Abstract
In this paper, Ag nanoparticles wrapped by N-doped carbon (Ag@NC) was successfully synthesized via a simple aging-pyrolysis-replacement strategy by using PVP/ZIF-8 as precursor. The synthesized Ag@NC was used as electrocatalyst for hydrogen evolution reaction (HER). After being carefully researched, Ag@NC exhibits excellent HER activity with a low onset potential of 189 mV and a small Tafel slope of 68.62 mV dec−1. Ag@NC also shows outstanding long-term stability. The superior HER performance of Ag@NC may be attributed to the synergistic effects between the Ag nanoparticles and N-doped carbon matrix. Ag@NC is expected to be used in the energy chemistry field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Traditional energy, such as oil, coal, natural gas, petroleum, kerosene, have been widely used in our live and industry during the past few decades. They highly improve our living standard. But they also produce harmful byproducts during consumption, such as SiO2, NOx and CO [1, 2]. It is urgent to generate clean and renewable energy source. Hydrogen, as one of eco-friendly and sustainable energies, is possible to replace the traditional energy and can be obtained by splitting water via electrochemical method [3, 4]. As an efficient way to produce hydrogen, hydrogen evolution reaction (HER) has attracted great attention and been widely studied. Many researchers have devoted themselves to exploring catalysts with superior HER performance. For example, Zhou et al., adopted hydrothermal reaction and subsequent selenylation process to fabricate self-assembled CoSe2 nanocrystals embedded into carbon nanowires (CoSe2@CNW)s electrocatalyst, which exhibited with a small Tafel slope of 41.3 mV dec−1 and a low onset potential of ~ 130 mV [5]. Chu and his partners have successfully synthesized carbon coated nickel-nickel oxide composites (Ni/NiO@C/GR-t-w) by directly carbonization of the composites of Ni-MOF-74 and graphene oxide (GO). They found that when the composites contained 8% of GO and carbonized at 900 °C, it exhibited the best electrocatalytic properties with an lower overpotential of 108 mV and a smaller Tafel slope of 44 mV dec−1 [6].
Metal-organic frameworks (MOFs), constructed by metal centers and organic ligands, have large surface areas, tunable pore size, regular void spaces [7,8,9]. They are widely used in various fields, such as drug delivery, as storage/separation, and catalysis [10,11,12]. Many researchers also adopted MOFs materials as precursors to fabricate metals/carbon hybrid materials through directly carbonization them at high temperature under inert atmosphere and explored their electrocatalytic properties [13, 14]. They found that this metals/carbon hybrid materials have high surface area and exhibit excellent conductive and catalytic performances. For example, Lin et al., synthesized MOFs derived cobalt diselenide (MOF-CoSe2) via in-situ selenization of Co-based MOFs and explored its HER performance. The obtained MOF-CoSe2 exhibits a small Tafel slope of 42 mV dec −1 and a low onset potential of 150 mV [15]. Xu and his cooperators adopted the Mo-based polyoxometalate-anion-incorporated as precursor to synthesize carbon-layer-coated Ni-decorated hollow molybdenum carbide structures [16]. The synergistic effect of the unique composition and configuration makes it exhibiting remarkable HER properties and stabilities.
Here, we, for the first time, adopted PVP/ZIF−8 as precursor to prepare Ag nanoparticles wrapped by N-doped carbon (Ag@NC) via a simple aging-pyrolysis-replacement strategy and explored its catalytic properties when it was used as catalyst for HER. The organic ligands of PVP/ZIF−8 were removed and transformed into porous carbon at high temperature. Zn2+, containing in ZIF−8, was reduced into Zn at high temperature under H2/Ar atmosphere. Then, Zn atoms, containing in porous carbon, were replaced by Ag + to prepare Ag nanoparticles wrapped by N-doped carbon (Ag@NC). Ag nanoparticles act as active sites and porous carbon can transport of ions and electrons when Ag@NC is used as electrocatalyst. After being carefully researched, Ag@NC exhibited excellent HER properties with a low onset potential of 189 mV and a small Tafel slope of 68.62 mV dec−1. Ag@NC also shown long-term stability.
2 Experimental
2.1 Chemicals
2-methylimidazole (C4H6N2), zinc nitrate hexahydrate (Zn(NO3)2·6H2O), silver nitrate (AgNO3), methanol, ethanol, and polyvinyl pyrrolidone (PVP, k30) were all analytical reagent and obtained from Sinopharm Chemical Reagent Co. Ltd. All of chemicals were directly used without any further treatment. Distilled water was used in this experiment.
2.2 Preparation of Ag nanoparticles wrapped by N-doped carbon
Ag nanoparticles wrapped by N-doped carbon (Ag/NC, NC is the N-doped carbon) was prepared including the fabrication of PVP/ZIF-8, carbonization of PVP/ZIF-8, formation of Ag@NC.
The fabrication of PVP/ZIF-8: Firstly, 3.32 g of C4H6N2 and 10 g of PVP were dissolved in 100 ml of CH3OH to form a homogeneous A solution. 3 g of Zn(NO3)2·6H2O was dissolved in 100 ml of CH3OH to form a homogeneous B solution. Then, A solution was slowly dropped into A solution under vigorous stirring. The mixture solution were aged at room temperature for 20 h to form PVP/ZIF-8. Finally, the formed PVP/ZIF-8 was centrifuged, washed with ethanol for three times, and dried at 60 °C for 6 h.
Carbonization of PVP/ZIF-8: The formed PVP/ZIF-8 was directly carbonized at 800 °C for 2 h with the heating rate of 2 °C/min under H2/Ar [V(H2): V(Ar) = 1:9] atmosphere to prepare the Zn@NC nanoparticles.
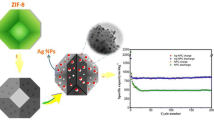
Formation of Ag@NC: Ag/NC nanoparticles were prepared through replacement reaction. Firstly, 0.128 g of AgNO3 was dissolved in a solution which was composed of 27 ml of ethanol and 3 ml of distilled water. Then, 0.5 g of Zn/NC was dispersed in the above solution and kept stirring for 30 min to form the Ag/NC nanoparticles. The formed Ag/NC nanoparticles were washed with ethanol and distilled water for three times and dried at 60 °C for 6 h. The schematic diagram for the formation of Ag@NC is shown in Fig. 1.
2.3 Characterization
The crystallinity and phase of the synthesized samples were analyzed via X-ray powder diffraction (XRD) data, which were obtained from a Bruker-Axsd8 diffractometer using Cu Ka radiation, operated at 40 kV and 40 mA. by The morphology of all samples were directly observed under scanning electron microscopy (SEM, a Hitachi SU70) with an accelerating voltage of 20 kV. The internal structure was obtained from the transmission electron microscopy (TEM, F30) operated at 200 kV. X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, ESCALAB 250) was used to analyze the elemental compositions of the synthesized samples.
2.4 Electrochemical characterization
The electrocatalytic properties of the synthesized catalysts were evaluated on an electrochemical station (CHI660D) using a typical three-electrode system in 1.0 M KOH solution. During preparation the working electrode, 10 mg of catalyst was homogeneously dispersed in 100 µL of solution which consisted of 90 µL of alcohol and 10 µL of Nafion (5%w/w in water) via ultrasonic processing. 10 µL of the above solution was dropped onto a glassy carbon electrode (GCE, 3 mm in diameter) and dried at room temperature to prepare the working electrode. An Ag/AgCl-saturated KCl was used as reference electrode. A Pt wire was used as counter electrode. Before HER measurement, the dissolved O2 in the electrolyte was excluded by continuous bubbling into the N2 for 30 min. Polarization curves were obtained according to the sweeping electrode potentials from 0 to − 0.4 V at a potential sweep rate of 2 mV s−1. The stability of the catalyst was evaluated by continuous measurement at 200 mV vs. RHE for 12 h. All date are presented with 95% iR compensation.
3 Results and discussion
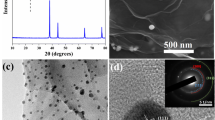
The crystallinity and phase of the synthesized samples were analyzed via X-ray powder diffraction (XRD). The results are shown in Fig. 2. As shown in Fig. 1a, Zn@NC just shown a broad band at about 26.1o, which is belonged to the diffraction of carbon [17]. There is no other peaks corresponding to Zn. It may be ascribed to the low content of Zn. Figure 1b is the XRD patterns of Ag@NC. Except to the broad band of carbon, there are new peaks at 38.12, 44.28, 64.43, and 77.47o, which are ascribed to the (111), (200), (220), and (311) reflections of Ag [18]. It indicates that Ag is successfully loaded on the catalyst.
Figure 3 shows the SEM images of Zn@NC and Ag@NC. Figure 3a is the SEM image of Zn@NC. Zn@NC nanoparticles exhibit the dodecahedral morphology. The particle size of Zn@NC is about 250 nm. From the previous research, we can know that PVP can help to keep the dodecahedral morphology of ZIF-8 at high temperature [19]. After being treated by 25 mM AgNO3, the nanoparticels keep the original morphology and exhibit the dodecahedral morphology (Fig. 3b).
Furthermore, XPS was used to investigate the surface composition and valence states of Ag@NC. The results is shown in Fig. 4. Figure 4a is the XPS survey spectrum of Ag@NC. It can be seen that Ag@NC is mainly composed of C, Ag, and N elements. O may be coming from the adsorbed oxygen. Figure 4b is the high-resolution XPS spectra of Ag 4d. It shows two different characteristic peaks at 368.48 and 374.33 eV, which are ascribed to the metallic Ag 3d5/2 and Ag 3d3/2, respectively [20]. There are no characteristic peaks belonging to oxidation state of Ag.
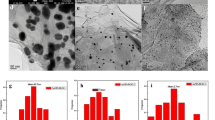
In addition, the internal structure of Ag@NC was directly observed on transmission electron microscopy. The results are shown in Fig. 5. Figure 5a is the low-magnification TEM image of Ag@NC nanoparticles. Ag@NC nanoparticles exhibit the dodecahedral morphology, which is in accordance with the SEM image (Fig. 3b). There are some black dots containing in Ag@NC nanoparticles. Figure 5b is the high-magnification TEM image of Ag@NC. It can be seen that the black dots show the lattice fringe of 0.205 nm, which is corresponding to the (200) space of Ag [21].
Zn@NC and Ag@NC were deposited on glassy carbon electrode and evaluated their HER performance using a typical three-electrode system in 1.0 M KOH solution, respectively. The HER performance of Zn@NC and Ag@NC are shown in Fig. 6. Figure 6a is the linear sweep voltammetry (LSV) curves, which are obtained at a scan rate of 2 mV s−1. Zn@NC shows very poor HER activity and needs the overpotential of 271 mV to drive 10 mA cm−2. Interestingly, Ag@NC exhibits an excellent HER activity and only demands overpotential of 189 mV to drive 10 mA cm−2. It may be attributed the rich catalytically active sites of the stable porous carbon. Ag can highly improve the HER activity of the catalyst. Figure 6b is the Tafel plots according to the LSV curves. Figure 5b is the Tafel plots of the catalysts according to their LSV curves (Fig. 6a). Zn@NC shows a Tafel slope of 92.34 mV dec−1. Noticeably, Ag (Ag@NC) exhibits a The Tafel slope of 68.62 mV dec−1, implying more favorable catalytic kinetics on Ag@NC.
The stability of Ag@NC was evaluated by continuous measurement of its current density for 12 h under 200 mV. The result is shown in Fig. 7. It can be seen that the current density just exhibits little change after 12 h of continuous measurement. It indicates that Ag@NC still has the excellent electrocatalytic performance for at least 12 h, implying the outstanding stability of Ag@NC.
4 Conclusions
In summary, we successfully synthesized Ag nanoparticles wrapped by N-doped carbon (Ag@NC) via simple hydrothermal treatment and replacement reaction by using PVP/ZIF-8 as precursor. Ag nanoparticles are small in size (about 15 nm) and well distributed in the N-doped carbon. Ag nanoparticles wrapped by N-doped carbon can effectively avoid the the aggregation of Ag nanoparticles during the hydrogen evolution reaction process. After being well tested, Ag@NC exhibits superior HER activity with overpotential of 189 mV at 10 mA cm−2 in the 1.0 M KOH solution and shows a Tafel slope of 68.62 mV dec−1. Except that, Ag@NC also shows outstanding stability. After 12 h of continuous measurement, the current density of Ag@NC just exhibits little change. The excellent HER performance of Ag@NC may be attributed to the synergistic effects between the Ag nanoparticles and N-doped carbon.
References
S. Kumar, V.C. Srivastava, S.M. Nanoti, Extractive desulfurization of gas oils: a perspective review for use in petroleum refineries. Sep. Purif. Rev. 46, 319–347 (2017)
M.A. Adnan, M.M. Azis, M.R. Quddus, M.M. Hossain, Integrated liquid fuel based chamical looping combustion-parametric study for efficient power generation and CO2 capture. Appl. Energ. 228, 2398–2406 (2018)
A. Eftekhari, Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrog. Energ. 42, 11053–11077 (2017)
Y.J. Zheng, L. Zhang, H.L. Huang, F.B. Wang, L.C. Yin, H.W. Jiang, D. Wang, J. Yang, G.H. Zuo, ZIE-67-derived Co, Ni and S co-doped N-enriched porous carbon polyhedron as an efficient electrocatalyst for oxygen evolution reaction (OER). Int. J. Hydrog. Energy 44, 27465–27471 (2019)
K. Zhou, J. He, X. Wang, J. Lin, Y. Jing, W. Zhang, Y. Chen, Self-assembled CoSe2 nanocrystals embedded into carbon nanowires as highly efficient catalyst for hydrogen evolution reaction. Electrochim. Acta 231, 626–631 (2017)
M. Chu, L. Wang, X. Li, M. Hou, N. Li, Y. Dong, X. Li, Z. Xia, Y. Lin, W. Cai, C. Zhang, Carbon coated nickel-Nickel oxide composites as a highly efficient catalyst for hydrogen reaction in acid medium. Electrochim. Acta 264, 284–291 (2018)
L. Zhu, X.Q. Liu, H.L. Jiang, L.B. Sun, Metal-organic frameworks for heterogeneous basic catalysis. Chem. Rev. 117, 8129–8176 (2017)
H. Kim, S. Yang, S.R. Rao, S. Narayanan, E.A. Kapustin, H. Furukawa, A.S. Umans, O.M. Yaghi, E.N. Wang, Water harvesting from air with metal-organic frameworks powered by nature sunlight. Science 356, 430–434 (2017)
Q.G. Zhai, X. Bu, X. Zhao, D.S. Li, P. Feng, Pore space partition in metal-organic frameworks. Acc. Chem. Res. 50, 407–417 (2017)
H. Zheng, Y. Zhang, L. Liu, W. Wan, P. Guo, A.M. Nyström, X. Zou, One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 138, 962–968 (2016)
M.I. Nandasiri, S.R. Jambovane, B.P. McGrail, H.T. Schaef, Adsorption, separation, and catalytic Properties of densified metal-organic frameworks. Coordin. Chem Rev. 311, 38–52 (2016)
S.M.J. Rogge, A. Bavykina, J. Hajek, A.I. Olios-Suarez, A. Sepúlveda-Escribano, A. Vimont, G. Clet, P. Bazin, F. Kapteijin, M. Daturi, E.V. Ramos-Fernadez, V.V. Speybroeck, J. Gascon, Metal-organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 46, 3134–3184 (2017). (Llabrés i Xamena)
J. Meng, C. Niu, L. Xu, J. Li, X. Liu, X. Wang, Y. Wu, X. Xu, W. Chen, Q. Liu, Z. Zhu, D. Zhao, I. Mai, General organic formation of carbon nanotubes from metal-organic frameworks. J. Am. Chem. Soc. 139, 8212–8221 (2017)
N. Wu, D. Xu, Z. Wang, F. Wang, J. Liu, W. Liu, Q. Shao, H. Liu, Q. Gao, Z. Guo, Achieving superior electromagnetic wave absorbers through the novel metal-organic frameworks derived magnetic porous carbon nanorods. Carbon 145, 433–444 (2019)
J. Lin, J. He, F. Qi, B. Zheng, X. Wang, B. Yu, K. Zhou, W. Zhang, Y. Li, Y. Chen, In-situ selenization of Co-based metal-organic frameworks as a highly efficient electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 247, 258–264 (2017)
X. Xu, F. Nosheen, X. Wang, Ni-decorated molybdenum carbide hollow structure derived from carbon-coated metal-organic framework for electrocatalytic hydrogen evolution reaction. Chem. Mater. 28, 6313–6320 (2016)
X. Yang, J. Chen, W. Yang, H. Lin, X. Luo, Influence of Zn and Co co-doping on oxygen evolution reaction electrocatalysis at MOF-derived N-doped carbon electrodes. Inorg. Chem. Front. 6, 3475–3481 (2019)
W. Ding, L. Zhao, H. Yan, X. Wan, X. Liu, X. Zhang, X. Huang, R. Hang, Y. Wang, X. Yao, B. Tang, Bovine serum albumin assisted synthesis of Ag/Ag2O/ZnO photocatalyst with enhanced photocatalytic activity under visible light. Coll. Surf. A. 568, 131–140 (2019)
N.L. Liu, S. Dutta, R.R. Salunkhe, T. Ahamad, S.M. Alshehri, Y. Yamauchi, C.H. Hou, K.C.W. Wu, ZIF-8 derived, nitrogen-doped porous electrodes of carbon polyhedron particles for high performance electrosorption of salt ions. Sci. Rep.-UK. 6, 28847 (2016)
X. Li, G. Zhu, L. Xiao, Y. Liu, Z. Ji, X. Shen, L. Kong, S.A. Shah, Loading of Ag on Fe-Co-S/N-doped carbon nanocomposite to achieve improved electrocatalytic activity for oxygen evolution reaction. J. Alloy. Compd. 773, 40–49 (2019)
G. Wang, Y. Sui, M. Zhang, F. Du, B. Zou, Ag ion kinetically tailored surface and interface engineering of Cu2O nanocrystals to modulate the Li-ion battery performance. J. Alloy. Compd. 774, 668–676 (2019)
Acknowledgements
The authors wish to acknowledge the eceshi (www.eceshi.cn) for the XPS analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, X., Li, Y. Ag nanoparticles wrapped by N-doped carbon as an efficient electricatalyst for hydrogen evolution reaction. J Porous Mater 27, 1213–1218 (2020). https://doi.org/10.1007/s10934-020-00899-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-020-00899-9