Abstract
Cadmium sulfide (CdS) nanoparticles were synthesized by hydrothermal process and have been characterized by x-ray diffraction (XRD), transmission electron microscopy (TEM), and energy-dispersive x-ray spectroscopy (EDXS) system. The effect of added CdS nanoparticles on the superconducting properties and flux pinning capability in (Bi,Pb)2Sr2Ca2Cu3Oy system (denoted as (Bi,Pb)-2223) has been reported. Hydrothermal method is an effective route to synthesize CdS nanoparticles with good crystallinity and having average grain size of about 12 nm. Then, small amounts (0–0.4 wt%) of nanosized CdS particles were added to Bi-2233 samples using a solid-state reaction route. The transport critical current densities and the electrical resistivity ρ(T, H) were performed using the four-probe technique. The results show that samples sintered by small amount of CdS nanoparticles (≤ 0.3 wt%) exhibit the higher critical current densities and energy pinning in applied magnetic fields compared to free added sample. Consequently, the addition of CdS could introduce effective pinning centers which account for the improvement in superconducting properties in the Bi-2223 materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor cadmium sulfide (CdS) nanoparticles have attracted considerable attention because of their wide direct band gap of 2.4 eV and their specific properties that are not observed by their bulk materials [1, 2]. Such nanoparticles are used in various fields such as optoelectronics, photonics, photovoltaics, and photocatalysis [2]. Based on previous studies, CdS nanostructures have been prepared using numerous physical and chemical techniques such as sol-gel [3], hydrothermal [1], solvothermal [4], co-precipitation [5], polyol [6], and microwave-assisted methods [7]. Among these different methods, low-temperature hydrothermal process is the most frequently used because of its non-toxicity, biocompatibility, eco-friendliness, and low-cost facility. Furthermore, by controlling various parameters such as temperature, reaction time, pressure, precursors, and surface ligands, one can get various dimensions, structure, and morphology of nanoparticles.
Since the discovery of Bi-based superconducting systems (BSCCO), several studies regarding the preparation methods, superconducting characteristics, and the structure of these compounds have been made [8,9,10]. Indeed, these materials have a particular place owing to their high critical transition temperatureT co and especially a good mechanical performance [11, 12]. Moreover, Bi-based superconducting materials are composed of the layered structures that make the mobile charge carriers confine to the Cu–O2 layers [13, 14]. In the Bi-based superconducting compounds, there are three basic superconducting phases with respect to the number of Cu–O2 planes in the unit cell. The phase which exhibits the high critical transition temperature T co = 110 K corresponds to Bi2Sr2Ca2Cu3O10(denoted Bi-2233). Nevertheless, the main constraints of BSCCO superconductors are weak links between the grains resulting in weak critical current density J c in bulk samples. The addition of nanosized particles or substitution of elements are one of the most effective ways for controlling the superconducting properties of high T c superconductors (HTS)[15,16,17,18,19,20,21]. The improvement or devastation of the superconducting properties is heavily dependent on the characteristics of the dopant in the crystal structure. A suitable amount of nanometer particles can cause a significant improvement in the flux pinning ability and transport properties of compounds. Among various classes of nanoparticles, semiconductor nanoparticles have emerged as important materials with promising applications and have proved its effectiveness in improving the critical current densities in superconducting materials [22,23,24]. Although many papers have been already reported as regards to the effect of nanoparticles on the enhancement of the J c , on the best of our knowledge, no report on the effect of CdS nanoparticles was published in the BSCCO system.
The first part of the present work concerns the synthesis of CdS nanoparticles via the hydrothermal method. Then, we have used the prepared nanoparticles as additives and we aim to study their effect on the superconducting properties and flux pinning mechanisms in BSCCO system.
2 Material and Methods
2.1 Nanoparticles Synthesis
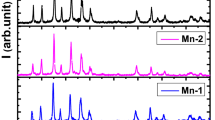
Hydrothermal method was used to prepare semiconductor CdS nanoparticles starting from cadmium chloride hydrate (CdCl2–H2O) and thiourea CH4N2S as precursors and using propanoic acid C3H6O2 as surface ligand, which is necessary in the stability of the nanocrystals. For the synthesis of CdS nanocrystals, 0.1006 g of CdCl2–H2O was dissolved in 5 ml of distilled water to obtain a 0.1-M solution, 0.0342 g of CH4N2S was dissolved in 4.5 ml of distilled water to obtain a 0.1-M solution, and 0.25 ml of propanoic acid was dissolved in 20 ml of distilled water. The cadmium chloride hydrate solution and propanoic acid solution were mixed together and then were dropped under vigorous stirring. To make a basic solution, a 2-M sodium hydroxide solution NaOH is added until a pH = 10. Thiourea solution is thereafter added to the previous solution as a source of sulfur. The result precursor mixture was stirred at room temperature for 24 h, placed into a teflon-lined autoclave, and finally subjected at heat treatment 180 °C for 7 h. The orange precipitates were collected, washed with ethanol for several times to remove soluble inorganic impurities, and then dried at 80 °C in air before further characterization. We have examined the structure, the average particle sizes, and the phase composition of the resulting product by powder XRD using a Philips 1710 diffractometer with CuK α radiation and FEI Tecnai G2 transmission electron microscopy coupled with an energy-dispersive x-ray spectroscopy (EDXS) system. Figure 1 displays the XRD patterns of CdS nanoparticles. Strong and sharp diffraction peaks have been observed indicating the good crystallinity of the product. The indexed diffraction peaks in Fig. 1 correspond to the hexagonal structure with lattice parameters a = 4.121 Å and c = 6.682 Å. Figure 2a is a transmission electron microscopy image showing an almost monodispersion of nanoentities with oval or spherical shape. EDXS analyses performed on areas with nanosized particle indicate the presence of cadmium (Cd) and sulfur (S) elements (Fig. 2b). Based on statistical analysis of more than 100 entities in a single region (Fig. 2c), the average size of nanoparticles is about 12 nm. While the diameter of CdS nanoparticles, estimated with the Debyee-Scherrer formula, was found to be 10 nm, which is in good agreement with the TEM observations.
2.2 Superconducting Samples Preparation
Details of the preparation of BSCCO polycrystalline samples have been presented elsewhere [21] and we give herein a brief description. Samples were synthesized using solid-state reaction route by considering three thermal cycles comprising additives at the beginning of each cycle. During the final treatment phase, the synthesized CdS nanoparticles were added to BSCCO. The additional amount of CdS, varied from x = 0 to x = 0.4 wt% of the total mass of sample. The mixture were pressed into pellets under a uniaxial pressure of 1 GPa and finally sintered in air at 835 °C for 72 h. The temperature dependence of the electrical resistivity ρ(T H) under applied magnetic fields ranging from 0 to 100 mT measurements and the current-voltage (I–V) characteristics were carried out using the four-probe technique. The critical current density (J c ) values were determined at various temperatures using a 5 μ V/cm criterion. A magnetic field was applied along the short axis of the sample and the excitation current was injected along the length axis of the samples.
3 Results and Discussion
Measurements of the resistivity dependence on the temperature ρ(T) for pure and CdS added Bi-2223 samples are shown in Fig. 3. All samples show (above the onset transition temperature (\(T_{c}^{\text {on}})\)) a metallic behavior in the normal state followed by a sharp superconducting transition to zero-resistivityT co confirming the decrease of the porosity, grain boundary resistivity, and grain boundary weak links in the system. The resistivity curves in the metallic behavior can be expressed by the linear equation such as:ρ n (T) = ρ o + α T, where ρ o is an indicator of the sample homogeneity and defect density and the slope α is considered as a parameter that depends on the intrinsic electronic interaction. The obtained values of critical temperatures(\(T_{c}^{\text {on}}\), T co), ρ o and α for pure and added samples are listed in Table 1. The data show that all CdS-added samples exhibit the lower normal-state resistivity and residual resistivity ρ o . This implies the good quality process of synthesis and positive effect of CdS doping. Furthermore, it is clearly seen from the inset of Fig. 3 that the zeroresistance temperature, T co, was practically maintained in Bi-2223 with different amounts of CdS. Also, we found an almost constant value of the onset temperature of the superconducting transition (\(T_{c}^{\text {on}}\) close to 114 K) for all sintered samples.
In order to examine the relevant effect of CdS addition under applied magnetic field (H), we have measured the resistivity of pure and CdS added samples with different values of magnetic fields. It is clearly seen from Fig. 4 that the lower region of the curves (T < T c ), where T c is the peak temperature of the \(\frac {d\rho } {dT}\) versus T curves, is strongly affected by the application of applied magnetic field. It is commonly known that the flow of superconducting carriers through the weak couplings and nanocrystallites is prevented by the application of a magnetic field. Indeed, an applied magnetic field is expected to suppress the critical current of a Josephson junction and consequently result in the appearance of a junction resistance. We note that the addition of CdS nanoparticles narrows the width of the resistive transition width ΔT = T co(0) − T co(H). The ΔT data are well adjusted according to a scaling relationship of the power law ΔT = a H −n. In our case, the parameter n is found to be similar 0.23 ± 0.01 for all sintered samples. However, the values of the factor, a, are lower for samples sintered with CdS concentration < 0.3 wt% as listed in Table 2. This result confirms the beneficial effect of CdS addition on the improvement of the electrical conduction in the material.
The dependence of ρ(T, H) is expected to provide information about the mechanism of the vortices pinning. Indeed, the magneto-resistivity ρ(T, H) curves in the polycrystalline HTSC has been found to obey to the Arrhenius relation as follows [25, 26]: \(\rho \left (T,H \right )=\rho _{o}\exp \left (-\frac {U_{o}\left (T,H \right )}{k_{B}T} \right )\) where U o is the flux pinning energy depends on both the temperature and the magnetic field and k B is the Boltzmann constant. The U o value can be directly determined from the linear data in the tail part of the slope of the plot of \(ln\frac {\rho } {\rho _{o}}\) versus \(\frac {1}{T}\); a typical curve is depicted in Fig. 5. Figure 6 displays the deduced U o , for different amounts of CdS addition. The value of U o decreases as the magnetic field increases for each sample. Compared to CdS-added samples, the pure one shows a rapid decrease of U o on magnetic field. Besides, it is clearly seen that U o for samples sintered with CdS concentration < 0.3 wt% is much higher than for a pure one in the whole range of the considered magnetic field. This result confirms the important role of CdS addition in improving the transport capabilities of Bi-2223 materials.
Figure 7 shows J c measurements under applied magnetic fields. All sintered samples exhibit deterioration of J c on increasing the magnitude of magnetic field. It is notable that J c is increased for samples sintered with CdS throughout the whole range of applied magnetic fields. For lower temperatures, the CdS-added samples exhibit less sensitivity to magnetic fields compared with the non-added one (inset of Fig. 7). These results suggest that the CdS nanoparticles act as effective pinning centers yielding to the improvement of critical current density behavior.
Figure 8 shows the temperature dependences of critical current densities at applied magnetic field for free and CdS-added samples. The critical current density increase monotonically on decreasing temperature from near T co down to T = 25 K for each sample. The samples sintered with CdS concentration ≤ 0.3 wt% exhibit the highest values of J c in almost the entire temperature range at self-magnetic field; however, when the addition of CdS exceeds 0.4 wt%, one can observe a decrease of J c (T) values. For an applied magnetic field (i.e., 100 mT), we note that the values of J c (T) remain very higher for samples sintered with a small amounts of CdS (≤ 0.3 wt%) compared to the non-added one. For clarity, we have plotted in Fig. 9 the temperature dependence J c (T) for 0.0, 0.1, and 0.2 wt% CdS-added samples. A comparison between samples shows that the critical current density of the added samples has increased for the whole-temperature range either on self or applied magnetic field. This effect could be the result of further homogeneity and better connection between the superconducting grains following the addition of CdS. Therefore, it seems that small amount of CdS nanoparticles are acting as conductors. So, it can enhance the connections of grains boundary of BSCCO compound and improve the transport the electrical current in the material.
In order to determine the contribution of each pinning mechanism for free and CdS-added samples, we have analyzed the critical current density dependences on temperature J c (T) based on the thermally activated flux motion and the collective flux-pinning models [27]. For HTS materials, the pinning mechanism can be classified into three types: (1) δ T c , which is due to the randomly distributed spatial variations in the transition temperature T c [28]; (2) δ l, mostly due to crystal lattice defects and related to spatial fluctuation of the charge-carrier mean free path [27]; and (3) δ ε, due to the stress/strain field [29]. According to these models, the global transport critical current density J c (T) is expressed as follows:
where J c (0) is the critical current density at 0 K, \( t=\frac {T}{T_{\text {co}}}\) is the reduced temperature, and the exponents α and β are related to the pinning mechanism;
Figure 10 shows the plots of the experimental data J c (T) for free, 0.1 and 0.2 wt% CdS-added samples. The theoretical curves of (2), (3), and (4) are also presented in the figure by the solid lines. As can be seen, the experimental J c (T) values at self-applied magnetic fields are in a good agreement with the model of δ T c pinning over the main temperature range for all sintered samples. However, for an applied magnetic field μ o H = 100 mT, the δ T c pinning mechanism suppressed completely and J c (T) values agree with the δ ε mechanism for pure sample while the experimental results of CdS added samples cannot be explained by a single model over the whole region of the J c (T) curves. Indeed, the δ l pinning mechanism is mainly responsible at low temperatures, decreased with increasing temperature, and then transformed to δ ε at temperatures close to T co. It can be noted that the addition of CdS nanoparticles inside the superconducting matrix causes the fluctuations in the mean free path that are responsible for the δ l pinning at low temperatures. Similar behavior has been found in the case of nano-Si-doped MgB2 superconductor [30]. These results provide strong evidence that the CdS nanoparticles produced very strong pinning centers in the Bi-2223 matrix.
Temperature dependence of the current density J c at magnetic fields of 0 and 100 mT for free and 0.1 and 0.2 wt% added samples. Scatters mark the experimental data. Lines are theoretical fits obtained based on the model of the δ l (blue curves), the δ T c (red curves), and the δ ε (black curves) pinning mechanisms
4 Conclusion
CdS nanoparticles with average grain size of about 12 nm were successfully synthesized by hydrothermal method. The effect of nanosized CdS particles addition on the superconducting properties of polycrystalline Bi-2223 compounds was systematically studied. CdS nanoparticles were added up to 0.4 wt% to Bi-2223 superconductors using solid-state reaction. The results of ρ(T) measurements show that the addition of CdS has acted in an effective manner and decreased the normal-state resistivity and the residual resistivity ρ o . It also showed that the onset temperature of the superconducting transition, \(T_{c}^{\text {on}}\), and the zero-resistance temperature, T co, values were maintained in Bi-2223 bulks with the addition of CdS nanoparticles. From the measurement of ρ(T H), we have found that samples sintered by small amount of CdS possess a narrower resistive transition width ΔT under applied magnetic fields and higher values of pinning energy indicating the beneficial role of CdS nanoparticles on the improvement of the electrical conduction in the material. Critical current densities J c were also investigated under applied magnetic fields at various temperatures. It is revealed that Bi-2223 bulk sintered with low concentration of CdS exhibits higher J c values under applied magnetic fields compared to the pure one. From the temperature dependence of the critical currents, we have evaluated the pinning mechanism. The results show that the addition of CdS nanoparticles favors more than one pinning mechanism. It therefore concluded that the CdS nanoparticles produced very strong pinning centers in the Bi-2223 matrix. So an enhancement can be beneficial in J c of CdS-added Bi-2223 compounds for the practical applications of these high temperature superconducting materials.
References
Chen, R., Han, B., Yang, L., Yang, Y., Xu, Y., Mai, Y.: Controllable synthesis and characterization of CdS quantum dots by a microemulsion-mediated hydrothermal method. J. Lumin. 172, 197–200 (2016)
Qutub, N., Sabir, S.: Optical, thermal and structural properties of CdS quantum dots synthesized by a simple chemical route. Int. J. Nanosci. Nanotechnol. 8, 111–120 (2012)
Zhang, H.: Effects of post-annealing treatment on the structure and photoluminescence properties of CdS/PS nanocomposites prepared by sol-gel method. Optoelectron. Lett. 12, 81–84 (2016)
Ren, B., Cao, M., Zhang, Q., Huang, J., Zhao, Z., Jin, X., Li, C., Shen, Y., Wang, L.: Controllable synthesis of CdS nanowire by a facile solvothermal method and its temperature dependent photoluminescent property. J. Alloys Compd. 659, 74–81 (2016)
Elavarthi, P., Kumar, A.A., Murali, G., Reddy, D.A., Gunasekhar, K.R.: Room temperature ferromagnetism and white light emissive CdS:Cr nanoparticles synthesized by chemical co-precipitation method. J. Alloys Compd. 656, 510–517 (2016)
Darwish, M., Mohammadi, A., Assi, N.: Microwave-assisted polyol synthesis and characterization of pvp-capped CdS nanoparticles for the photocatalytic degradation of tartrazine. Mater. Res. Bull. 74, 387–396 (2016)
Yang, H., Huang, C., Li, X., Shi, R., Zhang, K.: Luminescent and photocatalytic properties of cadmium sulfide nanoparticles synthesized via microwave irradiation. Mater. Chem. Phys. 90, 155–158 (2005)
Maeda, H., Tanaka, Y., Fukutomi, M., Asano, T.: A new high-Tc oxide superconductor without a rare earth element. Jpn. J. Appl. Phys. 27, L209 (1988)
Ozturk, O., Yegen, D., Yilmazlar, M., Varilci, A., Terzioglu, C.: The effect of cooling rates on properties of Bi1.7Pb0.35Sr1.9Ca2.1Cu3Oy superconductors produced by solid-state reaction method. Physica C 451, 113–117 (2007)
Michel, C., Hervieu, M., Borel, M.M., Grandin, A., Deslandes, F., Provost, J., Raveau, B.: Superconductivity in the Bi-Sr-Cu-O system. Z. Phys. B: Condens. Matter 68, 421–423 (1987)
Yildirim, G., Varilci, A., Akdogan, M., Terzioglu, C.: Role of annealing time and temperature on structural and superconducting properties of (Bi, Pb)-2223 thin films produced by sputtering. J. Mater. Sci: Mater. Electron. 23, 928–935 (2012)
Sarkar, K.A., Maartense, I., Peterson, T.L., Kumar, B.: Preparation and characterization of superconducting phases in the Bi(Pb)SrCaCuO system. J. Appl. Phys. 66, 3717–3722 (1989)
Zhang, H., Sato, H.: Universal relationship between Tc and the hole content in p-type cuprate superconductors. Phys. Rev. Lett. 70, 1697–1699 (1993)
Ghahfarokhi, S.E.M., Shoushtari, M.Z.: Structural and physical properties of Cd-doped Bi1.64Pb0.36Sr2Ca2−xCdxCu3Oy superconductor. Physica B 405, 4643–4649 (2010)
Halim, S.A., Khawaldeh, S.A., Mohamed, S.B., Azhan, H.: Superconducting properties of Bi2−xPbxSr2Ca2Cu3Oy system derived via sol-gel and solid state routes. Mater. Chem. Phys. 61, 251–259 (1999)
Dos Santos, C.A.M., Moehlecke, S., Kopelevich, Y., Machado, A.J.S.: Inhomogeneous superconductivity in Bi2Sr2Ca1−xPrxCu2O8+δ. Physica C 390, 21–26 (2003)
Biju, A., Abhilash Kumar, R.G., Aloysius, R.P., Syamaprasad, U.: Structural and superconducting properties of Bi1.7Pb0.4Sr2−xGdxCa1.1Cu2.1Oy system. Physica C 449, 109–115 (2006)
Awana, V.P.S., Agarwal, S.K., Narlikar, A.V., Das, M.P.: Superconductivity in Pr- and Ce-doped Bi2CaSr2Cu2Oy systems. Phys. Rev. B 48, 1211–1216 (1993)
Berger, H., Ariosa, D., Gaal, R., Saleh, A., Margaritondo, G., Lee, S.F., Huang, S.H., Chang, H.W., Chuang, T.M., Liou, Y., Yao, Y.D., Hwu, Y., Je, J.H., Gasparov, L.V., Tanner, D.B.: Coexistence of ferromagnetism and high-temperature superconductivity in Dy-Doped BiPbSrCaCuO. Surf. Rev. Lett. 9, 1109–1112 (2002)
Ghattas, A., Annabi, M., Zouaoui, M., Ben Azzouz, F., Ben Salem, M.: Flux pinning by Al-based nano particles embedded in polycrystalline (Bi,Pb)-2223 superconductors. Physica C 468, 31–38 (2008)
Zouaoui, M., Ghattas, A., Annabi, M., Ben Azzouz, F., Ben Salem, M.: Effect of nano-size ZrO2 addition on the flux pinning properties of (Bi, Pb)-2223 superconductor. Supercond. Sci. Technol. 21, 125005 (2008)
Ben Salem, M.K., Hannachi, E., Slimani, Y., Hamrita, A., Zouaoui, M., Bessais, L., Ben Salem, M., Ben Azzouz, F.: SiO2 nanoparticles addition effect on microstructure and pinning properties in YBa2Cu3Oy. Ceram. Int. 40, 4953–4962 (2014)
Guo, Y.C., Tanaka, Y., Kuroda, T., Dou, S.X., Yang, Z.Q.: Addition of nanometer SiC in the silver-sheathed Bi2223 superconducting tapes. Physica C 311, 65–74 (1999)
Ben Salem, M.K., Almessiere, M.A., Al-Otaibi, A.L., Ben Salem, M., Ben Azzouz, F.: Effect of SiO2 nano-particles and nano-wires on microstructure and pinning properties of YBa2Cu3O7−d. J. Alloys Compd. 657, 286–295 (2016)
Kim, J.J., Lee, H., Chung, J., Shin, H.J., Lee, H.J., Ku, J.K.: Flux-creep dissipation in epitaxial YBa2Cu3O7−δ film: Magnetic-field and electrical-current dependence. Phys. Rev. B 43, 2962–2967 (1991)
Koch, R.H., Foglietti, V., Gallagher, W.J., Koren, G., Gupta, A., Fisher, M.P.A.: Experimental evidence for vortex-glass superconductivity in Y-Ba-Cu-O. Phys. Rev. Lett. 63, 1511–1514 (1989)
Blatter, G., Feigel’man, M.V., Geshkenbein, V.B., Larkin, A.I., Vinokur, V.M.: Vortices in high-temperature superconductors. Rev. Mod. Phys. 66, 1125–1388 (1994)
Wen, H.H., Zhao, Z.X., Xiao, Y.G., Yin, B., Li, J.W.: Evidence for flux pinning induced by spatial fluctuation of transition temperatures in single domain (Y1−xPrx)Ba2Cu3O7−δ samples. Physica C 251, 371–378 (1995)
Wen, H.H., Zhao, Z.X., Wang, R.L., Li, H.C., Yin, B.: Evidence for the lattice-mismatch-stress-field induced flux pinning in (Gd1−xYx)Ba2Cu3O7−δ thin films. Physica C 262, 81–88 (1996)
Ghorbani, S.R., Wang, X.L., Hossain, M.S.A., Dou, S.X., Lee, S.I.: Coexistence of the δ l and δ T˙c flux pinning mechanisms in nano-Si-doped MgB2. Supercond. Sci. Technol. 23, 025019 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loudhaief, N., Labiadh, H., Hannachi, E. et al. Synthesis of CdS Nanoparticles by Hydrothermal Method and Their Effects on the Electrical Properties of Bi-based Superconductors. J Supercond Nov Magn 31, 2305–2312 (2018). https://doi.org/10.1007/s10948-017-4496-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-017-4496-4