Abstract
It is known that the use of the Dubinin–Radushkevich method in micro-mesoporous samples does not give adequate values of micropore volumes, unlike when the samples contain only microporous. Based on that, in this work, we propose an easy method to calculate a reliable micropore volume (VμP) of micro-mesoporous (nanopores) samples, separating the microporous region from the experimental isotherm. For this, the original isotherm is modified, estimating the thickness of the adsorbed layer (t) as a function of relative pressure and changing the external surface area (Sext) to obtain a Type I adsorption isotherm in the microporous region; then, the DR method can be applied to the modified isotherm. This proposal, named the DR_t method, allows the calculation of a reliable VμP of any nanoporous material using different adsorbates. Using this method, we analyzed adsorbents of distinct nature (i.e., carbons and silicas) with different adsorbates as N2 and O2 at 77 K, Ar at 87 K, and CO2 at 273 K. We used this method to calculate VμP in different samples and compare them with those obtained with the traditional DR method, highlighting that unlike the latter the DR_t method showed similar and consistent results with the different adsorbates. Therefore, the values of micropore volume calculated using the DR_t method demonstrate consistency across various adsorbates, not only for N2 but especially for CO2, which is suggested to analyze narrow micropore volumes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to the IUPAC [1], gas adsorption is a well-known technique to characterize porous solids and fine powders; this characterization requires various subcritical fluids (N2 at 77 K, Ar at 87 K, CO2 at 273 K, among others). In nanoporous materials, textural properties are essential. Particularly, the micropore volume (VμP) has a direct effect on the material performance, e.g., CO2 capture [2,3,4,5,6,7], H2 [8,9,10,11,12] and CH4 storage [13, 14], and electrochemical energy storage [15,16,17], among others. After measuring the adsorption isotherm and applying several procedures and models, we can evaluate the textural properties, i.e., specific surface area, pore volumes, and pore size distribution. In particular, VμP can be assessed by macroscopic methods (Dubinin–Raduschkevich (DR) and Dubinin–Ashtakov (DA)), semiempirical methods (αS-plot and t-plot), and microscopic methods based on molecular simulations such as Monte Carlo (MC) and Density Functional Theory (DFT), and the advantages/disadvantages of each method can be found elsewhere [1].
It is well known that microscopic methods (MC and DFT) provide the best description of fluid adsorption and phase behavior within pore structures at the molecular level due to statistical mechanics. However, access to these methods is still limited, and an adequate Kernel is required to describe a given porous system. On the other hand, the semiempirical methods (αS-plot and t-plot) are a suitable option for calculating VμP and the external surface area (Sext). However, they are susceptible to the selected relative pressure interval. Macroscopic methods based on the micropore filling theory and Polanyi’s potentials (as in DR) are commonly used due to their simplicity and reliable results (comparable with those obtained with microscopic methods) when the studied sample has a Type I isotherm. Nonetheless, the DR method overestimates the micropore volume in micro-mesoporous materials when the sample presents mesoporosity. This overestimation grows with the increase in mesoporosity [18,19,20,21].
The more accepted mechanisms for pore filling are those using adsorption potentials (as in the DR method) for the micropores region and those based on the capillary condensation theory (related to the external surface area of the material) in the mesopores region. Based on the above, Dubinin and Kadlec [22] proposed a method to evaluate the micropore volume and the external surface area by analyzing the adsorption data of N2 at 77 K or benzene at 293 K; this method, as planted, works well with materials with low mesoporosity.
Based on the theory and fundaments of Dubinin and Kadlec, and separating the microporosity and mesoporosity contribution in the adsorption isotherm, we developed an easy procedure to estimate the micropore volume, which agrees with those results calculated with αS-plot and DFT, regardless of the used adsorbate and the type of porous material.
2 Theory and methods of Dubinin
The Dubinin-Radushkevich theory is based on the Polanyi potential theory [23], which states that the adsorption potential, φ, corresponds to the increase in free energy of the adsorbate (taking the free energy of adsorbate in the liquid state as a reference value) at adsorption temperature, T, in equilibrium with its saturated vapor at a pressure pº. This means that the adsorption potential as a function of p/pº, \(\varphi \left(p/{p}^{{\text{o}}}\right)\), is given by Eq. 1:
where R is the ideal gas constant, and p is the absolute pressure of the adsorbate. The theory proposed by Dubinin used the concept of adsorption potential and assumed that micropores are filled with the adsorbate in the liquid state in a physical adsorption process [24]. In the linear form, the DR equation is expressed as in Eq. 2:
where \({V}_{ads}\left(p/{p}^{{\text{o}}}\right)\) is the adsorbed volume as a function of p/pº, VµP is the micropore volume, D is a constant equal to \(2.303\times {\left(\frac{R\times T}{E}\right)}^{2}\) and E is the adsorption energy.
As mentioned above, the DR method overestimates VμP in micro-mesoporous materials, and this overestimation grows with the increase of mesoporosity, as reported elsewhere [18,19,20,21].
According to the more accepted mechanisms, the micropore filling is based on the DR theory, and the mesopore filling is based on the capillary condensation theory (related to the materials’ external surface area). Dubinin and Kadlec [22] proposed a method to assess the micropore volume and the external surface area using the adsorption data of N2 at 77 K or benzene at 293 K between 1·10–5 and 0.3 of relative pressure, i.e., below the capillary condensation mechanism in mesopores. They used the t method (which considers the thickness of the adsorbed layer) and the F parameter (filling degree in micropores, estimated using the DR equation). Besides, they assumed that in this relative pressure interval, the following expression gives the total adsorbed volume in a porous solid:
where \({V}_{micro}\left(p/{p}^{{\text{o}}}\right)\) is the adsorbed volume in micropores, and \({V}_{meso+macro}\left(p/{p}^{{\text{o}}}\right)\) corresponds to the adsorbed volume on the surface of meso- and macropores, both volumes as a function of p/pº (in the range from 1·10–5 to 0.3). When the specific surface area of macropores is negligible compared to that of mesopores, it is possible to define the external surface area (Sext) as the mesopore specific surface area, and taking into account that \({\varvec{t}}\left({\varvec{p}}/{{\varvec{p}}}^{{\varvec{o}}}\right)\) is the thickness of the adsorbed layer, as a function of p/pº, on mesopores, in the same relative pressure interval, Eq. 3 transforms in:
Later, Dubinin and Kadlec, by using the DR equation (on the micropore adsorption), defined the micropore filling degree as a function of p/pº, \(F\left(p/{p}^{{\text{o}}}\right)\), as:
where VμP is the value of micropore volume, E is the characteristic energy of adsorption estimated by the DR equation between a relative pressure of 1·10–5 and 5·10–3, and \(\varphi \left(p/{p}^{{\text{o}}}\right)\) is the adsorption potential as a function of p/pº defined by Eq. 1.
Finally, combining Eq. 4 and 5, the Dubinin-Kadlec equation was obtained:
According to Eq. 6, a graphical representation of \({V}_{ads}\left(p/{p}^{{\text{o}}}\right)\)/\(F\left(p/{p}^{{\text{o}}}\right)\) versus \(t\left(p/{p}^{{\text{o}}}\right)\)/\(F\left(p/{p}^{{\text{o}}}\right)\) gives a straight line whose intercept corresponds to the micropore volume, and its slope is the external surface area of the studied sample.
The Dubinin-Kadlec method, as planted, gives good results in materials with little to no mesoporosity. However, the estimated VμP value presents a considerable error for materials with an important mesoporosity. In addition, depending on the selected p/po interval for the adjustment, different VμP and Sext values will be found. The latter fact generates uncertainty regarding the proper interval for applying this method.
Trying to correct the inconsistency of the values obtained by DR when compared with other techniques, Bouwer et al. [20], based on the same principle as the Dubinin–Kadlec method and, assuming a homogeneous surface, proposed a method that considers a proportionality constant (k* in cm3·m−2) relates the overestimated quantity in the micropore volume with the external surface area. However, k*, which is for a given adsorbate-adsorbent system, is defined as a constant independent of p/po, indicating that a 5 nm mesopore has the same filling capacity as a 50 nm pore, a fact without physical meaning because, in a porous system, the pore is more energetic when is smaller.
3 Proposed methodology: DR_t method
Now, to solve the issues mentioned above, instead of thinking that “the total adsorbed volume over a porous solid is the sum of the micro-and mesopore volume in a defined relative pressure range”, we propose that “the isotherm of a given micro-mesoporous material is the linear combination (or sum) of the individual contribution of both microporous and mesoporous isotherms”. It is possible to separate these two contributions (or individual isotherms) and thus evaluate the micropore volume from the individual micropore isotherm. This idea was proposed by Villarroel-Rocha [25]. Recently, a method to separate the partial isotherms for micropore and mesopore adsorption for the determination of pore size distribution in carbons with N2, was reported by Choma et al. [26].
The statement mentioned above means that the microporous part of a porous solid can be mathematically expressed using Eq. 4, but in the entire relative pressure range of the experimental adsorption isotherm:
where \({V}_{micro}\left(p/{p}^{{\text{o}}}\right)\) is the adsorption isotherm that only corresponds to the adsorption due to microporosity, Sext is the external surface area of the porous solid, and \(t\left(p/{p}^{{\text{o}}}\right)\) is the thickness of the adsorbed layer (on the external surface area) as a function of p/po, which is represented by Eq. 8:
where d is the molecular diameter of the adsorbed molecule on the surface and Vm is the monolayer adsorbed volume. Furthermore, an additional way to represent the data of a reference material is using αS(p/po) as show in Eq. 9:
here Vads0.4 is the adsorbed volume at 0.4 of p/po. For Eqs. 8 and 9Vads(p/po), Vm, and Vads0.4 correspond to the reference material. Combining Eqs. 8 and 9, we obtain the equation to calculate t(p/po):
here Ct is the conversion factor from αS to t (nm) (see Table 1), and given by \({C}_{t}=d\times \frac{{V}_{ads}^{0.4}}{{V}_{m}}\), where \({\alpha }_{{\text{S}}}\left(p/{p}^{{\text{o}}}\right)\) represents the isotherm data (in terms of αS) of the reference material, which must be carefully selected to ensure that it has the same chemical nature as the studied sample. Therefore, the individual isotherm of the microporous part of the studied solid will be the representation of \({V}_{micro}\left(p/{p}^{{\text{o}}}\right)\) versus p/po. From the adsorption isotherm data corresponding to micropores and the DR equation (Eq. 2), the micropore volume can be obtained. It is important clarify that in the case of materials analyzed with CO2 at 273 K it is not possible to determine \({V}_{ads}^{0.4}\) because the experimental isotherm with this adsorbate is measured up to 1000 kPa (i.e., 0.28 of p/po), therefore we change the reference point to 0.2 of p/po (\({V}_{ads}^{0.2}\)).
In Table 1 is also shown information about some reference materials that have not been previously reported.
4 Experimental
4.1 Materials
For this work, two different types of materials were used: On the one hand, three carbon samples, i.e., (1) an activated carbon (AC1), synthesized from coconut shells by chemical activation using zinc chloride followed by carbon dioxide activation (details of this procedure are described by García Blanco et al. [8]), (2) an ordered mesoporous carbon (CMK-8) synthesized according to the procedure of Barrera et al. [17], and (3) a carbon foam (EsFe) synthesized according to the procedure of De Araújo et al. [35]. On the other hand, two ordered mesoporous silica materials (SBA-16 and Al-modified SBA-15) were synthesized according to the nonhydrothermal procedure described by Villarroel-Rocha et al. [36].
4.2 Characterization
N2 (99.999%) and O2 (99.8%) adsorption–desorption isotherms at 77 K were measured in manometric adsorption equipment ASAP 2000 from Micromeritics and Autosorb iQ analyzer from Quantachrome Instruments. Ar (99.999%) adsorption–desorption isotherms at 87 K were measured in Autosorb iQ analyzer from Quantachrome Instruments coupled to a cryocooler (CTI-CRYOGENICS). Finally, CO2 (99.996%) adsorption isotherms at 273 K up to 1000 kPa were measured in manometric adsorption equipment ASAP 2050 from Micromeritics. Before each analysis, the samples were outgassed between 150 and 200 °C for 12 h.
5 Results and discussion
5.1 DR_t method procedure
To explain the methodology for obtaining Sext, N2 adsorption–desorption isotherms at 77 K of silica and carbon materials, i.e., SBA-16 (Fig. 1a) and activated carbon (AC1) (Fig. 2a), will be used as examples. Using Eq. 7, we obtain adsorption isotherms corresponding only to the microporosity, as shown in Figs. 1b and 2b. As shown, when Sext is increased in Eq. 7, the adsorbed volume of the new isotherm decreases. This because the mesoporous term (\({S}_{ext}\cdot t\left(p/{p}^{{\text{o}}}\right)\)) in Eq. 7 becomes more negative with the increase of the external surface area. Successive changes in Sext let us obtain a unique value of Sext for which the microporous isotherms correspond to a Type I isotherm (at low relative pressures); this is, the adsorbed volume will reach a horizontal plateau. This value is the correct one for the analyzed porous material, in this case 262 and 855 m2·g−1 are obtained with N2 at 77 K for SBA-16 and AC1, respectively. In Figs. 1b and 2b, the plateau is reached at 0.23 and 0.5 of p/po, respectively. For values higher than the correct value of Sext, it can be observed that the new isotherms decrease in adsorption as relative pressure increases, indicating that is incorrect using those values. For these examples, t(p/po) was estimated from the standard reference material LiChrospher Si-1000 [27], a macroporous silica, and CABOT BP280 [30], a non-graphitized carbon black.
After the obtention of Sext, VμP is obtained from the new adsorption isotherm (or modified isotherm), as shown in Figs. 1c and 2c for the above mentioned samples. SBA-16 and AC1 VμP are 0.18 and 0.25 cm3·g−1, respectively. In addition, Fig. 1c shows the modified isotherms of those shown in Fig. 1b at different Sext values. This figure shows that despite different Sext values generate straight lines, not only is necessary to have a good linear adjust, is more important to obtain a Type I isotherm to finally have unbiased and reliable VμP values.
Finally, the values of VμP, obtained with different methods for each sample, are displayed in Figs. 1d and 2d, respectively. The results show that the volumes calculated with our methodology are in good agreement with those obtained with αS -plot, DFT, and DK, but differ significantly from those obtained with the DR method; this latter is due to the presence of mesopores in the sample materials, which makes the model overestimates the VμP value, as discussed elsewhere [21]. Thus, regardless of the mesoporosity degree, the exposed methodology applies to any micro-mesoporous material to determine both Sext and VμP. To effectively utilize this methodology, it is essential to have a standard reference isotherm (for calculating t(p/po)) that accurately represents the studied sample.
5.2 Ordered porous carbon: CMK-8
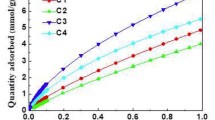
Figure 3a shows the adsorption isotherms of ordered porous carbon CMK-8 with different gases. This material exhibits, with N2 and O2 at 77 K, Type IV(a) isotherms according to the IUPAC classification [1], typical of mesoporous materials. On the other hand, CO2 adsorption shows a behavior like N2 adsorption isotherm up to ca. 0.28 in p/po. For this material, CABOT BP280 was used as reference material, where CO2 at 273 K and O2 at 77 K isotherms data previously reported elsewhere [31, 32]. This work shows these data in terms of αS in the Supplementary Information in Table S1 and S2 for CO2 and O2, respectively.
CMK-8: a adsorption–desorption isotherms with N2 (SBET = 1130m2g−1) and O2 at 77 K (blue and green), and CO2 adsorption isotherm at 273 K (black), b Sext variation to obtain the microporous isotherm (open symbols) from CO2 at 273 K and O2 at 77 K adsorption data, c VμP obtention from CO2 at 273 K adsorption data, and d comparison between the VμP values using DR and DR_t methods with the different gases (Color figure online)
The CO2 and O2 adsorption isotherms in the low relative pressure region are displayed in Fig. 3b. In each case, it can be observed that after subtracting the mesopores contribution to the original isotherm (filled symbols), a Type I isotherm is obtained (open symbols) for a specific Sext value, following the methodology described above. Once the isotherms are modified, the VµP is obtained as is usually done with the DR method (Fig. 3c). The micropore volumes for CMK-8 calculated with the DR_t methodology (Fig. 3d) from adsorption data using N2, O2, and CO2 were in good agreement among them. When comparing these values with those obtained with the DR method, the overestimation in VµP by this latter is seen. These results show that the DR method using CO2 adsorption data without modifications also overestimates the micropore volume; this is a worth mentioning result because it has been considered a reliable method to calculate VµP in carbon materials (with Type I isotherm) since it was proposed by Garrido et al. [37]. However, its use has been extended to other materials, regardless of their porosity (e.g., materials with Type IV isotherm). This observation was reported elsewhere [2].
5.3 Ordered porous silica: Al SBA-15
The adsorption isotherms of Al SBA-15 with different gases are shown in Fig. 4a, where this material exhibits, with N2 at 77 K and Ar at 87 K, Type IV(a) isotherms, and the CO2 adsorption shows a similar behavior to N2 and Ar adsorption isotherms up to ca. 0.3 in p/po.
Al SBA-15: a adsorption–desorption isotherms with N2 at 77 K (blue), SBET = 820m2g−1, and Ar at 87 K (red), and CO2 adsorption isotherm at 273 K (black), b Sext variation to obtain the microporous isotherm (open symbols) from CO2 at 273 K and Ar at 87 K adsorption data, c VμP obtention from CO2 at 273 K adsorption data, and d comparison between the VμP values using DR and DR_t methods with the different gases (Color figure online)
For this material, LiChrospher Si-1000 was used as reference material, where the isotherms data of N2 at 77 K [27] and Ar at 87 K [28] were used. In addition, macroporous silica X005 M (BioSepra Inc.) was also used as reference material, and the CO2 at 273 K and O2 at 77 K isotherms data are displayed in terms of αS in the Supplementary Information (Table S3 and S4). These data were obtained in this work and reported for the first time.
For this case, we show the analysis of CO2 and Ar adsorption isotherms at low relative pressures; where after subtracting the mesopores contribution to the original isotherm (filled symbols), we obtained a Type I isotherm (open symbols) for a defined Sext value; this is clearly observed in Fig. 4b. From this new Type I isotherm, VμP is estimated by using the DR equation (DR_t methodology), as observed in Fig. 4c for CO2 adsorption data. In Fig. 4d, a clear overestimation of the micropore volume values is observed when DR method is applied. When the DR_t method is used, the VμP values are similar, regardless the adsorbate used for the estimation of this value, highlighting that using the DR_t method, the micropore volume obtained with CO2 also agrees with those calculated with N2 and Ar, as in the previous case.
5.4 Carbon foam: EsFe
As last case, we analyze a carbon foam, and the adsorption isotherms are shown in Fig. 5a. In this case, it is observed that this carbon is mainly microporous, because at low relative pressures the sample exhibits a Type I-like isotherm. However, it is still necessary to correct the adsorption isotherm to obtain a net Type I isotherm (Fig. 5b) and to calculate a reliable VµP value. Therefore, we used the reference isotherm for this sample as in CMK-8 (i.e., CABOT BP280).
EsFe: a N2 adsorption–desorption isotherm at 77 K, SBET = 380m2g−1 and CO2 adsorption isotherm at 273 K, b Sext variation to obtain the microporous isotherm (open symbols) from N2 77 K and CO2 at 273 K adsorption data (left y axis for N2 and right y axis for CO2), c VμP obtention from CO2 at 273 K adsorption data, and d comparison between the VμP values using DR and DR_t methods with the different gases
In Fig. 5c, again, it is observed that, VμP values calculated with the DR_t method coincides with both adsorbates, and the difference with those obtained with the DR method is not as great as in the previous cases due to its nature, mostly microporous, where the mesoporous contribution (which is not as high as the other samples analyzed) is not high enough to contribute to a significant deviation in the DR method.
5.5 External surface area analysis
In Fig. 6 it is observed that there is a good agreement between the Sext values calculated with αS-plot and the DR_t method for all carbon materials and among all adsorbates (Fig. 6a). It is important to highlight that because CO2 adsorption isotherm only reaches 0.28 of p/po, it is not possible to apply αS-plot method. Regarding O2, specifically to the CMK-8 sample, we were unable to calculate VμP and Sext using αS-plot because it was impossible to find the region where only multilayer adsorption appears to apply this method, this was due to the presence of narrow mesopores.
In the case of silica materials (Fig. 6b) it is shown that the Sext values calculated with αS-plot are higher than those calculated with the DR_t method. For Al SBA-15, the Sext values obtained with N2 and CO2 are similar, different to the one obtained with Ar, which is lower; this difference is associated to the absence of quadrupole moment in the latter [36].
6 Conclusions
DR_t is a reliable method for obtaining the micropore volume, which agrees with those calculated with the αS-plot and DFT methods. We have shown that, regardless of the used adsorbate (N2, O2, Ar, and CO2), the results obtained using DR_t are similar among them, evidencing the consistency of this method. When the known DR method is applied in micro-mesoporous materials with all adsorbates, including CO2, VμP is overestimated. To our knowledge, DR_t is the first macroscopic method used to calculate reliable VμP using CO2 adsorption, the standard technique used to study narrow microporosity. In some cases, the αS-plot method is not easy to apply because it is sensitive to the relative pressure range where it is applied. It is necessary to have adsorption data up to 0.45 of p/po, and we must have a relative pressure range where only the multilayer adsorption occurs. Instead, the DR_t method is not susceptible to subjective analysis criteria (e.g., choosing p/po range). Finally, the DR_t method, having the proper reference isotherm that better describes the studied sample, allows the calculation of VμP even if the Ct value in Eq. 10 is unknown, which must have a value of 1. However, one must be aware that Sext is only a proportional value that does not reflect the external surface of the material. Finally, we reported the CO2 at 273 K and O2 at 77 K isotherms data of macroporous silica X005.
Data availability
Not applicable.
References
M. Thommes, K. Kaneko, A.V. Neimark, J.P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, K.S.W. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015)
K.Y. Montiel-Centeno, D. Barrera, J. Villarroel-Rocha, M.S. Moreno, K. Sapag, Hierarchical nanostructured carbons as CO2 adsorbents. Adsorption 25, 1287–1297 (2019)
M. Sevilla, J.B. Parra, A.B. Fuertes, Assessment of the role of micropore size and N-doping in CO2 capture by porous carbons. Appl. Mater. Interfaces 5, 6360–6368 (2013)
M.E. Casco, M. Martínez-Escandell, J. Silvestre-Albero, F. Rodríguez-Reinoso, Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 67, 230–235 (2014)
G. Srinivas, V. Krungleviciute, Z.-X. Guo, T. Yildirim, Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume. Energy Environ. Sci. 7, 335–342 (2014)
G. Sethia, A. Sayari, Comprehensive study of ultra-microporous nitrogen-doped activated carbon for CO2 capture. Carbon 93, 68–80 (2015)
J. Serafin, U. Narkiewicz, A.W. Morawski, R.J. Wróbel, B. Michalkiewicz, Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 18, 73–79 (2017)
A.A. García Blanco, J.C. Alexandre de Oliveira, R. López, J.C. Moreno-Piraján, L. Giraldo, G. Zgrablich, K. Sapag, A study of the pore size distribution for activated carbon monoliths and their relationship with the storage of methane and hydrogen. Colloids Surf. A Physicochem. Eng. Asp. 357, 74–83 (2010)
J. Serafin, B. Dziejarski, O.F. Cruz Junior, J. Sreńscek-Nazzal, Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage. Carbon 201, 633–647 (2023)
J.S. Mirzaei, A. Ahmadpour, A. Shahsavand, A.N. Pour, L. Lotfi Katooli, A.G. Asil, B. Pouladi, A. Arami-Niya, Experimental and simulation study of the effect of surface functional groups decoration on CH4 and H2 storage capacity of microporous carbons. Appl. Surf. Sci. 533, 147487 (2020)
L. Zubizarreta, E.I. Gomez, A. Arenillas, C.O. Ania, J.B. Parra, J.J. Pis, H2 storage in carbon materials. Adsorption 14, 557–566 (2008)
L. Zubizarreta, A. Arenillas, J.J. Pis, Carbon materials for H2 storage. Adsorption 34, 4575–4581 (2009)
S. Stelitano, G. Conte, A. Policicchio, A. Aloise, G. Desiderio, R.G. Agostino, Low pressure methane storage in pinecone-derived activated carbons. Energy Fuels 32, 10891–10897 (2018)
E. Mahmoud, Evolution of the design of CH4 adsorbents. Surfaces 3, 433–466 (2020)
F. Béguin, V. Presser, A. Balducci, E. Frackowiak, Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 26, 2219–2251 (2014)
P. Ratajczak, M.E. Suss, F. Kaasik, F. Béguin, Carbon electrodes for capacitive technologies. Energy Stor. Mater. 16, 126–145 (2019)
D. Barrera, M. Florent, M. Kulko, T.J. Bandosz, Ultramicropore-influenced mechanism of oxygen electroreduction on metal-free carbon catalysts. J. Matter. Chem. A. 7, 27110–27123 (2019)
M.J. Remy, G. Poncelet, A new approach to the determination of the external surface and micropore volume of zeolites from the nitrogen adsorption isotherm at 77 K. J. Phys. Chem. 99, 773–779 (1995)
E. Falabella Sousa-Aguiar, A. Liebsch, B.C. Chaves, A.F. Costa, Influence of the external surface area of small crystallite zeolites on the micropore volume determination. Microp. Mesop. Mater. 25, 185–192 (1998)
S. Brouwer, J.C. Groen, L.A.A. Peffer, The impact of mesoporosity on microporosity assessment by CO2 adsorption. Stud. Surf. Sci. Catal. 160, 145–152 (2007)
J. Villarroel-Rocha, D. Barrera, A.A. García Blanco, M.E. Roca Jalil, K. Sapag, Importance of the αS-plot method in the characterization of nanoporous materials. Adsorp. Sci. Technol. 31, 165–183 (2013)
M.M. Dubinin, O. Kadlec, New ways in determination of the parameters of porous structure of microporous carbonaceous adsorbents. Carbon 13, 263–265 (1975)
M.M. Dubinin, The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 60, 235–241 (1960)
J.M. Martín-Martínez, Physical adsorption of gases and vapors by coals, University of Alicante, Doctoral thesis, 1990 http://hdl.handle.net/10045/4291
J. Villarroel-Rocha, Adsorción de diferentes gases y vapores en la caracterización textural y estructural de materiales nanoporosos (Adsorption of different gases and vapors in the textural and structural characterization of nanoporous materials), Universidad Nacional de San Luis, Doctoral thesis, 2015 http://biblioteca.unsl.edu.ar/baea/search2.html?bool=Jhonny%20and%20Villaroel%20and%20Rocha&tipo=&biblio=B1
J. Choma, J. Jagiello, M. Jaroniec, Assessing the contribution of micropores and mesopores from nitrogen adsorption on nanoporous carbons: application to pore size analysis. Carbon 183, 150–157 (2021)
M. Jaroniec, M. Kruk, J.P. Olivier, Standard nitrogen adsorption data for characterization of nanoporous silicas. Langmuir 15, 5410–5413 (1999)
M. Kruk, M. Jaroniec, Accurate method for calculating mesopore size distributions from argon adsorption data at 87 K developed using model MCM-41 materials. Chem. Mater. 12, 222–230 (2000)
M. Kruk, M. Jaroniec, Determination of mesopore size distributions from argon adsorption data at 77 K. J. Phys. Chem. B 106, 4732–4739 (2002)
M. Kruk, M. Jaroniec, K.P. Gadkaree, Nitrogen adsorption studies of novel synthetic active carbons. J. Colloid Interf. Sci. 192, 250–256 (1997)
J. Jagiello, C. Ania, J.B. Parra, C. Cook, Dual gas analysis of microporous carbons using 2D-NLDFT heterogeneous surface model and combined adsorption data of N2 and CO2. Carbon 91, 330–337 (2015)
J. Jagiello, J. Kenvin, Consistency of carbon nanopore characteristics derived from adsorption of simple gases and 2D-NLDFT models. advantages of using adsorption isotherms of oxygen (O2) at 77 K. J. Colloid Interf. Sci. 542, 151–158 (2019)
K. Nakai, M. Yoshida, J. Sonoda, Y. Nakada, M. Hakuman, H. Naono, High resolution N2 adsorption isotherms by graphitized carbon black and nongraphitized carbon black – αS-Curves, adsorption enthalpies and entropies. J. Colloid Interf. Sci. 351, 507–514 (2010)
L. Gardner, M. Kruk, M. Jaroniec, Reference data for argon adsorption on graphitized and nongraphitized carbon blacks. J. Phys. Chem. B 105, 12516–12523 (2001)
M.J.G. De Araújo, J. Villarroel-Rocha, V.C. De Souza, K. Sapag, S.B.C. Pergher, Carbon foams from sucrose employing different metallic nitrates as blowing agents: application in CO2 capture. J. Anal. Appl. Pyrolysis 141, 104627 (2019)
J. Villarroel-Rocha, D. Barrera, J.J. Arroyo-Gómez, K. Sapag, Insights of adsorption isotherms with different gases at 77 K and their use to assess the BET area of nanoporous silica materials. Adsorption 27, 1081–1093 (2021)
J.J. Garrido, J.M. Martín-Martínez, M. Molina-Sabio, F. Rodríguez-Reinoso, R. Torregosa, Adsorption of hydrocarbons on CO2-reacted activated carbons. Carbon 24, 469–475 (1986)
Acknowledgements
The authors wish to thank UNSL, ANPCyT and CONICET for the financial support of this work. The authors also thank Dr. Jacek Jagiello from Micromeritics Instrument Corporation for providing the reference isotherm data of CABOT BP280 for CO2 and O2 at 273 and 77 K, respectively.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
J.V.R and K.S.: conceptualization; J.V.R: methodology; J.V.R, J.J.A.G., and D.B.: formal analysis; K.S.: resources; J.V.R, J.J.A.G., and D.B.: data curation; J.V.R, J.J.A.G., and D.B.: writing original draft; J.V.R, J.J.A.G., D.B., and K.S.: writing‒review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Declarations
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Villarroel-Rocha, J., Arroyo-Gómez, J.J., Barrera, D. et al. A new and reliable method to obtain micropore volume in nanoporous solids by gas adsorption based on Dubinin works and the thickness of the adsorbed layer. J Porous Mater 31, 1111–1120 (2024). https://doi.org/10.1007/s10934-024-01573-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-024-01573-0