Abstract
We investigate the single crystal growth of rutheno-cuprates, which are known to be magnetic superconductors. We attempted the single crystal growth of two systems, RuSr2GdCu2O8−δ (RuGd-1212) and RuSr2(Gd,Ce)2Cu2O10−δ (RuGd-1222), by the self-flux method with alumina boats. Fine cubic crystals of RuGd-1212 with a side length of approximately 5 μ m were obtained by slow cooling from 1350 to 940 ∘C. Plate-like RuGd-1222 crystals with a smooth plane were obtained at the surface of the precursor pellet. RuGd-1222 was more stable at high temperatures than RuGd-1212 and was also superconductive, but its superconductivity weakened as the cooling rate decreased. This weakening was induced by decomposition. Therefore, high-quality RuGd-1222 single crystals were difficult to obtain, and we failed to extract only the smooth planar crystal part. As a modified synthesis technique, we mixed a precursor containing Ru–Sr–Gd–Ce flux with RuGd-1222 powders and gradually cooled the mixture from 1350 to 1090 ∘C. A plate-like RuGd-1222 sample with a smooth surface and a size of 70 × 30μm2 was obtained from the side surface of the precursor pellet. By this technique, we could separate the planar crystal part. Semiquantitative composition analysis confirmed this planar crystal as a RuGd-1222 phase. The X-ray diffraction peak suggested the (0014) plane, implying that the plate-like precipitates are c-axis-oriented RuGd-1222 single crystals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ruthenium-based copper oxides (rutheno-cuprate) are high-temperature superconductors that have attracted considerable attention, because the ruthenium ions order antiferromagnetically in the ground state and ferromagnetically in a magnetic field. Consequently, rutheno-cuprate exhibits both magnetic order and superconductivity [1]. However, single-crystal growth in this system faces at least two difficulties: the incongruent melting of a complex oxide system comprising four or five cations and an oxygen anion, and the sublimability of ruthenium.
High-temperature superconducting rutheno-cuprate has two crystal structures, RuSr2GdCu2O8−δ (Ru-1212) and RuSr2(Gd,Ce)2Cu2O10−δ (Ru-1222), with different numbers of rare-earth layers. In the crystal structure of Ru-1212, the ruthenium ion and the periphery of octahedral oxygen replace the CuO chain of a 123-type superconductor, typified by YBa2Cu3O7−δ (YBCO) [2, 3]. Furthermore, the oxygen defects in Ru-1212 largely contribute to the conducting property of this material, and whether superconductivity is observed depends on the severe heat treatment conditions. Consequently, different experiments have yielded different results [1,2,3,4,5,6,7,8,9]. By replacing approximately 30% of the magnetically ordered ruthenium ions with copper ions, one can raise the superconducting transition temperature from around 50 K at the normal stoichiometric ratio to 70 K [3]. On the basis of these experimental results, researchers have deduced a superlattice structure for high-temperature superconducting rutheno-cuprates, in which a superconducting layer composed of copper oxide planes is periodically sandwiched between neighboring octahedral layers of magnetic ruthenium on atomic scales. Therefore, this rutheno-cuprate system is an ideal experimental platform for understanding the relationship between magnetism and superconductivity.
The bismuth-based copper-oxide high-temperature superconductors have been known to have a laminated structure of superconducting and blocking layers, which show an intrinsic Josephson junction effect [10,11,12]. Single crystals of the rutheno-cuprate system are also expected to display an intrinsic Josephson junction effect. As the block layer of the rutheno-cuprate high-temperature superconductor is magnetically ordered, it potentially possesses unique peculiar junction characteristics, such as those of a π junction. Intrinsic Josephson junction properties have been reported in single crystals of Ru-1212 [13, 14], but whether unique intrinsic Josephson junction properties arise in the Ru-1212 system remains questionable, because no peculiar properties have been observed.
In contrast, the Ru-1222 system contains two rare-earth layers between the CuO2 planes, and the ruthenium and CuO2 planes also exhibit magnetic ordering and superconductivity, respectively [15,16,17,18]. Although oxygen defects are less influential in the 1222 system than in the 1212 system, single-phase 1222 cannot be obtained unless the gadolinium in the rare-earth layer is replaced with cerium at ratios between 20 and 40%. Synthesis is also difficult. To our knowledge, Ru-1222 single crystals have never been previously obtained. No intrinsic junction characteristics have been reported in the Ru-1222 system. Moreover, whether superconductivity and ferromagnetism macroscopically coexist in single crystals is unclear. Therefore, if one can synthesize a single crystal of the Ru-1222 system and investigate the transport characteristics along its c-axis, the unique physical properties conferred by the magnetic order of the ruthenium blocking layer can be supposedly observed. Furthermore, by comparing the 1222 and 1212 systems, one might experimentally relate the distance between the two superconducting layers (copper oxygen layers) and the intrinsic Josephson junction properties. Notes that single crystals of only the Nb-1222 system, in which ruthenium is replaced with niobium, have been synthesized by the self-flux method in a platinum crucible [19]. The surfaces of these single crystals were coated with an insulating layer [19].
Recently, we reported the growth of uniform cubic single crystals of the magnetic superconductor Ru1−xSr2−yGd1 + yCu2 + xO8−δ (RuGd-1212) with a typical length of 100–150 μ m using the partial melting technique [20, 21]. Accordingly, we tried to grow Ru-1212 and Ru-1222 single crystals by the self-flux method, replacing the expensive platinum crucibles with alumina boats. To prevent the diffusion of aluminum, the Ru-1212 samples were sometimes wrapped in a thin platinum film. We investigated the single crystal growth conditions of rutheno-cuprate magnetic superconductors by changing the nominal composition and sintering temperature.
2 Sample Preparation
2.1 RuGd-1212
RuGd-1212 powder was synthesized by mixing weighed powder samples of RuO2 (3N or Ru 76.5 wt.%), SrCO3, Gd2O3, and CuO (3N). The heat treatment conditions have been determined as described in a previous report [2]. Briefly, the RuGd-1212 system was prepared at 800 ∘C for 72 h followed by 1000 ∘C for 24 h. The nominal composition of precursor RuGd-1212 was Ru:Sr:Gd:Cu = 1.8:2.0:1.4:3.7. The RuGd-1212 powder was prepared in an alumina crucible, and the calcined mixture was pressed into pellets of diameter 1 cm and thickness 2–5 mm to promote the interdiffusion of atoms and the chemical reaction. The pellets were heat treated in an alumina boat in an oxygen flow.
The calcination conditions of RuGd-1212 were as follows: the temperature was raised with 100 ∘C/h as mentioned in [13], maintained at its maximum (\(T_{\max }\), set to 1300, 1350, or 1375 ∘C) for 2 h of calcination, and then gradually decreased to 940 ∘C over 10 days. After slow cooling, the calcinated sample was immediately removed from the furnace and cooled to adjust the oxygen vacancy. To prevent impurities from the alumina boat, the sample was sometimes wrapped in a thin platinum film during the synthesis. Microcrystal grains of RuGd-1212 were confirmed in all cases of \(T_{\max }\ge \) 1350 ∘C in the absence of platinum film, and in all cases of \(T_{\max }\ge \) 1300 ∘C when using the platinum film.
2.2 RuGd-1222
RuGd-1222 powder was also prepared by adding powder sample of CeO2 (3N) together with the above-mentioned oxide and carbonate powders on the basis of the previous studies [2, 22]. This mixture was calcined at case I: 800 ∘C for 72 h followed by 1000 ∘C for 24 h, and case II: 600 ∘C for 12 h, 850 ∘C for 24 h, followed by 1050 ∘C for 24 h. The nominal composition of precursor RuGd-1222 was Ru:Sr:Gd:Ce:Cu = 1− x:2.0:1.4:0.6:2+ x, where − 0.3 ≤ x ≤ 1.0. The RuGd-1222 powders were prepared in an alumina crucible and pressed into pellets. The pellets were sintered in an alumina boat in an oxygen flow. At first, the decomposition temperature of the RuGd-1222 phase was identified by the solid-state reaction. Next, single crystals of RuGd-1222 were synthesized by the self-flux method. The calcination conditions of RuGd-1222 were as follows: the temperature was raised to 1350 ∘C for 12 h and held for 1 h, then, the temperature was gradually cooled to 1090 ∘C at different cooling rates (0.75–10 ∘C/h).
2.3 Experimental
The morphology and composition of the obtained sample were examined by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS), respectively, and the crystal structure was evaluated by X-ray diffraction. The temperature dependence of the electrical resistance was measured by a four-terminal method. The electrodes are formed using silver paste, and the size of each sample is approximately 4 mm3.
3 Results and Discussion
3.1 RuGd-1212
The largest cubic crystal of RuGd-1212 was obtained in the presence of platinum film at \(T_{\max }=\) 1350 ∘C. A SEM photograph of this sample is shown in Fig. 1. Cubic microcrystal grains suggesting single crystals were obtained, although the side length was only 15 μ m, in stark contrast with the morphology and shape of samples prepared by the usual solid-state reaction. According to the semiquantitative EDS analysis, the composition of the cubic crystal was Ru:Sr:Gd:Cu = 1.30:2.74:1.00:2.17, whereas that of the precursor was Ru:Sr:Gd:Cu = 3.31:4.60:1.00:2.50. This suggests that the cubic microcrystals were indeed RuGd-1212. The lower composition of Cu than the nominal value was attributed to melting of the copper oxide component during the slow cooling. The melted copper oxide fused to the bottom of the alumina boat. The composition ratio of the cubic crystals was consistent with that of RuGd-1212, although EDS cannot accurately determine.
The X-ray diffraction angle shifted to the lower-angle side by approximately 2% for all the fabricated samples, indicating the obtained samples contained mainly Sr2GdRuO6(2116) phase [23]. The microcubic crystals suggest that RuGd-1212 grew locally as a by-product. The platinum film, installed to prevent contamination by impurities, enhanced the growth of single crystals from 8 to 10 μ m or from 10 to 15 μ m on each side. Although the grown RuGd-1212 crystals were smaller than those grown in a platinum crucible [13], our results confirmed the fine RuGd-1212 single crystals can be grown in alumina containers [20, 24, 25]. The electrical resistance of the samples prepared by self-flux method remained nonzero at temperatures down to 4.2 K but dropped suddenly in a few samples, suggesting a superconducting transition.
3.2 RuGd-1222
3.2.1 Phase Stability
Subsequently, we now discuss the RuGd-1222 formed by the solid-state reaction method. Figure 2 shows the X-ray diffraction spectra of RuGd-1222 with a nominal composition of Ru:Sr:Gd:Ce:Cu = 1− x:2.0:1.4:0.6:2+ x, where 0.0 <= x<= 1.0. Here, the sample powders calcined at 600 ∘C followed by 850 ∘C were then sintered at 1050 ∘C for 24 h (case II). Observing the change in the diffraction pattern in the 2𝜃 range of 20–60∘ as x varies from 0 to 1, we find that the 1222 phase was stable at a sintering temperature of 1050 ∘C when x<= 0.5. For larger x, an impurity phase was precipitated. Figure 3 shows how the sintering temperature influences the X-ray diffraction patterns of RuGd-1222 with the nominal composition Ru:Sr:Gd:Ce:Cu = 1− x:2.0:1.4:0.6:2+ x, where x = 0.00 and 0.30. The sample powders calcined at 800 ∘C followed by 1000 ∘C (case I), were then sintered at 1070, 1090, and 1110 ∘C, and the sintering conditions for each sample are given in Fig. 3. Whereas the 1212 phase in RuGd-1212 decomposed at a sintering temperature of 1080 ∘C or higher [20], the 1222 phase of the RuGd-1222 phase with x = 0.0 stably existed up to 1110 ∘C. On the other hand, when x = 0.3, the 1222 phase existed as the main phase up to 1090 ∘C but decomposed at 1110 ∘C. A SEM image of the RuGd-1222 sample with x = 0.0 is shown in Fig. 4. When sintered at 1110 ∘C, the sample exhibited a smooth surface, suggesting single crystals with a plate-like faceted structure. Moreover, the sample with x = 0.3 sintered at 1070 ∘C also formed plate-like crystals with a smooth surface. The semiquantitative EDS analysis suggests a Ru:Sr:Gd:Ce:Cu composition of 0.91:2.00:1.30:0.58:1.83 for the x = 0.0 sample and 0.49:2.00:1.63:0.66:2.54 for the x = 0.3 sample. The former is consistent with the RuGd-1222 phase, but the latter deviates from RuGd-1222. Single crystals of RuGd-1222 may naturally form plates because the Ru-1222 system has two rare-earth layers between the CuO2 planes. The increased length of the c-axis increases the anisotropy of the crystal structure, which may result in change of crystal shape from cubic (RuGd-1212) to plate (RuGd-1222).
In summary, the RuGd-1222 phase with a nominal composition of x = 0.0 was stable up to 1110 ∘C, meaning that its thermal stability was higher than that of RuGd-1212. When the nominal composition of ruthenium was partially replaced with copper, the decomposition temperature decreased depending on the substitution amount, and consequently, the 1222 phase was easy to decompose. We also measured the temperature dependence of the resistance of these samples. The sample with nominal composition x = 0.0 was superconductive with Tc−onset = 39 K. The samples with composition x = 0.3 or sintered at temperatures above 1170 ∘C were also superconductive, but the absolute values of their resistivities were increased by one order of magnitude, and their Tc−onset values decreased to 28 K (x = 0.3) and 24 K (> 1170 ∘C).
3.2.2 Single Crystal Growth
Finally, we synthesized single crystals of RuGd-1222 by the self-flux method. The nominal composition was Ru:Sr:Gd:Ce:Cu = 1− x:2.0:1.4:0.6:2+ x, with − 0.3 ≤ x ≤ 1.0. The temperature was raised with 100 ∘C/h. After 2 h at \(T_{\max }\) (1350 ∘C), the temperature was gradually reduced to 1090 ∘C at different cooling rates (10–0.75 ∘C/h). Figure 5a shows the X-ray diffraction spectra of the RuGd-1222 samples (x = 0.0) as the cooling rate reduced from 10 to 1.5 ∘C/h. When cooled at 10 ∘C/h, the sample retained its 1222 phases as the main phase, but when the cooling rate was reduced to 2.5 and 1.5 ∘C/h, the 1222 phases decomposed. In SEM surface observations, the sample cooled at 10 ∘C/h appears as fine crystals with side lengths of approximately 10 μ m. Moreover, the composition is Ru:Sr:Gd:Ce:Cu = 0.99:2.00:1.40:0.52:1.77, which suggests the RuGd-1222 phase. Although this sample transited to superconductivity at 30 K, the critical current was as small as 50 μ A at 4.2 K, reflecting supercurrent between the grain boundaries. When the cooling rate was lowered to 1.5 ∘C/h, the RuGd-1222 phase was almost decomposed, but plate crystals appeared on the surface of the precursor pellet (Fig. 5b). The plate crystals were approximately 100 × 40μm2 in size, and the RuGd-1222 phase was inferred as Ru:Sr:Gd:Ce:Cu = 0.98:2.00:1.35:0.61:1.90 by EDS analysis. Temperature dependence of the electrical resistance of these samples at cooling rate of 2.5 and 1.5 ∘C/h are shown in Fig. 6. We measured the resistance of polycrystalline lumps containing such plate crystals. Both samples show superconductivity at 21 K. Clearly, reducing the rate at which the temperature is lowered from \(T_{\max }\) increases the decomposition extent of the main 1222 phase, but superconductivity can still be experimentally confirmed. The decomposition of the parent phase is responsible for reducing the transition temperature of 30 to 21 K as the cooling rate is lowered at 10 to 1.5 ∘C/h.
a X-ray diffraction spectra of RuGd-1222 samples obtained by the self-flux method. The cooling rate of the sample (x = 0.0) was changed from 10 to 1.5 ∘C/h. b Optical micrograph of plate-like RuGd-1222 (x = 0.0) crystals on the sample surface, obtained in the self-flux method with a cooling rate of 1.5 ∘C/h
To increase the size of the RuGd-1222 crystals, we changed the nominal value of x in the synthesis. The temperature lowering rate was fixed at 1.5 ∘C/h, and the samples were analyzed by X-ray diffraction. The results are shown in Fig. 7. Most of the RuGd-1222 phases decomposed but were partially retained at x = − 0.3. The sample with x = − 0.3 was also superconductive, but the transition temperature was low (20 K), and the resistance did not completely vanish down to 4.2 K. Another problem with the self-flux method is the difficulty of extracting single grains, because the plate-like crystal part on the surface fuses with the base material.
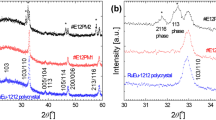
Finally, we confirmed the growth of plate crystals from precursor RuGd-1222 pellets with a Ru–Sr–Gd–Ce flux as the RuGd-1222 was cooled from 1350 ∘C at 0.75 ∘C/h. The flux composition was optimized as Ru:Sr:(Gd,Ce) = 3.0:2.0:2.0, and the flux-to-1222 phase ratio was 17:9 based on ruthenium. A SEM photograph of the formed plate crystal is shown in Fig. 8a. The Ru:Sr:Gd:Ce:Cu:O composition was inferred as 0.98:2.00:1.46:0.54:2.00:11.68 by EDS, suggesting a RuGd-1222 phase. The displayed planar crystal is approximately 70 × 30μm2 in size. Furthermore, as the plate crystals grow like whiskers from the pellet surfaces, only the plate crystals are extracted. Although the present size of plate crystals is not sufficient for transport measurements, the X-ray diffraction spectrum was successfully obtained (see Fig. 8b). The diffraction peak corresponds to the (0014) plane of RuGd-1222, and the plate crystal might be oriented along its c-axis. Generally, such c-axis-oriented crystal shows the 00l reflection peaks, but only the 0014 reflection was confirmed in the sample. Although the 0014 reflection was ascertained for several samples, observation of additional 00l reflection peaks is a future task to be solved.
4 Conclusion
We attempted to grow single crystals of RuGd-1212 and RuGd-1222 by the self-flux method with alumina boats. RuGd-1212 microcrystals with side lengths of approximately 15 μ m were successfully prepared. The largest cubic crystal of RuGd-1212 was obtained in the presence of platinum film at \(T_{\max }=\) 1350 ∘C. The RuGd-1222 phase was more thermally stable at high temperatures than RuGd-1212 in solid-state reaction. In the self-flux method, plate-like RuGd-1222 crystals sized approximately 100 × 40μm2 were grown from the surface of the precursor pellet. Although the sample lump including the planar crystals was superconductive, the transition temperature was lower than in normal polycrystalline RuGd-1222 (x = 0.0), and the 1222 phase part of the crystal was restricted to the pellet surface. However, under Ru–Sr–Gd–Ce flux, we successfully synthesized a plate-like single crystal of RuGd-1222 with possible c-axis orientation from the surface of the precursor pellet. Although the plate crystal was small (only 70 × 30μm2), it may be the first-reported single crystal of the RuGd-1222 system. As it precipitated from the surface of the precursor pellet, the planar crystals could be separated from the precursor pellet. Increasing the size of rutheno-cuprates single crystals by an appropriately developed technology is crucial for elucidating the coexistence of magnetism and superconductivity.
References
Bernhard, C., Tallon, J.L., Niedermayer, C., Blasius, T., Golnik, A., Brucher, E., Kremer, R.K., Noakes, D.R., Stronach, C.E., Ansaldo, E.J.: Phys. Rev. B 59, 14099 (1999)
Otzschi, K., Mizukami, T., Hinouchi, T., Shimoyama, J., Kishio, K.: J. Low Temp. Phys. 117, 855 (1999)
Klamut, P.W., Dabrowski, B., Kolesnik, S., Maxwell, M., Mais, J.: Phys. Rev. B 63, 224512 (2001)
Tokunaga, Y., Kotegawa, H., Ishida, K., Kitaoka, Y., Takigawa, H., Akimitsu, J.: Phys. Rev. Lett. 86, 5767 (2001)
Tallon, J., Bernhard, C., Bowden, M., Gilberd, P., Stoto, T., Pringle, D.: IEEE Trans. Appl. Supercond. 9, 1696 (1999)
McCrone, J.E., Cooper, J.R., Tallon, J.L.: J. Low Temp. Phys. 117, 1199 (1999)
Lynn, J.W., Keimer, B., Ulrich, C., Bernhard, C., Tallon, J.L.: Phys. Rev. B 61, 14964 (2000)
Henn, R.W., Friedrich, H., Awana, V.P.S., Gmelin, E.: Phys. C 341–348, 457 (2000)
Masini, R., Artini, C., Cimberle, M.R., Costa, G.A., Carnasciali, M., Ferretti, M. In: Noce, C. et al. (eds.) Ruthenate and Rutheno-Cuprate Materials, p 222. Springer, Berlin (2002)
Kleiner, R., Steinmeyer, F., Kunkel, G., Mueller, P.: Phys. Rev. Lett. 68, 2394 (1992)
Kleiner, R., Mueller, P.: Phys. Rev. B 49, 1327 (1994)
Oya, G., Aoyama, N., Irie, A., Kishida, S., Tokutaka, H.: Jpn. J. Appl. Phys. 31, L829 (1992)
Lin, C.T., Liang, B., Ulrich, C., Bernhard, C.: Phys. C 364–365, 373 (2001)
Nachtrab, T., Koelle, D., Kleiner, R., Bernhard, C., Lin, C.T.: Phys. Rev. Lett. 92, 117001 (2004)
Felner, I., Asaf, U., Levi, Y., Millo, O.: Phys. Rev. B 55, R3374 (1997)
Sonin, E.B., Felner, I.: Phys. Rev. B 57, R14000 (1998)
Felner, I., Asaf, I., Goren, U., Goren, S.D., Korn, C.: Phys. Rev. B 57, 550 (1998)
Felner, I., Asaf, U., Levi, Y., Millo, O: Physica C 334, 141 (2000)
Samata, H., Takahashi, S., Masuda, T., Nagata, Y., Uchida, T., Ohtsuka, M., Lan, M.D.: J. Phys. Chem. Solids 59, 1585 (1998)
Yamaki, K., Bamba, Y., Irie, A.: Jpn. J. Appl. Phys. 57, 033101 (2018)
Yamaki, K., Bamba, Y., Mochiku, T., Funahashi, S., Matsushita, Y., Irie, A.: Phys. C 548, 40 (2018)
Watanabe, M., Hai, D.P., Kakeya, I., Kadowaki, K.: Physica B 359–361, 433 (2005)
Papageorgiou, T.P., Herrmannsdoerfer, T., Dinnebier, R., Mai, T., Ernst, T., Wunschel, M., Braun, H.F.: Phys. C 377, 383 (2002)
Mele, P., Artini, C., Costa, G.A., Carnasciali, M.M., Ferretti, M., Masini, R.: Int. J. Mod. Phys. B 17, 716 (2003)
Mohan, R., Gaur, N.K., Bhattacharya, S., Gupta, S.K.: Optoelectron. Adv. Materials-Rapid Commun. 4, 1740 (2010)
Acknowledgments
This study was partially supported by a Grant-in-Aid for Scientific Research (Grant No. 18K04921) from the Japan Society for the Promotion of Science (JSPS) and the Casio Science Promotion Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaki, K., Bamba, Y. & Irie, A. Preparation of Fine Single Crystals of Rutheno-Cuprates by the Self-Flux Method with Alumina Boats. J Supercond Nov Magn 32, 1171–1177 (2019). https://doi.org/10.1007/s10948-018-4803-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-018-4803-8