroscopy properties are successively investigated. The XRD patterns and the Rietveld refinement confirm the cubic spinel-type structure for all samples. Two principal absorption bands are shown from FTIR spectra that are attributed to the stretching vibration of tetrahedral and octahedral sites. The saturation magnetization and conductivity values were also affected by the increase of ST as a consequence of the gradual increase of average crystallite size. Dielectric constants decrease with frequency due to the decrease in polarization. The variation of electrical modulus shows the presence of an electrical relaxation phenomenon with non-Debye type in the prepared ferrites. From the Nyquist diagram analysis, we found that the resistances of the prepared ferrites resulted mostly from the grain boundary effect.
References
Meng, Y.Y., Liu, Z.W., Dai, H.C., Yu, Y., Zeng, D.C., Shukla, S., et al.: Powder Technol. 229, 270 (2012)
Kolekar, Y.D., Sanchez, L., Rubio, R.E., et al.: Solid State Commun. 184, 34 (2014)
Reddy, M.P., Madhuri, W., Ramana, M.V., Reddy, N.R., Kumar, K.V.S., Murthy, V.R.K., Kumar, K.S., Reddy, R.R.: J. Magn. Magn. Mater. 322, 2819 (2010)
Patil, R.P., Hankare, P.P., Garadkar, K.M., Sasikala, R.: J. Alloys Compd. 523, 66 (2012)
Kannan, Y.B., Saravanan, R., Srinivasan, N., Ismail, I.: J. Magn. Magn. Mater. 423, 217 (2017)
Rahimi, M., Kameli, P., Ranjbar, M., Salamati, H.: J. Nanopart. Res. 15, 1865 (2013)
Rietveld, H.M.: J. Appl. Crystallogr. 2, 65 (1969)
Hakim, M.A., Nath, S.K., Sikder, S.S., Maria, K.H.: J. Phys. Chem. Solids. 74, 1316 (2013)
Lohar, K.S., Patange, S.M., Mane, M.L., Shirsath, S.E.: J. Mol. Struct. 1032, 105 (2013)
Houshiar, M., Jamilpanah, L.: Mater. Res. Bull. 98, 213 (2018)
Gholizadeh, A., Jafari, E.: J. Magn. Magn. Mater. 422, 328 (2017)
Pailhé, N., Wattiaux, A., Gaudon, M., Demourgues, A.: J. Solid State Chem. 181, 1040 (2008)
Verma, K., Kumar, A., Varshney, D.: J. Alloys Compd. 526, 91 (2012)
Anwar, M.S., Ahmed, F., Koo, B.H.: Acta Mater. 71, 100–107 (2014)
Ahmed, M.A., Afify, H.H., El Zawawia, I.K., Azab, A.A.: J. Magn. Magn. Mater. 324, 2199 (2012)
Oumezzine, E., Hcini, S., Hlil, E.K., Dhahri, E., Oumezzine, M.: J. Alloys Compd. 615, 553 (2014)
Khot, V.M., Salunkhe, A.B., Phadatare, M.R., Pawar, S.H.: Chem. Phys. 132, 782 (2012)
Rahaman, M.D., Mia, M.D., Khan, M.N.I., Hossain, A.K.M.A.: J. Magn. Magn. Mater. 404, 238 (2016)
Zaki, H.M., Dawoud, H.A.: Physica B. 405, 4476 (2010)
Shaikh, P.A., Kambale, R.C., Rao, A.V., Kolekar, Y.D.: J. Alloys Compd. 492, 590 (2010)
Sabri, K., Rais, A., Taibi, K., Moreau, M., Ouddane, B., Addou, A.: Physica B. 501, 38 (2016)
Ahmed, Y.M.Z.: Ceram. Int. 36, 969 (2010)
Waje, S.B., Hashim, M., Ismail, I.: J. Magn. Magn. Mater. 323, 1433 (2011)
Ortega, N., Kumar, A., Bhattacharya, P., Majumder, S.B., Katiyar, R.S.: Phys. Rev. B. 77, 014111 (2008)
Funke, K.: Prog. Solid State Chem. 22, 111 (1993)
Atiq, S., Majeed, M., Ahmad, A., Abbas, S.K., Saleem, M., Riaz, S., Naseem, S.: J. Magn. Magn. Mater. 43, 2486 (2017)
Chakrabarty, S., Pal, M., Dutta, A.: Mater. Chem. Phys. 153, 221 (2015)
Maxwell, J.C.: Electricity and Magnetism. Oxford University Press, London (1973)
Jadhav, P.A., Devan, R.S., Kolekar, Y.D., Chougule, B.K.: J. Phys. Chem. Solids. 70, 396 (2009)
Vasoya, N.H., Jha, P.K., Saija, K.G., Dolia, S.N., Zankat, K.B., Modi, K.B.: J. Electron. Mater. 45, 917 (2016)
Saha, S., Sinha, T.P.: Phys. Rev. B. 65, 1341 (2005)
Padmasree, K.P., Kanchan, D.D., Kulkami, A.R.: Solid State Ionics. 177, 475 (2006)
Bergman, R.: J. Appl. Phys. 88, 1356 (2000)
Rao, K.S., Krishna, P.M., Prasad, D.M., Gangadharudu, D.: J. Mater. Sci. 42, 4801 (2007)
Sivakumar, N., Narayanasamy, A., Ponpandian, N., Govindaraj, G.: J. Appl. Phys. 101, 084116 (2007)
Selmi, A., Hcini, S., Rahmouni, H., Omri, A., Bouazizi, M.L., Dhahri, A.: J. Phase Transit. 90, 942 (2017)
Sekulic, D.L., Lazarevic, Z.Z., Sataric, M.V., Jovalekic, C.D., Romcevic, N.Z.: J. Mater. Sci. Mater. Electron. 26, 1291 (2015)
Dhaou, M.H., Hcini, S., Mallah, A., Bouazizi, M.L., Jemni, A.: Appl. Phys. A Mater. Sci. Process. 123, 8 (2017)
Kotnala, R.K., Dar, M.A., Verma, V., Singh, A.P., Siddiqui, W.A.: J. Magn. Magn. Mater. 322, 3714 (2010)
Guo, J., Zhang, H., He, Z., Li, S., Li, Z.: J. Mater. Sci. Mater. Electron. 29, 2491 (2018)
Omri, A., Bejar, M., Dhahri, E., Es-Souni, M., Valente, M.A., Graça, M.P.F., Costa, L.C.: J Alloy. Compd. 536, 173 (2012)
Kallel, S., Nasri, A., Kallel, N., Rahmouni, H., Peña, O., Khirouni, K.: M. Oumezzine Physica B. 406, 2172 (2011)
Khadhraoui, S., Triki, A., Hcini, S., Zemni, S., Oumezzine, M.: J. Alloys Compd. 574, 290 (2013)
Johnson, D.: ZView: a software program for IES analysis, version 2.8. Scribner Associates, Inc., Southern Pines (2008)
Shinde, S.S., Rajpure, K.Y.: J. Solid State Chem. 183, 2886 (2010)
Funding
This work was supported by the Deanship of Scientific Research at Al Qassim University under the research Project Number 5090-cosabu-2018-1-14-S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kouki, N., Hcini, S., Aldawas, R. et al. Structural, Infrared, Magnetic, and Electrical Properties of Ni0.6Cd0.2Cu0.2Fe2O4 Ferrites Synthesized Using Sol-Gel Method Under Different Sintering Temperatures. J Supercond Nov Magn 32, 2209–2218 (2019). https://doi.org/10.1007/s10948-018-4951-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-018-4951-x