Abstract
For N2-to-NH3 fixation, electrocatalytic N2 reduction has become one promising alternative to Haber–Bosch process for its superior environmental protection and low-energy consumption. During this electrochemical conversion, adopting a suitable catalytic material is frequently the first consideration that involves in cost and production efficiency. Herein, Ag nanoparticles-reduced graphene oxide hybrid (Ag NPs-rGO) is designed as a novel Ag-based catalyst that exhibits high-efficiency electrocatalytic performance for N2-to-NH3 conversion. Introducing rGO into the catalyst results in a significant improvement of catalytic activity, for the advanced electrical conductivity of rGO and dispersed Ag NPs anchored on this support material. In a 0.1 M Na2SO4 solution, the Ag NPs-rGO delivers Faradaic efficiency of 3.60% and NH3 yield of 18.86 μg h−1 mg−1 cat. at − 0.7 V versus reversible hydrogen electrode (RHE). By contrast, the Ag NPs attains inferior Faradaic efficiency of 2.25% with NH3 yield of 9.43 μg h−1 mg−1 cat. (− 0.7 V vs RHE). The Ag NPs-rGO also maintains good stability and sustainability during the electrocatalytic process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the most general nitrogen compounds, ammonia (NH3) is widely utilized in the fields of the chemical industry, agriculture, medicine, etc. [1,2,3]. However, the conversion from N2 to NH3 is difficult to accomplish for the high N≡N bond energy and low polarizability of N2 [4,5,6]. At present, it remains to be the most effective technique to synthesize NH3 according to Haber–Bosch process at harsh conditions, meanwhile accompanied with high energy consumption and heavy emissions of CO2 [7, 8]. Therefore, it is essential to develop a both eco-friendly and sustainable method for producing NH3 to replace the Haber–Bosch process.
In recent reports, electrochemical nitrogen reduction reaction (NRR) under ambient conditions has the tendency to be conducted as a novel approach for efficient electrocatalytic N2-to-NH3 conversion [9,10,11,12,13,14,15]. During this process, the selection of suitable electrocatalysts plays an important role in ammonia production efficiency. A large number of the studies reported in the past decade have shown that noble metal materials (Au, Pd, Ru, etc.) can greatly promote N2 reduction reaction [16,17,18,19]. Nevertheless, the defects such as scarcity and high cost of these noble metals could obstruct their application in large-scale ammonia production [20, 21]. Among these noble metal electrocatalysts, Ag is the most promising electrocatalyst due to its lowest price and high catalytic activities, which has been applied in electrochemical hydrogen evolution reaction (HER) or oxygen evolution reaction (OER) [22,23,24]. But the severe self-aggregation of small-size Ag nanoparticles leads to not only the decrease in activity but also reduction in conductivity, resulting in a poor electrocatalytic performance [25,26,27].
Reduced graphene oxide (rGO), a novel two-dimensional carbon material, exhibits super-high surface area and excellent chemical properties, which is an ideal support material for nanoparticles to be well dispersed [28, 29]. Moreover, the unique π–π conjugate sp2 carbon structure of graphene provides an effective path for electron migration, endowing graphene nanocomposites with strengthened conductivity [30, 31]. These advantages of rGO contribute to a tremendous possibility in promoting the electrocatalytic activity of metallic Ag at NRR, which has not been reported before.
In this investigation, Ag nanoparticles-reduced graphene oxide hybrid (Ag NPs-rGO) is synthesized as an improved Ag-based catalyst for electrochemical N2 reduction. In a 0.1 M Na2SO4 electrolyte, the Ag NPs-rGO attains the NH3 yield of 18.86 μg h−1 mg−1 cat. and Faradaic efficiency (FE) of 3.60% at − 0.7 V vs. reversible hydrogen electrode (RHE), evidently higher than that of Ag NPs (9.43 μg h−1 mg−1 cat., 2.25%). In addition, this catalyst shows good stability and excellent selectivity during the NRR experiment.
Results and discussion
Ag NPs-rGO is prepared by a modified method of hydrothermal reaction (see the Supporting Information for preparation details) [32]. Figure 1a shows the X-ray diffraction (XRD) patterns of Ag NPs and Ag NPs-rGO. The XRD pattern for Ag NPs shows diffraction peaks at 38.1°, 44.3°, 64.4° and 77.3° that correspond to the (111), (200), (220) and (311) planes of the Ag phase (PDF No. 87-0597), respectively. Ag NPs-rGO also shows the coincident peaks characteristic of Ag, and the weak diffraction peak at about 23.3° is ascribed to the (002) plane of few-layer rGO. The result indicates that GO was successfully reduced and the precursor of silver was decomposed into metallic silver through the hydrothermal process. The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of Ag NPs-rGO are shown in Fig. 1b and c, proving the formation of well-dispersed Ag NPs adsorbed on the surface of rGO through reduction reaction. The high-resolution TEM (HRTEM) image (Fig. 1d) reveals well-resolved lattice fringes with an interplanar distance of 0.232 nm of Ag NPs-rGO, which is matched to the (111) plane of Ag NPs. Selected area electron diffraction (SAED) image (inset in Fig. 1d) shows the polycrystalline structure of Ag NPs-rGO indexed to the (111), (200) and (311) planes of the Ag phase.
Raman spectroscopy is an important method to characterize graphene-based materials and has the advantage of nondestructive testing [33]. The Raman spectra of GO and Ag NPs-rGO are presented in Fig. 2a at a spectral range of 1200–2000 cm−1. Obviously, two feature peaks at ~ 1350 cm−1 and at ~ 1593 cm−1 are attributed to D band (corresponding to the disorder sp3 carbon) and G band (corresponding to the graphitic sp2 carbon) of both GO and Ag-rGO samples [34]. Since D band is related to defects of graphene, the intensity ratio of D band to G band (ID/IG) can be utilized as an index of disorder in GO or rGO. Ideally, the defects will decrease after reduction of GO, with a lower value of ID/IG [33]. From the calculation, the ratio of ID/IG increases from 0.92 of GO to 1.02 of Ag NPs-rGO. The reverse result is probably due to the reason that the oxygen-containing functional groups were reduced, and the conjugated sp2 carbon was reconstructed with smaller size than the original one [35]. Figure 2b displays the X-ray photoelectron spectroscopy (XPS) survey spectrum of Ag NPs-rGO, implying the presence of Ag, N, O and C elements. In the region of C 1s (Fig. 2c), three peaks at the BEs of 284.8 eV, 286.2 eV and 287.8 eV correspond to the sp2-hybridized carbon (C–C/C=C), C–O, and C=O, respectively [36]. The weaker peaks of the carbon–oxygen species signals in Ag NPs-rGO demonstrate a high degree of deoxygenation and successful reduction from the GO to rGO. The Ag 3d spectra (Fig. 2d) shows two peaks of Ag0 3d5/2 (368.2 eV) and Ag0 3d3/2 (374.2 eV) at both Ag NPs and Ag NPs-rGO, indicating that the synthesized Ag is mostly at zero valence state [32, 37].
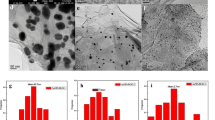
Under ambient conditions, 0.1 mg Ag NPs-rGO was loaded on a 1 × 1 cm2 carbon paper as the working electrode for NRR experiment with continuous N2 inputting in 0.1 M Na2SO4. All potentials at the analytical process were converted to a RHE scale. Figure S1 shows the linear sweep voltammetry (LSV) curve for Ag NPs-rGO in Ar- and N2-saturated 0.1 M Na2SO4 solution. It is evident that Ag NPs-rGO attains a high current density in the N2-saturated solution, revealing that it is active for the electrocatalytic reaction to N2. As shown in Fig. 3a, the time-dependent current density curves at different potentials keep relative stability under 2 h of electrolysis. The production of NH3 was determined by using the indophenol blue method [38], and the corresponding calibration curve is presented in Fig. S2. Figure 3b shows the UV–Vis absorption spectra of catalytic electrode stained with the indophenol indicator after NRR electrocatalysis for 2 h with potential ranging from − 0.5 to − 0.9 V. Then, calculated by the linear formula of fitting curve, NH3 yields and FEs of Ag NPs-rGO at different potentials are obtained in Fig. 3c. The maximum NH3 yield rate of 18.86 μg h−1 mg−1 cat. and a high FE of 3.60% can be reached at − 0.7 V, while the Ag NPs-rGO shows an excellent performance compared with most reported aqueous-based NRR electrocatalysts listed in Table 1 [10, 11, 39,40,41,42,43,44]. When the applied potential is at − 0.5 V, Ag NPs-rGO achieves a high FE of 4.77% due to its minimal current density. But the inferior NH3 yield of 9.43 μg h−1 mg−1 cat. suggests its unsatisfactory catalytic activity for NRR at this potential. From previous reports, the activity of carbon materials would lead to the possibility of electrocatalytic properties at NRR experiments [10, 11, 22]. To further testify the optimal performance of Ag NPs-rGO, comparative experiments were performed with Ag NPs, rGO and blank CP at − 0.7 V or at open-circuit (OC) potential. As shown in Fig. 3d and Fig. S4a, the NH3 yields and FEs of CP (2.96 μg h−1 mg−1 cat., 0.75%), rGO (3.62 μg h−1 mg−1 cat., 0.77%) and Ag NPs (9.43 μg h−1 mg−1 cat., 2.25%) exhibit deficient catalytic activities for NRR, compared with Ag NPs-rGO. For the unusual NH3 production of blank CP, it can be attributed to the possible activated C, significant conductivity or other catalytic elements. XPS analysis of CP indicates the presence of abundant F that is frequently doped into catalysts for NRR experiments (Fig. S3) [45,46,47]. In this series of experiments, comparative data at a different atmosphere is vital to eliminate background contributions such as dissolved N2, remnant NH4+ absorbed on Nafion membrane or N-containing species in chemicals. And the experiments under an Ar atmosphere were conducted at the same applied potentials. In Fig. S4b, it is clear that the generated NH3 concentrations at various potentials are extremely approaching 0 μg mL−1 by continuously supplying Ar. Thus, the colorimetric data can not only indicate that the background contributions are almost ignorable, but verify that the N atom of NH3 is generated from N2 via NRR. From the colorimetric data of electrolytes in the anodic compartment, NH4+ crossover is inevitable in Nafion membranes that would cause the final calculated NH3 production rate of catalysts to get a smaller value (Fig. S5). Furthermore, electrochemical double-layer capacitance (Cdl) is related to the active surface area of the electrocatalyst [48]. Thus, high Cdl value of Ag NPs-rGO ensures the sufficient active sites for NRR experiments (Fig. S6). The superiority of Ag NPs-rGO is mostly attributed to the enhancement of rGO to nanoparticles at dispersivity and conductivity. The electrochemical impedance spectroscopy (EIS) data show that Ag NPs-rGO has a smaller radius of the semicircle than that of Ag NPs (Fig. S7). The values of charge transfer resistance (Rct) for Ag NPs-rGO and Ag NPs are 14.84 Ω and 17.86 Ω obtained from the equivalent circuit. Thus, a lower Rct of Ag NPs-rGO exhibits preferable electrical conductivity, causing faster NRR kinetics. Moreover, hydrazine (N2H4) may be a possible by-product in the NRR, which is determined using the method of Watt and Chrisp [49]. Figure S8a and S8b presents UV–Vis absorption spectra with various concentrations of N2H4 and the corresponding calibration curves, respectively. Figure S9 shows that no obvious N2H4 can be detected at various potentials, which proves the excellent selective catalytic activity of Ag NPs-rGO.
a Time-dependent current density curves for Ag NPs-rGO at different potentials in 0.1 M Na2SO4. b UV–Vis absorption spectra of the electrolytes stained with an indophenol indicator after NRR electrolysis at a series of potentials for 2 h. c NH3 yields and FEs for Ag NPs-rGO at a series of potentials in 0.1 M Na2SO4. d NH3 yields and FEs for different electrodes at − 0.7 V or at OC after 2 h of electrolysis under ambient conditions
Furthermore, stability is also an important factor in evaluating the performance of catalysts in long-term utilization. During the six times consecutive cycling tests at − 0.7 V, Ag NPs-rGO has a minimal variation in the NH3 yields and FEs, exhibiting the strong recycling sustainability of the catalyst (Fig. 4a). In Fig. 4b, the time-dependent current density curve at − 0.7 V is maintained at an invariable range all the time. It is inferred that Ag NPs-rGO can hold a stable catalytic activity for at least 24 h, indicating its sustainable stability. For the catalytic mechanism of Ag (111) for NRR, according to previous reports [50, 51], it follows the associative mechanism where the nitrogen molecules are step-by-step reduced with protons and electrons. As illustrated in Fig. 4c, the potential-determining step for NRR on Ag surface is the step of *N2 combined with the first H (where * denotes a surface active site). With the introduced rGO as supporter, the electron transfer reaction will be accelerated and Ag NPs are uniformly distributed on this carbon layers, which maximizes both the exposure of active sites and availability of catalysts.
Conclusions
In summary, Ag NPs-rGO nanocomposites are experimentally conducted as one modified silver-based NRR electrocatalyst under ambient conditions. With the high specific surface area of rGO, the aggregated Ag NPs are dispersedly anchored on the surface of the rGO sheets, leading to more exposure of active sites. Meanwhile, rGO greatly enhances the conductivity of catalyst, increasing its catalytic performance for NRR. At the potential of − 0.7 V versus RHE, Ag NPs-rGO achieves a high NH3 yield rate (18.86 μg h−1 mg−1 cat.) and FE (3.60%) in 0.1 M Na2SO4, accompanied with excellent selectivity and electrochemical stability. This study not only improves the catalytic activity of Ag NPs at the NRR, but also verifies the enormous effect of rGO on nanocatalysts.
References
Foster SL, Bakovic SIP, Duda RD et al (2018) Catalysts for nitrogen reduction to ammonia. Nat Catal 1(7):490–500
Schlogl R (2003) Catalytic synthesis of ammonia-a “never-ending story”? Angew Chem Int Ed Engl 42(18):2004–2008
Rosca V, Duca M, de Groot MT, Koper MTM (2009) Nitrogen cycle electrocatalysis. Chem Rev 109(6):2209–2244
Lu YH, Yang Y, Zhang TF et al (2016) Photoprompted hot electrons from bulk cross-linked graphene materials and their efficient catalysis for atmospheric ammonia synthesis. ACS Nano 10(11):10507–10515
Honkala K, Hellman A, Remediakis IN, Logadottir A, Carlsson A, Dahl S, Christensen CH, Norskov JK (2005) Ammonia synthesis from first-principles calculations. Science 307(5709):555–558
Yu GS, Guo HR, Kong WH et al (2019) Electrospun TiC/C nanofibers for ambient electrocatalytic N2 reduction. J Mater Chem A 7(34):19657–19661
Chu K, Liu YP, Li YB, Wang J, Zhang H (2019) Electronically coupled SnO2 quantum dots and graphene for efficient nitrogen reduction reaction. ACS Appl Mater Interfaces 11(35):31806–31815
Chu K, Liu YP, Li YB, Zhang H, Tian Y (2019) Efficient electrocatalytic N2 reduction on CoO quantum dots. J Mater Chem 7(9):4389–4394
Li PP, Liu ZC, Wu TW et al (2019) Ambient electrocatalytic N2 reduction to NH3 by metal fluorides. J Mater Chem A 7(30):17761–17765
Xia L, Li BH, Zhang Y et al (2019) Cr2O3 Nanoparticle-reduced graphene oxide hybrid: a highly active electrocatalyst for N2 reduction at ambient conditions. Inorg Chem 58(4):2257–2260
Zhang XX, Liu Q, Shi XF, Asiri AM, Luo YL, Sun XP, Li TS (2018) TiO2 nanoparticles-reduced graphene oxide hybrid: an efficient and durable electrocatalyst toward artificial N2 fixation to NH3 under ambient conditions. J Mater Chem A 6(36):17303–17306
Chen SM, Perathoner S, Ampelli C, Mebrahtu C, Su DS, Centi G (2017) Room-temperature electrocatalytic synthesis of NH3 from H2O and N2 in a gas–liquid–solid three-phase reactor. ACS Sustain Chem Eng 5(8):7393–7400
Chen SM, Perathoner S, Ampelli C, Mebrahtu C, Su DS, Centi G (2017) Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst. Angew Chem Int Ed Engl 56(10):2699–2703
Zhang XP, Kong RM, Du HT, Xia L, Qu F (2018) Highly efficient electrochemical ammonia synthesis via nitrogen reduction reactions on a VN nanowire array under ambient conditions. Chem Commun 54(42):5323–5325
Zhang L, Ji XQ, Ren X, Luo YL, Shi XF, Asiri AM, Zheng BZ, Sun XP (2018) Efficient electrochemical N2 reduction to NH3 on MoN nanosheets array under ambient conditions. ACS Sustain Chem Eng 6(8):9550–9554
Shi MM, Bao D, Wulan BR, Li YH, Zhang YF, Yan JM, Jiang Q (2017) Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions. Adv Mater 29(17):1606550
Wang J, Yu L, Hu L, Chen G, Xin HL, Feng XF (2018) Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential. Nat Commun 9:1795
Kordali V, Kyriacou G, Lambrou C (2000) Electrochemical synthesis of ammonia at atmospheric pressure and low temperature in a solid polymer electrolyte cell. Chem Commun 17:1673–1674
Oshikiri T, Ueno K, Misawa H (2016) Selective dinitrogen conversion to ammonia using water and visible light through plasmon-induced charge separation. Angew Chem Int Ed 55(12):3942–3946
Du HT, Guo XX, Kong RM, Qu FL (2018) Cr2O3 nanofiber: a high-performance electrocatalyst toward artificial N2 fixation to NH3 under ambient conditions. Chem Commun 54(91):12848–12851
Bao D, Zhang Q, Meng FL et al (2017) Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle. Adv Mater 29(3):1604799
Ji XQ, Liu BP, Ren X, Shi XF, Asiri AM, Sun XP (2018) P-Doped Ag nanoparticles embedded in N-doped carbon nanoflake: an efficient electrocatalyst for the hydrogen evolution reaction. ACS Sustain Chem Eng 6(4):4499–4503
Hou YH, Liu YP, Gao RQ et al (2017) Ag@CoxP core–shell heterogeneous nanoparticles as efficient oxygen evolution reaction catalysts. ACS Catal 7(10):7038–7042
Ashok A, Kumar A, Matin MA, Tarlochan F (2018) Synthesis of highly efficient bifunctional Ag/Co3O4 catalyst for oxygen reduction and oxygen evolution reactions in alkaline medium. ACS Omega 3(7):7745–7756
Zhou YZ, Yang J, He TT, Shi HF, Cheng XN, Lu YX (2013) Highly stable and dispersive silver nanoparticle-graphene composites by a simple and low-energy-consuming approach and their antimicrobial activity. Small 9(20):3445–3454
Yang YK, He CE, He WJ, Yu LJ, Peng RG, Xie XL, Wang XB, Mai YW (2011) Reduction of silver nanoparticles onto graphene oxide nanosheets with N, N-dimethylformamide and SERS activities of GO/Ag composites. J Nanopart Res 13(10):5571–5581
Begum R, Farooqi ZH, Aboo AH, Ahmed E, Sharif A, Xiao JL (2019) Reduction of nitroarenes catalyzed by microgel-stabilized silver nanoparticles. J Hazard Mater 377:399–408
Wu JS, Pisula W, Mullen K (2007) Graphenes as potential material for electronics. Chem Rev 107(3):718–747
Chen JH, Jang C, Xiao SD, Ishigami M, Fuhrer MS (2008) Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat Nanotechnol 3(4):206–209
Zhang YP, Li HB, Pan LK, Lu T, Sun Z (2009) Capacitive behavior of graphene-ZnO composite film for supercapacitors. J Electroanal Chem 634(1):68–71
Sun XY, Liu X, Shen X, Wu Y, Wang ZY, Kim JK (2017) Reprint of graphene foam/carbon nanotube/poly (dimethyl siloxane) composites for exceptional microwave shielding. Compos A 92:190–197
Meng XH, Shao X, Li HY, Liu FZ, Pu XP, Li WZ, Su CH (2013) One-step hydrothermal synthesis, characterization and visible-light catalytic property of Ag-reduced graphene oxide composite. Mater Res Bull 48(4):1453–1457
He B, Shen YX, Ren ZJ et al (2014) Defect-controlled synthesis of graphene based nano-size electronic devices using in situ thermal treatment. Org Electron 15(3):685–691
Liu FC, Sun YY, Zheng YP, Tang NJ, Li M, Zhong W, Du YW (2015) Gram-scale synthesis of high-purity graphene quantum dots with multicolor photoluminescence. RSC Adv 5(125):103428–103432
Shen JF, Shi M, Yan B, Ma HW, Li N, Ye MX (2011) One-pot hydrothermal synthesis of Ag-reduced graphene oxide composite with ionic liquid. J Mater Chem 21(21):7795–7801
Wang X, Song L, Yang HY, Xing WY, Kandola B, Hua Y (2012) Simultaneous reduction and surface functionalization of graphene oxide with POSS for reducing fire hazards in epoxy composites. J Mater Chem 22(41):22037–22043
Zhou YZ, Yang J, Cheng XN, Zhao N, Sun HB, Li D (2013) Transparent and conductive reduced graphene oxide/silver nanoparticles multilayer film obtained by electrical self-assembly process with graphene oxide sheets and silver colloid. RSC Adv 3(10):3391–3398
Zhu D, Zhang LH, Ruther RE, Hamers RJ (2013) Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat Mater 12(9):836–841
Ji L, Shi SF, Asiri AM, Zheng BZ, Sun XP (2018) Nanostructured bromide-derived Ag film: an efficient electrocatalyst for N2 reduction to NH3 under ambient conditions. Inorg Chem 57(23):14692–14697
Huang HH, Xia L, Shi SF, Asiri AM, Sun XP (2018) Ag nanosheets for efficient electrocatalytic N2 fixation to NH3 under ambient conditions. Chem Commun 54(81):11427–11430
Lee HK, Koh CSL, Lee YH, Liu C, Phang IY, Han XM, Tsung CK, Ling XY (2018) Favoring the unfavored: selective electrochemical nitrogen fixation using a reticular chemistry approach. Sci Adv 4(3):3208
Gao WY, Hao YC, Su X et al (2019) Morphology-dependent electrocatalytic nitrogen reduction on Ag triangular nanoplates. Chem Commun 55(72):10705–10708
Huang H, Gong F, Wang Y et al (2019) Mn3O4 nanoparticles@reduced graphene oxide composite: an efficient electrocatalyst for artificial N2 fixation to NH3 at ambient conditions. Nano Res 12(5):1093–1098
Xie HT, Geng Q, Li X et al (2019) Ceria-reduced graphene oxide nanocomposite as an efficient electrocatalyst towards artificial N2 conversion to NH3 under ambient conditions. Chem Commun 55(72):10717–10720
Liu YP, Li YB, Zhang H, Chu K (2019) Boosted electrocatalytic N2 reduction on fluorine-doped SnO2 mesoporous nanosheets. Inorg Chem 58(15):10424–10431
Zhu XJ, Liu ZC, Wang HB et al (2019) Boosting electrocatalytic N2 reduction to NH3 on –FeOOH by fluorine doping. Chem Commun 55(27):3987–3990
Zhao JX, Zhang L, Xie XY et al (2018) Ti3C2Tx (T=F, OH) MXene nanosheets: conductive 2D catalysts for ambient electrohydrogenation of N2 to NH3. J Mater Chem A 6(47):24031–24035
Li LQ, Tang C, Xia BQ, Jin HY, Zheng Y, Qiao SZ (2019) Two-dimensional mosaic bismuth nanosheets for highly selective ambient electrocatalytic nitrogen reduction. ACS Catal 9(4):2902–2908
Watt G, Chrisp JD (1952) A spectrophotometric method for the determination of hydrazine. Anal Chem 24(12):2006–2008
Skulason E, Bligaard T, Gudmundsdottir S et al (2012) A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys Chem Chem Phys 14(3):1235–1245
Montoya JH, Tsai C, Vojvodic A, Norskov JK (2015) The challenge of electrochemical ammonia synthesis: a new perspective on the role of nitrogen scaling relations. ChemSusChem 8(13):2180–2186
Acknowledgements
This study was funded by Sichuan Province Science and Technology Program (2018GZ0459).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Xie, H. & Mao, J. Ag nanoparticles-reduced graphene oxide hybrid: an efficient electrocatalyst for artificial N2 fixation to NH3 at ambient conditions. J Mater Sci 55, 5203–5210 (2020). https://doi.org/10.1007/s10853-020-04371-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04371-6