Abstract
Low-lying Arctic coastal environments are threatened by marine storm surges, which are predicted to increase in frequency and intensity as a result of decreasing sea ice, rising sea levels and altered intensity and frequency of storm activity. The Mackenzie Delta of Canada’s Northwest Territories, a vast, low-lying wetland ecosystem, is particularly susceptible to such storm surges, because much of the outer alluvial plain is below 2-m elevation. A large storm-surge event in September 1999 flooded >13,000 ha of alluvial terrain and impacted the terrestrial and freshwater ecosystems of the region. Previous research on the limnological impacts of the storm surge recorded a shift from freshwater to brackish diatom taxa, and a change in cladoceran assemblages to more saline-tolerant species. We examined the remains of Chironomidae (Insecta, Diptera) in sediment cores from two lakes impacted by the 1999 saltwater inundation to determine whether the storm surge also affected benthic macroinvertebrate communities, which are particularly important to lake ecosystem function in Arctic regions. We observed an increase in the relative abundance of saline-tolerant taxa in the two impacted lakes, including Paratanytarsus and Cricotopus/Orthocladius, and decreases in saline-intolerant Sergentia and Corynocera oliveri-type, coincident with the 1999 storm. We observed no major assemblage changes after 1999 in a control lake located beyond the zone of inundation. The number of head capsules recovered from sediments of the impacted lakes increased after the 1999 storm, suggesting no negative impact on overall chironomid abundance as a result of the shift to brackish conditions. There has, however, been no recovery of the chironomid community to the pre-1999 composition. Earlier assemblage changes in both impacted lakes likely tracked regional climate warming in the region, known to have begun in the late nineteenth century.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climate change will impact low-lying coastal ecosystems through sea-level rise and intensified storm activity (Church et al. 2013). In the circumpolar Arctic, decreased sea-ice extent further exacerbates the risk of storm surge impacts (Pisaric et al. 2011; Kokelj et al. 2012; Vermaire et al. 2013). The potential for inundation of low-lying Arctic environments is increased by the longer duration and extent of open-water periods from sea ice loss (Serreze et al. 2007), resulting in greater fetch and wave action (Lintern et al. 2011; Overeem et al. 2011). The potential for storm surge impacts is compounded by rising sea levels (Nicholls and Cazenave 2010) and more frequent and intense Arctic storms (Comiso 2006; Comiso et al. 2008; Sepp and Jaagus 2011). Of particular note is the increase in the strength of late-season storms related to decreasing sea ice (Simmonds and Keay 2009).
The Mackenzie Delta region (NT, Canada) is the world’s second largest Arctic delta ecosystem, and a wetland of global significance (Burn and Kokelj 2009). The low-lying outer Mackenzie Delta alluvial plain, much of which is <2 m above mean sea level, makes this lake-rich, freshwater environment particularly susceptible to the impacts of storm surges (Rampton 1988). A large storm surge in September 1999 was among the most intense on record (Manson and Solomon 2007), resulting in the highest water level ever recorded at the delta front (Kokelj et al. 2012). The storm surge and associated saltwater inundation resulted in severe impacts to at least 13,000 ha of alluvial vegetation, flooding the majority of the landscape within 20–30 km of the coast (Pisaric et al. 2011; Kokelj et al. 2012). The recent increase in the frequency of storm surges that impact the outer Mackenzie Delta region has been shown to be strongly linked to changes in sea ice and climate (Vermaire et al. 2013). Aquatic ecosystems across the outer delta were impacted (Thienpont et al. 2012), with both diatom (Pisaric et al. 2011; Thienpont et al. 2012) and cladoceran communities (Deasley et al. 2012) exhibiting shifts to those tolerant of brackish conditions. These changes were inferred to have been unmatched in the recent past, and in at least one location, unprecedented in the history of the lake. To date, little or no chemical or biological recovery has been documented in affected lakes of the region (Thienpont et al. 2012).
The dipteran family Chironomidae is one of the most widely distributed and abundant groups of insects in freshwater ecosystems, particularly in Arctic regions (Armitage et al. 1995; Bennike et al. 2004). The capacity for different Chironomidae taxa to thrive across a wide range of limnological gradients related to pH, water depth, dissolved oxygen concentration, temperature and salinity, enables species to occupy the vast majority of aquatic environments (Porinchu and MacDonald 2003). The impact of the intense 1999 storm surge on the chironomid communities in the Mackenzie Delta region had not been investigated, which represented a gap in our understanding of the response of biota in these ecosystems to saltwater inundation. Because there is little to no limnological monitoring of lakes on the outer Mackenzie Delta alluvial plain, lake sediment records, as utilized in previous studies (Pisaric et al. 2011; Deasley et al. 2012; Thienpont et al. 2012; Vermaire et al. 2013), are necessary to infer changes that occurred as a consequence of the storm surge event. Chironomid assemblages in lake sediment have been used widely to infer salinity changes in both inland (Walker et al. 1995; Eggermont et al. 2006) and coastal regions (Hofmann 1987; Dickson et al. 2014), but had hitherto not been used to reconstruct past marine flooding frequency in high-latitude regions.
The objectives of this study were to: (1) determine the ecological impact of the 1999 storm surge on the chironomid assemblages in three freshwater lakes of the outer Mackenzie Delta, two of which were subject to saltwater intrusion, and one of which served as an unaffected control site. In particular, we examined whether chironomid taxa were lost from these lakes or if community composition changed as a result of the rapid shift from freshwater to brackish conditions. Second, using paleolimnological approaches, we assessed the resilience of individual chironomid taxa to large, sudden increases in lakewater conductivity, and thereby increased our understanding of chironomid salinity tolerance in coastal Arctic lakes that are vulnerable to marine storm surges. Third, we compared the changes in the chironomid community to those described previously for diatom and cladoceran assemblages in the same sediment cores, to provide a holistic view of biotic change. Given the transient and sensitive nature of delta ecosystems, coupled with changes associated with recent climate warming (Smol and Douglas 2007), saltwater intrusions are likely to have increasingly important ecological impacts on these Arctic systems in the future (Marsh and Schmidt 1993; Kokelj et al. 2012).
Site description
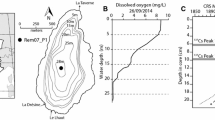
The Mackenzie Delta is comprised of alluvial sediments deposited by the Mackenzie and Peel rivers (Rampton 1988). The outer Delta is a productive alluvial ecosystem that extends approximately 250 km northward from its apex at Point Separation to the Beaufort Sea coast (Fig. 1). The Mackenzie Delta contains approximately 20,000 lakes of different sizes and spans an area of approximately 13,000 km2, making it the largest cold-region delta in North America (Marsh and Schmidt 1993; Burn and Kokelj 2009; Goulding et al. 2009). The delta includes traditional lands of the Inuvialuit and Gwich’in, who rely on subsistence resources from the region. Topographic relief throughout the delta is low and surfaces within 10–20 km of the coast are <1 m above sea level. The low relief that characterises the outer Mackenzie Delta makes the region highly susceptible to storm surges (Cahoon 2006).
Vegetation in the outer Mackenzie Delta is dominated by willow (Salix spp.) and alder (Alnus spp.), which grow on aggrading point bars. Sedges (Cyperaceae) and mosses dominate the poorly drained wetlands (Mackay 1963; Kokelj et al. 2012). The climate is characterized by cold winters that last up to 8 months, and short, cool summers (Burn and Kokelj 2009). Mean annual air temperature and total precipitation at Tuktoyaktuk, the nearest climate station, which is 120 km east of our study sites and has operated since 1948, are −9.8 °C and 151 mm, respectively (Burn and Kokelj 2009). Average summer air temperature for 2011, the most recent record available, was 9.6 °C.
Lake selection was accomplished using a combination of satellite and ground observations. DZO-29 (unofficial name; 69°09′20.5″N, 135°56′52.1″W) is a small (~3.5 ha), moderately shallow (zmax = ~4.1 m) lake with a circular shape (Fig. 1). DZO-30 (unofficial name; 69°09′ 12.5″N, 135°57′ 02.0″W) has a larger surface area (~8.7 ha), is more elongate and shallower (zmax = ~1.8 m). These lakes are located close to each other (Fig. 1), approximately 7 km from the present-day coastline and within the 1999 surge-affected area. Lake C-27 (unofficial name; 69º06′41.64″N, 135º18′15.12″W) is a larger (~258 ha) lake with a maximum depth of ~3 m, located approximately 28 km inland, and beyond the area impacted by the 1999 storm surge (Kokelj et al. 2012). C-27 served as the control site for this study. Specific conductance in the impacted lakes remains elevated (DZO-29: 12,722 µS cm−1, DZO-30: 6,855 µS cm−1) compared to the value in the more distant control lake (C27: 217 µS cm−1). Complete contemporary (2009) water chemistry data are presented in Thienpont et al. (2012).
Materials and methods
Sediment coring and radiometric dating
Sediment cores were collected from Lake C-27 in August 2009, and from Lakes DZO-29 and DZO-30 in July 2010, using a 7.6-cm diameter Glew-type gravity corer (Glew 1989). Cores from each lake were sectioned at 0.25-cm intervals for the top 5 cm of the core, and 0.5-cm intervals for the remainder of the core using a Glew (1988) vertical extruding device, and samples were stored at <4 °C prior to analysis. Select sediment intervals from the C-27 and DZO-30 lake cores were 210Pb-dated by alpha spectroscopy at MyCore Scientific Inc. (Deep River, ON, Canada). For Lake DZO-30, the constant rate of supply (CRS) model was used for sediment age determination (Appleby and Oldfield 1978). Because of the small amount of sediment from DZO-29 that remained following chironomid and other analyses, there was insufficient material for radiometric sediment age determination.
Laboratory and statistical analyses
Sediments were prepared for chironomid analysis using standard procedures (Walker 2001). Samples were gently washed in warm (~30 °C) 5 % KOH for approximately 30 min, then sieved through an 80-µm mesh. For each interval, approximately 50 head capsules (Quinlan and Smol 2001) were picked from the processed sediment using a Bogorov counting chamber, and mounted on microscope slides using Permount™. The amount of wet sediment required to obtain the minimum number (50) of chironomid head capsules recommended for robust statistical analyses (Quinlan and Smol 2001) ranged from 1.0 to 5.0 g. Because of limited sediment availability and low head capsule counts in selected intervals from all three cores, some adjacent 0.25-cm intervals were combined into 0.5-cm intervals. This ensured that all depths in the cores were analyzed throughout and that each interval contained as close to 50 head capsules as possible, while still maintaining reasonably high temporal resolution for analysis. Chironomids were identified to the lowest possible taxomomic level based on head capsule features at 200–400× magnification, using a Nikon Eclipse 80i light microscope and standard texts (Oliver and Roussel 1983; Brooks et al. 2007). Given the abundance of broken, partially missing or disintegrated head capsules, identification in most cases could only be made to the genus level.
Relative abundances of each taxon were determined for each sediment interval. Additionally, the total number of chironomid head capsules per gram dry sediment was calculated to estimate changes in chironomid density throughout the sediment cores. Relative abundance diagrams were prepared for each core (taxa >5 % relative abundance) using the computer program TGView v2.0.2 (Grimm 2011), with taxa ordered from lowest to highest salinity tolerances, based on Walker et al. (1995). Constrained incremental sum of squares (CONISS) cluster analyses (Grimm 1987), based on the complete chironomid datasets for each lake, are included on each stratigraphic plot. Significant biostratigraphic zones were determined by comparison to a broken stick model (Bennett 1996) using the vegan (Oksanen et al. 2010) and rioja (Juggins 2009) packages for the R software environment (R Development Core Team 2011). Detrended correspondence analysis (DCA) was conducted using vegan for R on the complete assemblage data for the three study lakes in order to track changes in DCA axes 1 and 2 over time. In addition, DCA axis 1 scores were determined in the storm surge-impacted lakes for which sediment core dates were available, applied to the chironomids presented here from DZO-30, as well as to the Cladocera from DZO-29 (Deasley et al. 2012) and diatoms from DZO-29 and -30 (Thienpont et al. 2012). Detrended canonical correspondence analysis (DCCA), the direct form of DCA, with species assemblage changes constrained to sediment age as the sole environmental variable, were conducted to estimate biological community turnover for each taxonomic group. Only indicators from inundation-impacted lakes that had dating controls were included in this analysis. This technique was used in previous paleolimnological studies in Arctic regions to assess quantitatively the response of biological indicators to stressors, including climate warming and permafrost thaw (Smol et al. 2005; Thienpont et al. 2013).
Results
A total of 19 chironomid taxa were recovered from the three sediment cores analyzed in this study. In DZO-30, the earliest significant change in the chironomid assemblage occurred at a core depth of approximately 15 cm (Fig. 2). Below a core depth of ~15 cm, the chironomid assemblage was dominated by Cricotopus/Orthocladius van der Wulp (with relative abundances ranging from 20 to 50 %) and Chironomus Meigen (~20 % of identified head capsules) (Fig. 2). Cricotopus/Orthocladius relative abundance decreased steadily from the bottom of the sediment core to 15 cm. Tanytarsus van der Wulp and Psectrocladius Kieffer were also common below 15 cm, with relative abundances ranging from 10 to 20 % (Fig. 2). Other taxa, including Corynocera oliveri-type Zetterstedt, Procladius Skuse, Paratanytarsus Thienemann & Bause, Pseudochironomini Malloch and Sergentia Kieffer, were observed at low (<10 %) abundances in the early part of the DZO-30 record. Beginning at ~15 cm depth, the major change observed in the chironomid assemblage was an increase of C. oliveri-type, from ~10 to >50 % abundance (Fig. 2). This directional increase, beginning in the late nineteenth century, continued until ~4.0 cm, i.e. the late 1990s. During this period, C. oliveri-type was the dominant taxon in the DZO-30 assemblage. Other taxa common in the assemblage during this period include Tanytarsus (10–20 %), Chironomus (10–25 %), Paratanytarsus (10–25 %), Cricotopus/Orthocladius (10–20 %), and Sergentia (<10 %) (Fig. 2). Remains of Procladius were not found above 7 cm (~1975) in DZO-30. The most significant change in the chironomid assemblage of DZO-30, as determined by CONISS compared with the broken stick model, occurred at 3.5 cm, and was characterized by a rapid decline in the abundance of Sergentia and C. oliveri-type, coincident with increases in Paratanytarsus and Cricotopus/Orthocladius (Fig. 2). This assemblage change occurred ~1999, coincident with the timing of the major storm surge. The number of chironomid head capsules recovered after ~1999, i.e. above 3.5 cm, was greater than in the lower portion of the sediment core, increasing from 500–1,000 to >1,000 head capsules g−1 dry weight (Fig. 2).
Relative abundance diagram of the most common (>5 %) chironomid taxa from impacted Lake DZO-30, scaled by depth with 210Pb-derived sediment age included as a second y-axis. The number of chironomid head capsules (per gram dry sediment) recovered per interval is included as a line plot. A constrained incremental sum of squares (CONISS) cluster analysis dendrogram is included to illustrate biostratigraphic zones
The chironomid assemblage from nearby DZO-29 contained similar taxa, and experienced similar assemblage shifts as DZO-30 (Fig. 3). The sediment core from DZO-29 was longer than the one from DZO-30, and extended to 45 cm depth. From the base of the core to ~37 cm the assemblage was dominated by Cricotopus/Orthocladius, Chironomus and Paratanytarsus, and also included Sergentia, C. oliveri-type, Tanytarsus and Chironomus anthracinus-type Zetterstedt (Fig. 3). At approximately 37 cm, a large increase in Corynocera oliveri-type occurred, coincident with a decrease in Paratanytarsus and Cricotopus/Orthocladius. From 37 to ~9 cm, the relative abundance of C. oliveri-type fluctuated, representing between 15 and 75 % (Fig. 3). The most significant change in the DZO-29 assemblage occurred between 9 and 4.25 cm, and was characterized by a rapid decrease in C. oliveri-type and increase in Paratanytarsus (Fig. 3). At a core depth of 4.25 cm, the chironomid assemblage recorded a rapid and dramatic rise in the relative abundance of Cricotopus/Orthocladius from <30 to >60 %, as well as an increase in abundance of Tanytarsus (Fig. 3). Paratanytarsus remained an important component of the assemblage above 4.25 cm, though at slightly lower abundance than between 9 and 4.25 cm. Above 4.25 cm, C. oliveri-type was not observed in the sediments, and Sergentia was found in only one interval at very low abundance. The number of chironomid head capsules recovered from the DZO-29 sediment core was <1,000 head capsules g−1 from the bottom of the core to ~4 cm, at which point an abrupt increase to between 2,000 and 5,000 head capsules g−1 was recorded (Fig. 3).
Relative abundance diagram of the most common (>5 %) chironomid taxa from impacted Lake DZO-29, scaled by depth. The number of chironomid head capsules (per gram dry sediment) recovered per interval is included as a line plot. A constrained incremental sum of squares (CONISS) cluster analysis dendrogram is included to illustrate biostratigraphic zones
The chironomid assemblage from control Lake C-27 exhibited few directional changes during the period represented by this relatively short (17 cm) sediment core (~1895–2009). The assemblage was composed primarily of Chironomus, Cricotopus/Orthocladius, Psectrocladius, and Paratanytarsus (Fig. 4). Unlike DZO-29 and DZO-30, C. oliveri-type was not an important component of the assemblage at any point during the recent past. No significant changes in the assemblage were inferred using the CONISS cluster analysis, and no directional change was observed corresponding to the 1999 storm surge, ~4.5 cm core depth (Fig. 4). The number of chironomid head capsules recovered from the sediment core from Lake C-27 was lower than DZO-29 or DZO-30, and remained <1,000 head capsules g−1 throughout the record (Fig. 4). A short-duration, relatively small increase in head-capsule number was observed between 6 and 3.5 cm (1990–2000), as well as in the surface sediment (Fig. 4).
Relative abundance diagram of the most common (>5 %) chironomid taxa from control Lake C-27, scaled by depth with 210Pb-derived sediment age included as a second y-axis. The number of chironomid head capsules (per gram dry sediment) recovered per interval is included as a line plot. A constrained incremental sum of squares (CONISS) cluster analysis dendrogram is included to illustrate biostratigraphic zones
Analysis of the detrended correspondence analysis (DCA) axes 1 and 2 site scores for each lake showed that the chironomid assemblage from the impacted lakes, DZO-29 and DZO-30, were markedly different at the top of the sediment core when compared to the bottom of the sediment core, whereas the assemblage in control Lake C-27 did not exhibit any strong directional changes over the recent past (Fig. 5). DCA axis 1 site scores for the chironomid assemblage from DZO-30 (Fig. 2), the cladoceran assemblage from nearby DZO-29 (Deasley et al. 2012), and the diatom assemblages from both DZO-29 and DZO-30 (Thienpont et al. 2012) show the strongest directional changes during the recent past, since 1999 or 2000 (Fig. 6). Species assemblage turnover (i.e. beta diversity), estimated using detrended canonical correspondence analysis, was highest for diatoms from DZO-29 (1.49 SD) and DZO-30 (1.21 SD), followed by chironomids from DZO-30 (0.47 SD) and cladocerans from DZO-29 (0.31 SD).
Detrended correspondence analysis axis 1 site scores for the chironomid assemblage from the sediment core collected from Lake DZO-30, the diatom assemblages from DZO-29 and DZO-30 (Thienpont et al. 2012), and the cladoceran assemblage from DZO-29 (Deasley et al. 2012), scaled by 210Pb date since 1950. The sediment depth corresponding to the 210Pb date of 1999, the year of the storm surge, is shown by a dotted line. Detrended canonical correspondence analysis (constrained to sediment age as the sole environmental variable) derived estimates of species turnover (i.e. beta diversity, scaled in standard deviation units) are included in bold text next to each indicator name
Discussion
Chironomid response to the 1999 storm surge event
The chironomid assemblages from cores DZO-30 and DZO-29 exhibit consistent and dramatic shifts, coincident with the timing of the saltwater inundation caused by the September 1999 storm surge (Kokelj et al. 2012). The marked decrease in the relative abundances of Sergentia and Corynocera oliveri-type head capsules and the dramatic increase in Cricotopus/Orthocladius and Paratanytarsus abundances following the 1999 surge indicate the storm surge had an immediate ecological impact on chironomid communities in these lakes. Previous paleolimnological studies showed Sergentia to be poorly represented in higher-salinity lakes, and generally found in dilute waters (0–100 mg L−1) (Walker et al. 1995). Thus, decreased abundance of Sergentia in Lakes DZO-29 and DZO-30 after the 1999 storm surge (current chloride concentrations of 5,030 and 2,530 mg L−1, respectively; Thienpont et al. 2012) is not surprising. In both impacted lakes, the 1999 storm surge caused large declines in the abundance of C. oliveri-type, a taxon that had been increasing in abundance in both lakes, the onset of which was dated to the late nineteenth century in DZO-30. This taxon is also common in ion-poor lakes in western Canada (Walker et al. 1995), as well as the Hudson’s Bay Lowlands (HBL; Dickson et al. 2014). These coincident decreases illustrate the ecological impact the 1999 surge event had on saline-intolerant taxa in both lakes.
Following the rapid inundation with saline/brackish water in September 1999, Paratanytarsus and Cricotopus/Orthocladius, two cold-water taxa that include species known to be tolerant of saline water (Walker et al. 1995), became abundant in DZO-30 and DZO-29, and both continue to be important components of the chironomid assemblage in these lakes. This assemblage shift from saline-intolerant to saline-tolerant taxa corresponds directly to the increase in brackish-water diatoms in DZO-30 (Thienpont et al. 2012), and closely matches the timing of the rapid increase in brackish diatoms recorded in DZO-29, which was analyzed in a different sediment core and dated to exactly 1999 (Pisaric et al. 2011). Cricotopus/Orthocladius has been shown to have variable salinity optima in different regions, being observed in lower-salinity ponds in the HBL (Dickson et al. 2014) than in British Columbia (Walker et al. 1995). Dickson et al. (2014) concluded that this taxon was likely capable of tolerating a wide range of salinity conditions, and presented findings that suggested a high median lethal dose (LD50) for this group in response to salinity in a laboratory setting. This variability likely reflects the fact that different species within this group are found in waters of varying salinities. This suggests the salinity tolerances and taxa of Cricotopus/Orthocladius present in these lakes from the Mackenzie Delta region, which could not be identified to the species level, may be more aligned with saline-tolerant species from the interior of western Canada than the coastal HBL. Paratanytarsus increased in relative abundance strongly coincident with the 1999 storm surge. Previous research has observed members of the subtribe Tanytarsina, including Paratanytarsus, across a very broad salinity range (0–15,000 mg L−1; Walker et al. 1995), again likely related to taxonomic resolution. The increase in the relative abundance of Paratanytarsus following the 1999 storm surge further reinforces the designation of members of this genus as saline-tolerant. In both DZO-29 and DZO-30, the relative abundance of Chironomus and Tanytarsus taxa, both identified as saline-tolerant (Walker et al. 1995), changed little as a consequence of the 1999 storm surge, suggesting these groups were able to tolerate the influx of brackish water. The increases in these taxa, combined with the limnological changes inferred from other indicator taxa, reinforce the impact the 1999 storm had on the biota of lakes in this region.
Previous paleolimnological research determined that no biological recovery of the cladoceran (Deasley et al. 2012) or diatom (Pisaric et al. 2011) assemblages in Lake DZO-29 occurred following the 1999 storm surge. This lack of recovery in diatoms was documented across the outer delta (Thienpont et al. 2012). Similar to diatoms, the potential for re-colonization by saline-intolerant taxa following chemical recovery is likely high for chironomids, as winged chironomid adults are known to colonize new sites through both active and passive dispersal (Armitage et al. 1995). As with the other paleolimnological indicator taxa studied previously, however, we observed no major decreases in saline-tolerant Paratanytarsus or Cricotopus/Orthocladius in sediments that post-date 1999. This suggests that little or no biological recovery from the 1999 saltwater inundation has occurred. Furthermore, no recovery in the relative abundance of the saline-intolerant Sergentia or C. oliveri-type groups has occurred. This corroborates the inferred lack of chemical recovery based on the spatial analyses of water chemistry variables across the outer delta (Thienpont et al. 2012). Analysis of the assemblage trajectory based on detrended correspondence analysis show that the complete assemblages of the two impacted lakes are different in the most recent sediment compared to pre-impact, bottom sediments (Fig. 5). To date, no algal, zooplankton or benthic invertebrate community studied in the impacted lakes of the outer Mackenzie Delta has recovered from the 1999 storm surge, despite more than a decade during which no new major storm surge events have been documented (Kokelj et al. 2012). Saline conditions persist in these aquatic ecosystems because of the high salt concentrations that remain in the surrounding soils (Kokelj et al. 2012). These salts are slow to be removed because of a frozen active layer and persistent lake-ice cover during the spring freshet, combined with limited summer precipitation and poor drainage. This further illustrates the sensitivity of these coastal freshwater ecosystems to episodic saltwater flooding.
In comparison to the impacted “DZO” lakes, we recorded no significant shifts in the chironomid assemblage of control Lake C-27 at any time in the recent past, including in sediment layers that coincide with the 1999 storm surge. This provides further support, and confirms our remote sensing observations, that this site was not impacted by this recent saltwater inundation event (Kokelj et al. 2012). We did not observe a decrease in C. oliveri-type, which was not an important component of the chironomid assemblage, or Sergentia in Lake C-27 during the period recorded by this relatively short sediment core. In contrast to DZO-29 and DZO-30, Cricotopus/Orthocladius decreased slightly in C-27 after 1999, and there was no increase in the salt-tolerant taxon Paratanytarsus.
The number of chironomid head capsules recovered from the sediment sections of the two impacted lakes increased after the 1999 storm surge, suggesting that, although the species assemblages in the lakes changed because of the saltwater inundation, overall chironomid production was not adversely affected by the saltwater intrusion. These data suggest that chironomids increased in number following the storm surge, however concentration data must be interpreted cautiously in paleolimnological studies and are prone to many biases (Smol 2008). Whereas studies of the biological response of freshwater ecosystems to rapid flooding by seawater are limited, Clair and Paterson (1976) found that the chironomid community was temporarily extirpated from a small, shallow, eutrophic lake in New Brunswick, which was inundated by seawater from an intense gale, though most taxa recolonized the site following the return of freshwater conditions, a process that has not yet occurred in the outer Mackenzie Delta. The number of chironomid head capsules recovered from C-27 was lower than in the impacted lakes, and showed no major changes throughout the recent past. One potential explanation for the increase in chironomid numbers following inundation may be that other benthic macroinvertebrate groups with which the non-biting midges compete for resources, were negatively affected by the rapid influx of saline water, allowing the saline-tolerant chironomid taxa to exploit resources under decreased competition. Alternatively, it is possible the storm surge resulted in increased nutrient levels in the impacted lakes, which could have resulted in increased primary, and thus secondary, production.
Climate-driven chironomid assemblage changes
Although the most significant chironomid changes in both DZO-29 and DZO-30 occurred in the post-1999 sediments, both sediment cores record earlier assemblage shifts. One change recorded in the earlier part of the records from both DZO-29 and DZO-30 was a directional increase in Corynocera oliveri-type. This increase occurred at ~15 cm in DZO-30, which was dated to the late nineteenth century, which matches the timing of diatom assemblage changes related to increased periphytic community development (Thienpont et al. 2012). The increase in C. oliveri-type in DZO-29 first occurred at ~38 cm, was followed by a rapid decline at ~26 cm, after which the relative abundance increased again from 26 to 9 cm. Previous climate-based chironomid studies have suggested C. oliveri-type has a temperature optimum of 12.3 °C, compared with Cricotopus/Orthocladius and Paratanytarsus, with lower temperature optima of 11.1 °C (Barley et al. 2006). The gradual replacement of the cool-water taxa by C. oliveri-type in both DZO-29 and DZO-30 in the earlier part of these records could potentially be tracking the gradual warming that has occurred in the region over the last century (Porter et al. 2009; Thienpont et al. 2013). These trends suggest that regional temperature change may have been one important driver of chironomid community change prior to the 1999 storm surge. This conclusion, though tentative as it is based on only two records, one of which lacks robust chronological control, is supported by diatom (Thienpont et al. 2012), cladoceran (Deasley et al. 2012) and particle size data (Vermaire et al. 2013). This suggests that limnological responses to changing regional climate may have been driving chironomid community composition until the 1999 surge, when changes in salinity became the dominant control factor. The reason for the rapid decline in C. oliveri-type in DZO-29 at 26 cm is difficult to explain, particularly given the lack of dating control for this core. This period can be tentatively estimated to correspond to the 1700s–1800s (Vermaire et al. 2013), a period of relatively cool conditions in the Arctic (Kaufman et al. 2009). The decrease in the relative abundance of C. oliveri-type at ~26 cm, coincident with increases in taxa with cooler temperature optima, may be related to changing climate conditions in the region. Despite a lack of dating control, the similarities in timing and direction of chironomid assemblage changes in Lakes DZO-29 and DZO-30 suggest that similar environmental drivers, including recent climate warming and storm surge impacts, were responsible for the chironomid community changes in both lakes before and after the 1999 storm surge event.
Whole ecosystem biological changes following the storm surge
This assessment of the Chironomidae complements work utilizing other paleolimnological indicators and provides strong evidence that broad-scale ecosystem changes occurred in the freshwater systems of the outer delta as a result of the 1999 storm surge. A comparison of the impacts on chironomids, diatoms and cladocerans, three groups commonly used as key indicators of ecosystem health, shows the changes that occurred coincident with the 1999 saltwater inundation were the most significant in the recent past (Fig. 6). As expected, the magnitude of the response varied among the three indicator groups (Fig. 6). Diatoms exhibited the greatest assemblage changes, a near replacement of freshwater taxa by brackish species following the storm surge (Thienpont et al. 2012). This reflects the fact that diatoms show greater taxonomic diversity and are likely directly impacted by changing water chemistry, whereas higher trophic levels may be responding indirectly, as has been observed in other regions (Bigler et al. 2006). Among the zoological indicators, chironomids show a stronger response than Cladocera. Chironomid species exhibit more discrete salinity tolerances in these lakes, and, unlike the more subtle assemblage shifts observed for the cladocerans (Deasley et al. 2012), a marked chironomid assemblage turnover to saline-tolerant species was observed. Furthermore, the response of Cladocera to the storm surge was muted because of the lack of taxonomic resolution among the Alona spp., which had to be grouped to the generic level (Deasley et al. 2012). Despite the variable magnitude of response, these paleolimnological analyses clearly show the importance of marine storm surges as a stressor on whole-lake biology in Arctic coastal ecosystems.
Conclusions
Chironomid assemblages in sediment cores from two lakes located in the outer Mackenzie Delta record significant, synchronous increases in saline-tolerant taxa, in particular Paratanytarsus and Cricotopus/Orthocladius, coincident with the flooding of these lakes by a large marine storm surge that impacted the region in September 1999. Saline-intolerant, freshwater taxa, including Sergentia and Corynocera oliveri-type, decreased in relative abundance following the storm surge, and were clearly less competitive in the brackish conditions following the inundation. No similar changes were observed in a control lake located further inland, beyond the extent of the storm surge. The chironomid assemblage did not return to a community resembling the pre-impact composition, providing evidence that these lakes have exhibited little to no chemical or biological recovery more than a decade after the 1999 event. This observation corroborates similar conclusions reached in previous paleolimnological studies of diatom and cladoceran assemblages from the same lakes. The number of head capsules recovered from post-1999 sediment sections was higher than in pre-impact deposits, which contrasts with studies from other regions that showed marine incursions resulted in temporary extirpation of the invertebrate fauna. Prior to the 1999 storm surge, subtle changes in chironomid assemblage structure were potentially driven by regional climate warming, and closely matched previous paleoclimate inferences based on diatoms and spectral inferences of increasing primary production. This research highlights the ability of acute, localized stressors to alter the primary driver of biotic assemblages influenced by regional forcing mechanisms, such as climate warming.
References
Appleby PG, Oldfield F (1978) The calculation of 210Pb assuming a constant rate of supply of unsupported 210Pb to the sediments. Catena 5:1–8
Armitage PD, Cranston PS, Pinder LC (eds) (1995) The Chironomidae: the biology and ecology of nonbiting midges. Chapman and Hall, London
Barley EM, Walker IR, Kurek J, Cwynar LC, Mathewes RW, Gajewski K, Finney BP (2006) A northwest North American training set: distribution of freshwater midges in relation to air temperature and lake depth. J Paleolimnol 36:295–314
Bennett KD (1996) Determination of the number of zones in a biostratigraphical sequence. New Phytol 132:155–170
Bennike O, Brodersen KP, Jeppesen E, Walker IR (2004) Aquatic invertebrates and high latitude paleolimnology. In: Pienitz R, Douglas MSV, Smol JP (eds) Long-term environmental change in Arctic and Antarctic Lakes. Springer, Dordrecht, pp 159–186
Bigler C, Heiri O, Krskova R, Lotter AF, Sturm M (2006) Distribution of diatoms, chironomids and cladocera in surface sediments of thirty mountain lakes in south-eastern Switzerland. Aquat Sci 68:154–171
Brooks SJ, Langdon PG, Heiri O (2007) The identification and use of Palaearctic Chironomidae larvae in palaeoecology. Quaternary Research Association, London
Burn CR, Kokelj SV (2009) The environment and permafrost of the Mackenzie Delta area. Permafrost Periglac 20:83–105
Cahoon DR (2006) A review of major storm impacts on coastal wetland elevations. Estuaries Coasts 29:889–898
Church JA, Clark PU, Cazenave A, Gregory JM, Jevrejeva S, Levermann A, Merrifield MA, Milne GA, Nerem RS, Nunn PD, Payne AJ, Pfeffer WT, Stammer D, Unnikrishnan AS (2013) Sea level change. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 1137–1216
Clair T, Paterson CG (1976) Effect of a salt water intrusion on a freshwater Chironomidae community: a palaeolimnological study. Hydrobiologia 48:131–135
Comiso JC (2006) Abrupt decline in the Arctic winter sea ice cover. Geophys Res Lett 33:L18504. doi:10.1029/2006GL027341
Comiso JC, Parkinson CL, Gersten R, Stock L (2008) Accelerated decline in the Arctic sea ice cover. Geophys Res Lett 35:L01703. doi:10.1029/2007GL031972
Deasley K, Korosi JB, Thienpont JR, Kokelj SV, Pisaric MFJ, Smol JP (2012) Investigating the response of Cladocera to a major saltwater intrusion event in an Arctic lake from the outer Mackenzie Delta (NT, Canada). J Paleolimnol 48:287–296
Dickson TR, Bos DG, Pellatt MG, Walker IR (2014) A midge-salinity transfer function for inferring sea level change and landscape evolution in the Hudson Bay Lowlands, Manitoba, Canada. J Paleolimnol 51:325–341
Eggermont H, Heiri O, Verschuren D (2006) Fossil Chironomidae (Insecta: Diptera) as quantitative indicators of past salinity in African lakes. Quat Sci Rev 25:1966–1994
Glew JR (1988) A portable extruding device for close interval sectioning of unconsolidated core samples. J Paleolimnol 1:235–239
Glew JR (1989) A new trigger mechanism for sediment samplers. J Paleolimnol 2:241–243
Goulding HL, Prowse TD, Beltaos S (2009) Spatial and temporal patterns of break-ups and ice-jam flooding in the Mackenzie Delta, NWT. Hydrol Process 23:2654–2670
Grimm EC (1987) CONISS—a FORTRAN-77 program for stratigraphically constrained cluster-analysis by the method of incremental sum of squares. Comput Geosci 13:13–35
Grimm EC (2011) Tilia v1.6 computer program. Illinois State Museum. Research and Collections Center, Springfield
Hofmann W (1987) Stratigraphy of Cladocera (Crustacea) and Chironomidae (Insecta: Diptera) in three sediment cores from the Central Baltic Sea as related to paleo-salinity. Int Revue Ges Hydrobiol 72:97–106
Juggins S (2009) Rioja: analysis of quaternary science data. R package version 0.7-3. http://CRAN.Rproject.org/package=rioja
Kaufman DS, Schneider DP, McKay NP, Ammann CM, Bradley RS, Briffa KR, Miller GH, Otto-Bliesner BL, Overpeck JT, Vinther BM, Arctic Lakes 2k Project Members (2009) Recent warming reverses long-term Arctic cooling. Science 325:1236–1239
Kokelj SV, Lantz TC, Solomon S, Pisaric MFJ, Keith D, Morse P, Thienpont JR, Smol JP, Esagok D (2012) Utilizing multiple sources of knowledge to investigate northern environmental change: Regional ecological impacts of a storm surge in the outer Mackenzie Delta, N.W.T. Arctic 65:245–366
Lintern DG, Macdonald RW, Solomon SM, Jakes H (2011) Beaufort Sea storm and resuspension modeling. J Mar Syst. doi:10.1016/j.jmarsys.2011.11.015
Mackay JR (1963) The Mackenzie Delta area, N.W.T. Memoir 8. Department of Mines and Technical Surveys, Geographic Branch, Ottawa
Manson GK, Solomon SM (2007) Past and future forcing of Beaufort Sea coastal change. Atmos-Ocean 45:107–122
Marsh P, Schmidt T (1993) Influence of a Beaufort Sea storm surge on channel level in the Mackenzie Delta. Arctic 48:35–41
Nicholls RJ, Cazenave A (2010) Sea-level rise and its impact on coastal zones. Science 328:1517–1520
Oksanen J, Blanchet FG, Kindt R, Legendre P, O’Hara RB, Simpson GL et al (2010) Vegan: community ecology package. R package version 1.17-4. http://CRAN.Rproject.org/package=vegan
Oliver DR, Roussel ME (1983) The insects and arachnids of Canada. In Part 11: the genera of larval midges of Canada. Agriculture Canada, Ottawa
Overeem I, Anderson RS, Wobus CW, Clow GD, Urban FE, Matell N (2011) Sea ice loss enhances wave action at the Arctic coast. Geophys Res Lett 38: doi:10.1029/2011GL048681
Pisaric MFJ, Thienpont JR, Kokelj SV, Nesbitt H, Lantz TC, Solomon S, Smol JP (2011) Impacts of a recent storm surge on an Arctic delta ecosystem examined in the context of the last millennium. Proc Natl Acad Sci USA 108:8960–8965
Porinchu DF, MacDonald GM (2003) The use and application of freshwater midges (Chironomidae: Insecta: Diptera) in geographical research. Prog Phys Geog 27:378–422
Porter TJ, Pisaric MFJ, Kokelj SV, Edwards TWD (2009) Climatic signals in δ13C and δ18O of tree-rings from white spruce in the Mackenzie Delta region, northern Canada. Arct Antarct Alp Res 41:497–505
Quinlan R, Smol JP (2001) Setting minimum head capsule abundance and taxa deletion criteria in chironomid-based inference models. J Paleolimnol 26:327–342
Rampton VN (1988) Quaternary geology of the Tuktoyaktuk coastlands, Northwest Territories. Geological Survey of Canada, Natural Resources Canada, Ottawa
R Development Core Team (2011) R: a language and environment for statistical computing. R foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Sepp M, Jaagus J (2011) Changes in the activity and tracks of Arctic cyclones. Clim Change 205:577–595
Serreze MC, Holland MM, Stroeve J (2007) Perspectives of the Arctic’s shrinking ice cover. Science 315:1533–1536
Simmonds I, Keay K (2009) Extraordinary September Arctic sea ice reductions and their relationships with storm behavior over 1979–2008. Geophys Res Lett 36:L19715. doi:10.1029/2009GL039810
Smol JP (2008) Pollution of Lakes and Rivers: a paleoenvironmental perspective, 2nd edn. Wiley-Blackwell, Oxford
Smol JP, Douglas MSV (2007) From controversy to consensus: making the case for recent climatic change in the Arctic using lake sediments. Front Ecol Environ 5:466–474
Smol JP, Wolfe AP, Birks HJB, Douglas MSV, Jones VJ, Korhola A, Pienitz R, Rühland K, Sorvari S, Antoniades D, Brooks SJ, Fallu M, Hughes M, Keatley BE, Laing TE, Michelutti N, Nazarova L, Nyman M, Paterson AM, Perren B, Quinlan R, Rautio M, Saulnier-Talbot E, Siitonen S, Solovieva N, Weckström J (2005) Climate-driven regime shifts in the biological communities of arctic lakes. Proc Natl Acad Sci USA 102:4397–4402
Thienpont JR, Johnson D, Nesbitt H, Kokelj SV, Pisaric MFJ, Smol JP (2012) Arctic coastal freshwater ecosystem responses to a major saltwater intrusion: a landscape-scale palaeolimnological analysis. The Holocene 22:1451–1460
Thienpont JR, Rühland KM, Pisaric MFJ, Kokelj SV, Kimpe LE, Blais JM, Smol JP (2013) Biological responses to permafrost thaw slumping in Canadian Arctic lakes. Freshw Biol 58:337–353
Vermaire JC, Pisaric MFJ, Thienpont JR, Courtney Mustaphi C, Kokelj SV, Smol JP (2013) Arctic climate warming and sea ice declines lead to increased storm surge activity. Geophys Res Lett 40:1386–1390. doi:10.1002/grl.50191
Walker IR (2001) Midges: Chironomidae and related Diptera. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments. Volume 4: Zoological Indicators. Kluwer, Dordrecht, pp 43–66
Walker IR, Wilson SE, Smol JP (1995) Chironomidae (Diptera): quantitative palaeosalinity indicators for lakes of western Canada. Can J Fish Aquat Sci 52:950–960
Acknowledgments
This research was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada through Discovery grants to MFJP and JPS, Polar Continental Shelf Program support to MFJP, Northern Scientific Training Program funding to JRT and CS, a Fonds de Recherche du Québec—Nature et Technologies (FQRNT) Postdoctoral Fellowship to JCV and the NWT Cumulative Impact Monitoring Program. We thank Drs. Dave Porinchu and Joshua Kurek for assistance with chironomid identification. We thank the two reviewers for comments that improved the quality of the manuscript. This is NWT Geoscience Office contribution #82.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thienpont, J.R., Steele, C., Vermaire, J.C. et al. Synchronous changes in chironomid assemblages in two Arctic delta lake ecosystems after a major saltwater intrusion event. J Paleolimnol 53, 177–189 (2015). https://doi.org/10.1007/s10933-014-9815-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-014-9815-1