Abstract
Aerially transported contaminants from the industrial development of the bituminous sands in the Athabasca Oil Sands Region (AOSR) of western Canada may threaten the water quality and community structure of the region’s lakes. This environmental threat is further compounded by the region’s changing climate (i.e. increased temperatures and reduced moisture). Because environmental monitoring began ~ 30 years after the initiation of the region’s bitumen-based industry (~ 1967), a paleolimnological approach is required to document pre-disturbance conditions and to determine how lakes have changed, if at all, in response to environmental stressors. Our bottom–top (before and after) study of dated sediment records compared pre-disturbance (~ 1850) and modern subfossil diatom assemblages from 18 shallow, isolated lakes (which are typical of the region), located along a spatial gradient (up to ~ 110 km) relative to the main area of local industry. Despite the region’s substantial environmental stressors, the changes in mostly benthic-dominated diatom communities were minor at 15 of the 18 lakes. We conclude that the muted biological responses may be a consequence of naturally high nutrient concentrations and/or the typically subtle nature of changes in assemblages dominated by benthic generalist taxa. At all sites, including three lakes with marked changes, we found no evidence of a diatom assemblage response to airborne inputs of contaminants (i.e. dibenzothiophenes) and nutrients (i.e. bioavailable nitrogen) from the AOSR industry. Instead, it is likely that regional warming played a role in the modest diatom assemblage changes observed in these shallow lakes, with responses mediated by lake-specific characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Athabasca Oil Sands Region (AOSR) is well-known for its enormous bitumen extraction and processing industry that began with the development of the first commercial upgrader in 1967 (Government of Alberta 2014). The Athabasca deposit is the largest of three bitumen deposits in Alberta and Saskatchewan, with current production rates of ~ 1.76 million barrels per day, and forecast rates of ~ 3.16 million barrels per day by 2030 (Alberta Energy Regulator 2015; Canadian Association of Petroleum Producers 2015). The environmental concerns that accompany this large industrial expansion are becoming increasingly apparent. For example, development of facilities, mines, and supporting infrastructure has resulted in habitat fragmentation (Rooney et al. 2012), ground deformation (Stancliffe and van der Kooij 2001), and ultimately the loss of ecosystem services (Rooney et al. 2012). Moreover, bitumen-based industrial processes require large volumes of freshwater, generate copious amounts of tailings (Sauchyn et al. 2015), and can contaminate the ground (e.g. flow-to-surface events; Korosi et al. 2016), water (Kelly et al. 2009, 2010), and atmosphere (Kurek et al. 2013a; Kirk et al. 2014; Manzano et al. 2017).
Industrial airborne contaminants from upgrader stacks and machinery, as well as fugitive dusts from mine sites, industrial waste piles, and exposed bitumen (Landis et al. 2012; Jautzy et al. 2013; Zhang et al. 2016; Manzano et al. 2017), are deposited across the AOSR. Paleolimnological data suggest that contaminants may reach aquatic environments at least 90 km from the main industrial area (Kurek et al. 2013a). The industrial airborne contaminants include a suite of potentially toxic compounds (Kelly et al. 2009, 2010; Kirk et al. 2014). For example, dibenzothiophenes (DBTs) are a group of polycyclic aromatic compounds (PACs) that are among the industrial contaminants released to the atmosphere by the oil sands operations (Jautzy et al. 2013; Kurek et al. 2013a) and are an established proxy for exposure of an AOSR aquatic system to industrial airborne contamination (Jautzy et al. 2013; Kurek et al. 2013a, b; Summers et al. 2016). Several studies that explored atmospheric contamination in the AOSR also detected elevated concentrations of crustal, anthropogenic, and priority pollutant elements (Guéguen et al. 2016; Kelly et al. 2010; Kirk et al. 2014; Landis et al. 2012). Biologically available nitrogen, which can potentially enhance primary production and alter biotic communities, is known to be emitted in large quantities by oil sands activities. For example, 102 tonnes of dissolved inorganic nitrogen were deposited in 2014 springtime snowpack within 50 km of the main industiral oil sands development (Summers et al. 2016).
Consistent with Canada’s prairie provinces, warming temperatures and a drying landscape characterize the AOSR (Schindler and Donahue 2006). During the past ~ 100 years, mean annual temperatures in the prairie provinces have increased by ~ 1–4 °C, while total annual precipitation has declined by ~ 25% (Schindler and Donahue 2006). Previous paleolimnological studies investigating the effects of industrial activity in the AOSR, and downwind, originally focused on the effects of acid deposition from emissions of nitrogen and sulphur oxides (Hazewinkel et al. 2008; Curtis et al. 2010; Laird et al. 2013). Although they produced no consistent evidence of widespread surface water acidification, these studies noted potential increases in primary production, which Summers et al. (2016) identified as a regional effect that was most closely linked to recent climate warming and not the deposition of bioavailable nitrogen. Other recent paleolimnological studies that investigated biological responses to environmental stressors in the AOSR found an overriding influence of climate warming, instead of industrial inputs of contaminants and nutrients, in structuring aquatic communities of lakes (Hazewinkel et al. 2008; Kurek et al. 2013a, b; Laird et al. 2017; Mushet et al. 2017; Summers et al. 2017).
Direct long-term monitoring data and baseline conditions are largely lacking for the AOSR (Dowdeswell et al. 2010). Thus, paleolimnological methods, which use physical, chemical, and biological proxies preserved in lake sediments (Smol 2008), are required to understand past environmental conditions of these aquatic systems. In this study, we used diatoms as the primary proxy to advance our understanding of the effects of industrial airborne pollution (i.e. contaminants and nutrients) and climate change on shallow lake ecosystems. Subfossil remains of diatoms are one of the most commonly used biological proxies in paleolimnological and biomonitoring studies and are used as indicators of past changes in pH, salinity, nutrients, and climate-mediated conditions, as well as many other variables (Smol and Stoermer 2010).
Previous diatom studies tracked temporal changes in the AOSR in specific lakes of interest (Curtis et al. 2010; Hazewinkel et al. 2008; Laird et al. 2017; Summers et al. 2017). To explore the regional patterns of temporal change in diatom assemblage composition, however, we used a top–bottom (i.e. diatom assemblages in recent sediments compared to assemblages preserved in pre-disturbance sediments) paleolimnological approach (Smol 2008), including 18 shallow study lakes situated within ~ 110 km of the main industrial activities. Sites were selected to represent shallow, isolated lakes that receive different amounts of airborne pollution from the local bitumen industry. For all sites, pre-disturbance (bottom) sediment sections were dated and are known to represent ~ 1850. We addressed the following research questions: (1) Do modern diatom assemblages differ from pre-disturbance assemblages? (2) Has aerially transported industrial pollution (i.e. contaminants and bioavailable nitrogen) affected diatom assemblage composition? (3) What physical and chemical variables best explain the spatio-temporal patterns of change in diatom assemblages among shallow AOSR lakes?
Study area
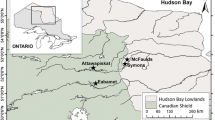
The AOSR is situated primarily in the northeast corner of Alberta, Canada, and extends into northern Saskatchewan (Fig. 1). Decades of bitumen-based industry and development have altered this once-remote part of the Boreal Plains ecozone. The landscape is characterized by boreal forest, peatlands, uplands featuring jack pine (Pinus banksiana) and trembling aspen (Populus trembuloides), and many shallow lakes and wetland systems (Natural Regions Committee 2006). The region’s surficial geology is composed mainly of coarse glacio-fluvial till, which overlies sand, shale, bituminous sands, and carbonate-dominated limestone and dolomite bedrock (Canadian Society of Petroleum Geologists 1973). The winters are long and cold (2014 mean winter air temperature = − 19.7 °C), and the summers are short and warm (2014 mean summer air temperature = 17.2 °C; Environment and Climate Change Canada’s Fort McMurray temperature station; ID: 3062696; 56°36′00.0″N, 111°13′12.0″W; www.ec.gc.ca/dccha-ahccd). Most precipitation falls as rain between June and October (Kerkhoven and Gan 2011), with an annual average of 400–500 mm (Leong and Donner 2015).
Locations of the 23 cored study lakes, two local communities, and the footprint of industrial oil sands development. Black circles mark the 18 shallow sites included in the histogram of the changes in diatom assemblages from the top–bottom sediment analyses (Fig. 3) and statistical analyses. White circles mark sites that were not included in the study because their chronologies do not extend to pre-disturbance times or there is a lack of diatoms in the pre-disturbance sediment sections (see text for details)
Materials and methods
Site selection
The 18 lakes used in this top–bottom paleolimnological survey are a subset of shallow lakes that were strategically selected to represent a range of airborne contamination from local bitumen-based industry (Table 1; Fig. 1). Given that this project explores aerially transported industrial contamination and nutrient deposition, the selected study lakes are isolated basins that are not directly part of a larger hydrologic system, such as the Athabasca River, and have small catchments relatively undisturbed by industry at the time of coring. The sites are located at different distances (within ~ 110 km) in all directions from the main area of industrial bitumen-related activities (Fig. 1).
Sample collection
As reported in earlier AOSR studies, including Kurek et al. (2013a) and Summers et al. (2016), sediment cores and water samples were collected in early March 2011–2015 as part of the Canada-Alberta Joint Oil Sands Monitoring (JOSM) program (www.jointoilsandsmonitoring.ca). All lakes are located on provincially owned and publicly accessible lands that were accessed by helicopter. Three sediment cores were collected through the ice from the deepest part of each shallow lake using a Uwitec gravity coring system and were sectioned using a Uwitec vertical extruder immediately upon returning to the field base (www.uwitec.at). Given that the cores were sectioned contiguously every 0.5 cm down to a depth of 20 cm and contiguously every 1.0 cm thereafter, the top sections for each lake were 0.5 cm thick and the bottom sections were 1.0 cm thick. The core sections were frozen until analysed. The primary core from each site was used for radiometric dating (Flett Research Ltd., Winnipeg, Manitoba), PAC analyses (AXYS Analytical Services, Sidney, British Columbia), and bioindicator and sedimentary chlorophyll-a analyses (Paleoecological Environmental Assessment and Research Laboratory (PEARL), Queen’s University, Kingston, Ontario). Replicate cores were retained for additional analyses. At the time of coring, water samples were collected through the ice at each site and were processed following standard techniques at the National Laboratory for Environmental Testing (NLET; Burlington, Ontario). Measures of environmental variables used in numerical analyses were obtained from field measurements, map measurements, sediment samples, and water samples (Electronic Supplementary Material [ESM] Table S1).
Radioisotopic analyses
Radioisotope activity and age modelling were completed by Flett Research Ltd and were previously reported in Summers et al. (2016, 2017). Briefly, 210Po and 226Ra activities were measured using alpha spectrometry as proxies for total and background (supported) 210Pb, respectively. Age-depth relations (± 2 SD) of the measured sediments in which total 210Pb exceeded supported 210Pb were calculated using the constant rate of supply (CRS) age model (Appleby 2001). Ages for the remaining sediment sections were estimated by fitting a polynomial of the lowest order with a reasonable fit to the cumulative dry mass of the measured sediment, and setting the intercept to the time of coring (Summers et al. 2016, 2017).
The top–bottom paleolimnological approach
A top–bottom paleolimnological approach (reviewed in Smol 2008) was used to compare two temporal snap shots (present and ~ 1850) of diatom communities from 18 shallow, isolated sites across the AOSR. Top–bottom analyses are time-efficient and useful for exploring environmental change across broad spatial scales; however, the approach does not identify the specific timing of historical changes (Smol 2008). The uppermost available sediment section from each core was used as the top, providing an integrated sample from the whole basin that represents the most recent (ca. 2010–2014) conditions (ESM Table S2). Sediments below 0–0.5 cm were used only if no sediments from the original top section were available (sites 2014-Z, RAMP 268, and 274). Given that chronologies for all of the sediment cores were previously established (Summers et al. 2016, 2017), 210Pb-estimated dates of sediment deposition were used to select the section from each lake that was most representative of ~ 1850 (available sections ranged from ~ 1825 to ~ 1897). These sections were used as the bottom (pre-disturbance) sections for each lake and provided an integrated sample of conditions preceding industrial oil sands development and anthropogenic climate change in this region (ESM Table S2). In this study, the top sections were 0.5 cm thick and the bottom sediment sections were 1.0 cm thick, which, in addition to the effects of downcore sediment compaction, resulted in the bottom sections representing a longer period of sediment deposition than the top sections. In this study, however, the time intervals for each sediment section were known (based on 210Pb dating) and the differences between tops and bottoms were investigated through the use of multiple sites.
Diatom sample preparation and analyses
Diatom samples were prepared from either wet or freeze-dried sediment generally following standard methods (Battarbee et al. 2001). Briefly, sediment samples were digested in a 1:1 molar solution of strong nitric and sulphuric acids and heated in a hot water bath at 80 °C for at least 2 h. The samples were allowed to settle for 24 h before the acid was removed and the remaining slurry was diluted with deionized water. The settling and dilution procedure was repeated until neutral pH was achieved. At least four dilutions of the digested slurry were plated onto coverslips and mounted onto slides using Naphrax®. Enumeration and identification of subfossil diatom remains (minimum = 303 valves, average = 418 valves) was completed at 1000 × magnification using a Leica DMR light microscope fitted with differential interference contrast optics and oil immersion lenses. Taxonomic references primarily included Krammer and Lange-Bertalot (1986–1991) and Camburn and Charles (2000). Identified diatom taxa were expressed as relative abundances (%) of the total number of valves counted in each sediment section.
Numerical analyses
Environmental variables
A principal components analysis (PCA) was performed on modern environmental data (including physical, chemical, and industrial data) collected from the 18 study lakes to examine the variation of sites with respect to modern environmental variables. Prior to inclusion in any ordination, the Vegan (Oksanen et al. 2012) and Analogue (Simpson and Oksanen 2016) packages in the R software environment (R Development Core Team 2015) were used to test the environmental variables for normality, and non-normal variables were transformed as necessary (using square-root, log, or log(x + 1) transformations; Supplemental Table 1). A Pearson correlation matrix with a Bonferroni correction was completed with R’s Hmisc package (Harrell 2017) and was used to calculate correlations among the environmental variables. The PCA was performed using CANOCO version 5.0 (ter Braak and Šmilauer 2012). The analysis was completed on a correlation matrix with variables centred and standardized to account for the different units of measurement.
Diatom assemblages
For most statistical analyses using diatom assemblage data, unless stated otherwise, rare taxa (not exceeding 2% in at least one sediment section) were excluded. Additionally, relative abundances of diatoms were square-root transformed in most statistical analyses to reduce the influence of dominant taxa and increase the weight of taxa with lower abundances, thereby equalizing the variance among taxa and preventing subtle trends from being obscured (Borcard et al. 2011).
Changes in the diatom communities were quantified in several ways. First, the trajectories of change between pre-disturbance times and the present day were determined using a detrended correspondence analysis (DCA). A DCA is an unconstrained ordination method that assesses compositional changes between species and between cases (Hill and Gauch 1980), which were bottom and top sediment sections in this scenario. Modern assemblage data were actively included in the DCA, whereas pre-disturbance assemblage data were included passively to compare the trajectories of assemblage change within the context of the modern samples. The results were similar when both modern and pre-disturbance assemblage data were run actively. The DCA was performed with CANOCO version 5.0 software (ter Braak and Šmilauer 2012) using the default settings of detrending by segments and no additional down-weighting of rare species. Trajectories of change in DCA space between pre-disturbance and present day (ΔDCA1 and ΔDCA2) were determined by subtracting bottom sediment section DCA axis 1 and 2 site scores from the corresponding top sediment section DCA axis 1 and 2 sites scores (Quinlan et al. 2003; Perren et al. 2008; Sweetman et al. 2008). DCAs are scaled in standard deviation (SD) units, which are units of species turnover along the first underlying gradient of compositional variation (Birks 2007). This metric of beta diversity quantifies assemblage change in a species-based framework (Birks 2007) and is thus potentially more ecologically relevant than other metrics commonly used to understand biological change (Birks 2007; Perren et al. 2008).
To explore trends down to the species level across the 18 study lakes, pre-disturbance (bottom) and modern (top) samples of DCA sample scores, species richness, species diversity, and relative abundances of select taxa were compared in 1:1 scatter plots. DCA sample scores (axes 1 and 2) for bottom sediments were plotted against DCA sample scores (axis 1 and 2) for top sediments with a 1:1 line to make comparisons between the two time periods. Differences between modern and pre-disturbance species richness and Hill’s N2 diversity (alpha diversity), were included in plots in the same way. Species richness and Hill’s N2 diversity were calculated using the Vegan (for both analyses; Oksanen et al. 2012) and the Rioja (for Hill’s N2 diversity; Juggins 2015) packages in R. Prior to richness and diversity calculations, raw count data, with rare taxa included, were rarified to a common sum to account for differences in the total number of diatoms counted in each sediment section (Birks 2012). Species richness is expressed as the number of taxa in a sample, whereas Hill’s N2 diversity incorporates richness and evenness in a sample, and is relatively easy to interpret ecologically because it is measured in units of very abundant taxa within a sample (Birks 2012). Percent relative abundances of common taxa or groups of taxa were also included in scatter plots with a 1:1 line to assess and compare compositional changes between modern and pre-disturbance assemblages. Finally, temporal assemblage changes in DCA 1 and 2 axis scores (ΔDCA1 and ΔDCA2), richness, diversity, and the taxa included in the 1:1 scatter plots were displayed as histograms and arranged along gradients of environmental variables and PCA axis 1 and 2 scores.
Results
Core chronologies
Radioisotope activity profiles and age-depth models from all the lakes included in this study are shown in ESM Fig. S1. Most of the chronologies were published previously in Summers et al. (2016). 210Po (a proxy for total 210Pb) in the majority of cores exhibited a typical exponential decay curve with some irregularity, although some cores showed low 210Po in the surface sediments. Sediment cores from sites 274 and RAMP 271 only extended to ~ 1897 and ~ 1880, respectively. Thus, those sediment sections, although more than 30 years younger than ~ 1850, were selected as the closest representations of ~ 1850. Pre-disturbance sediment sections selected from the other lakes ranged from ~ 1825 to 1865.
Top–bottom sediment availability
In this study, 23 lakes were originally sampled; however, only 18 sites were ultimately included in the analyses. One sediment core (RAMP 227) only extended to ~ 1953 (41–42 cm; ESM Fig. S1) and was thus inappropriate for inclusion. Furthermore, despite ample diatoms in modern sediments, four sites (SE22, RAMP 223, NE20, 2014-B) did not have a sufficient number of diatoms to count in the pre-disturbance sections and were therefore also excluded from the study. On average, based on our estimated 210Pb dates, the bottom sediment sections in our study represent approximately 6 years whereas the top sections represent approximately 1 year of diatom accumulation. The pre-disturbance sections with no/low diatoms were high in siliciclastic materials, whereas diatoms and other autochthonous siliceous indicators (e.g. chrysophyte cysts and scales) were scarce but well preserved, ruling out dissolution as the cause for no/low diatoms. There were no apparent patterns or common features in the lakes lacking pre-disturbance diatoms. Although 18 study lakes is a relatively low number for a regional assessment, the sites cover a sufficient range of industry contamination to justify an exploratory survey of lakes (dibenzothiophene enrichment factors (DBT EFs) 0.7–37.4; Summers et al. 2016).
Limnological trends across study lakes
All environmental variables actively included in the PCA, except for pH (which is already expressed on a log scale), were either normally distributed or displayed an acceptably normal distribution following transformation (ESM Table S1). The sources of the measurements for each environmental variable are included in ESM Table S1, and ranges are in Table 1. Sixteen environmental variables were actively included in the PCA, and six variables were included passively (ESM Table S1). The passive variables included specific conductance and five ions (chloride, calcium, magnesium, potassium, and sodium) that were positively correlated to and thus represented by dissolved inorganic carbon (DIC), which was actively included in the PCA. In addition to conventional water chemistry measures and physical lake characteristics, the PCA included representative variables of industrial impact such as DBT enrichment factors (DBT EFs), DBT concentrations from the uppermost sediment section (top [DBT]), and sulphate (SO42−) from the water column. DBT EFs were calculated in Summers et al. (2016) and compare DBT concentrations in modern sediment sections to concentrations in sediment sections immediately preceding industrial activity in the AOSR (~ 1955–1970). The EFs characterize the change in DBT concentrations at a site over time and are thus useful as metrics of industrial airborne contaminants (Summers et al. 2016). The top [DBT] is not a measure of industrial impact, but rather, is the combined natural and industrially sourced DBT concentration in the lake sediments deposited most recently. Sulphate is included independently because AOSR pet coke, a bitumen industry waste product, is rich in sulphur (Alberta Energy Regulator 2015; Manzano et al. 2017).
The PCA demonstrated that water chemistry and other limnological variables were generally dissimilar among the lakes, as no lakes clustered together in the ordination (Fig. 2). The amount of variance explained was similarly distributed between the two main axes, with PCA axis 1 explaining 25% and axis 2 explaining 23%. Although total nitrogen loaded the most strongly of all variables onto axis 1 (score = − 0.91), the axis represented a complex gradient of nutrients as well as ions, represented by DIC (score = − 0.72). Axis 2 represented a gradient of multiple variables, but mostly represented physical characteristics, including surface area and depth, which loaded most strongly onto axis 2 (score = 0.74 and 0.68, respectively; Fig. 2). Interestingly, sulphate was not positively correlated to DBT contaminant variables.
Principal components analysis (PCA) plot including the first two ordination axes of measured environmental variables from the 18 lakes included in statistical analyses. Lake surface area (SA), coring depth (Depth), pH, dissolved inorganic carbon (DIC), dissolved organic carbon (DOC), total nitrogen (TN), total phosphorus (TP), ammonia (NH3), silicon dioxide (Si), chlorophyll-a (Chla), dibenzothiophene enrichment factor (DBT EF), top sediment DBT concentrations (Top [DBT]), and sulphate (sulph) were included actively and are marked in solid black. Specific conductance (Scond), chloride (Cl), calcium (Ca), magnesium (Mg), potassium (K), and sodium (Na) were included passively and are marked in grey. Site names are abbreviated (Table 1)
Trends in diatom assemblages
A total of 311 diatom taxa were identified in the 18 lakes. A total of 97 taxa remained when a cut-off criterion was applied retaining only taxa exceeding 2% in at least one sediment section. Fourteen taxa are shown in the top–bottom relative abundance histogram, including taxa exceeding 5% relative abundance in at least one sediment section and demonstrating notable change in abundance from bottom to top in at least one site (Fig. 3). Small, benthic fragilarioid taxa were common to most sediment sections and, in fact, were dominant in the majority of both top and bottom samples (Fig. 3). NE13 was an exception. NE13 is a marl lake characterized by many benthic taxa (e.g. Brachysira neoexilis, cymbelloid taxa, and long Navicula taxa including, for example, Navicula cryptocephala, Navicula cryptotenella; Fig. 3).
Relative abundances of select diatom taxa (> 5% relative abundance in at least one interval and demonstrating notable abundance change from bottom-to-top in at least one site) in the modern (top of each shaded pair) and pre-disturbance (bottom of each shaded pair) sediment sections from the 18 study lakes. Where applicable, the number of taxa included in the complexes and summed groups are indicated in parentheses following the group name. Taxa included in complexes and summed groups are listed in ESM Table S4. Lakes are arranged from top to bottom in order of increasing dibenzothiophene enrichment factor (DBT EF; Table 1)
The trajectories of overall assemblage change through time (i.e. the differences between the pre-disturbance and the modern DCA sample scores from each lake) showed modest changes with no consistent directional change among sites (Fig. 4). It is evident, however, that pre-disturbance diatom assemblages shared more similarities among lakes than modern assemblages. The plot of DCA sample scores (Fig. 4) highlighted the differences among assemblages, with only modest separation of sites (except for the marl lake, NE13). DCA axis 1 explained 13.5% of the variance and axis 2 explained 9.9% (Fig. 4). Examining the diatom taxonomic loadings on the DCA axes (i.e. species scores), Aulacoseira taxa and pennate planktonic Fragilaria taxa were most important to DCA axis 1 (ESM Fig. S2, ESM Table S3). Three lakes demonstrated notable changes between pre-disturbance and modern assemblages (2014-D, 2014-Z, and Pushup; Fig. 3). Sites 2014-D, 2014-Z, and Pushup demonstrated clear compositional turnover from high relative abundances of small, benthic fragilarioid taxa to high relative abundances of centric planktonic taxa (Fig. 3). Diatom assemblage changes in other sites were less pronounced (Fig. 4), but nevertheless exhibited changes among numerous benthic taxa, mostly transitioning from Staurosira construens and Staurosirella pinnata taxa to other benthic taxa with more specialized habitat preferences, or from benthic taxa to pennate planktonic taxa in one case (Fig. 4).
Detrended correspondence analysis (DCA) plot including site scores from the first two ordination axes. Black circles mark the actively included modern diatom assemblages for each site. White circles mark the passively included pre-disturbance diatom assemblages for each site. The length and direction of the lines connecting pre-disturbance and modern assemblages indicates the magnitude and direction of assemblage change over time at each site. Site names are abbreviated as in Table 1
An examination of trends in the dataset’s most common taxa highlighted distinct differences between pre-disturbance and modern relative abundances (Fig. 5). One of the clearest changes was a decrease in the relative abundances of taxa in the dominant, benthic fragilarioid complex (Staurosira construens and Staurosirella pinnata) in the modern assemblages of most study lakes (Fig. 5). Notable relative abundance increases in the modern samples are evident in plots of Achnanthidium minutissimum; the small, benthic naviculoid complex (Eolimna minima, Sellaphora atomoides, and Sellaphora seminulum); the long, benthic Navicula complex (Navicula cryptocephala, Navicula cryptotenella, Navicula pseudolanceolata, Navicula radiosa, Navicula rhynchocephala, and Navicula vulpina); and all Nitzschia taxa summed (Fig. 5 plots).
Scatter plots of assemblage metrics and select common diatom taxa from the pre-disturbance (bottom; x axis) and modern (top; y axis) sediment sections of the 18 Athabasca Oil Sands Region (AOSR) study lakes. The benthic fragilarioid complex includes Staurosira construens and Staurosirella pinnata. The small benthic naviculoid complex includes Eolimna minima, Sellaphora atomoides, and Sellaphora seminulum. The long benthic Navicula complex includes Navicula cryptocephala, Navicula cryptotenella, Navicula pseudolanceolata, Navicula radiosa, Navicula rhynchocephala, and Navicula vulpina. Black circles denote sites with coring water depths ≤ 2 m. Grey circles denote sites with coring water depths > 2 m. Where applicable, the number of taxa included in the complexes and summed groups are indicated in parentheses following the group name. A 1:1 line is included. Samples that plot above the 1:1 line indicate greater values in the modern assemblages than in the pre-disturbance assemblages
Scatter plots of pre-disturbance and modern species richness and Hill’s N2 diversity (rarified n = 303) showed no directional trend among the 18 lakes (Fig. 5). However, as is the case in all paleolimnological studies, changes in species diversity through time probably involve different time spans in sediment sections and thus should be interpreted with caution (Smol 1981). Plots of DCA Axis 1 scores demonstrated minimal change between the two time periods investigated, and mostly plotted along the 1:1 line, while several sites had noticeably larger DCA axis 2 site scores in modern diatom assemblages, indicating marked diatom assemblage changes at those sites (Fig. 5).
Environmental variables and their relation to diatom assemblages
Temporal assemblage changes in richness, diversity, DCA 1 and 2 axis scores (ΔDCA1 and ΔDCA2), and the taxa included in the scatter plots were displayed as histograms and arranged along gradients of environmental variables and PCA axis 1 and 2 scores. The ordered histograms did not show consistent, directional patterns of biological change (ESM Fig. S3). It is notable that these metrics of assemblage change, when plotted along gradients of DBT EF and distance to nearest major industrial source (Landis et al. 2017), which are proxies for aerial industrial oil sands impact, showed no clear directional trends (ESM Fig. S3).
Discussion
Collectively, there is little change between pre-disturbance and modern diatom assemblages in our 18 study sites. When assemblage changes are examined at individual sites, however, lake-specific responses are observed. Furthermore, the absence of directional patterns in top–bottom metrics of assemblage change (i.e. species richness, Hill’s N2, DCA axis 1 and 2 sample scores, and the relative abundances of common taxonomic groups), when plotted along measured environmental gradients (e.g. pH, nutrients, DBT enrichment factor, etc.) indicate that there are no clear relationships between the degree of change and any measured environmental variables in these shallow lakes (ESM Fig. S3).
Importantly, the absence of consistency in diatom assemblage responses along industrial gradients (i.e. DBT EF, distance to nearest major industrial source) suggests that airborne pollution from local oil sands industry, including contaminants and bioavailable nitrogen, has not notably altered the assemblages of the mostly benthic algae of these lakes. The lack of a biological response to deposition of industrial airborne pollution is consistent with findings from other studies across the AOSR (Kurek et al. 2013a, b; Laird et al. 2017; Mushet et al. 2017; Summers et al. 2017) and may be a consequence of naturally high nutrient concentrations. Given that our study lakes have high modern concentrations of nutrients (Table 1) and that there are lakes in Alberta with known nutrient-rich histories prior to settlement (Blais et al. 2000; Bayley et al. 2007), it is plausible that our study lakes were historically rich in nutrients. Furthermore, the majority of our study lakes are phosphorus-limited (Table 1; TN:TP column; > 14 = P-limited) and, as such, it is logical that the addition of growth-stimulating nitrogen (Summers et al. 2016) has not yielded marked changes in biological assemblages. The absence of biological changes corresponding to elevated levels of aerially deposited industrial contamination suggests that the diatom assemblages in our study lakes are resistant to the atmospheric pollution from the local activities.
Lake 2014-D is a unique case in this study. 2014-D is a shallow lake (0.9 m) that demonstrated a marked diatom compositional turnover from small, benthic fragilarioid taxa (> 85%) in its pre-disturbance assemblage to over 60% abundance of Aulacoseria granulata in its modern assemblage. Given that 2014-D is very high in total phosphorus (TP; 2014-D = 341 μg/L) and that A. granulata is commonly found in meso-eutrophic to eutrophic conditions (Kilham and Kilham 1975), it is possible that the assemblage changes at this site were caused by increasing nutrient concentrations. 2014-D, however, is > 80 km away from the nearest major industrial source and its DBT EF is 1.6 (i.e. minimal “industrial impact”; Summers et al. 2016); thus, the community changes are not attributed to industrial inputs of biologically available nitrogen.
The muted diatom community changes in most of our shallow study sites are consistent with known responses to climate change in this region. In the AOSR, mean annual air temperatures have increased markedly since the beginning of the record in 1916 and, since the 1970s, have mostly exceeded the record-long average (Laird et al. 2013). Moisture in the region has declined with an increase in evaporation driven by warming that has outpaced the cyclical patterns of precipitation (Schindler and Donahue 2006). The shallow depths of our study lakes mostly preclude high abundances of planktonic taxa and, as such, the observed changes in the diatom communities are mainly, but not exclusively, among benthic taxa. In the majority of our shallow sites, we found changes from pre-disturbance assemblages dominated by Staurosira construens and Staurosirella pinnata (small, epilithic, generalist, fragilarioid taxa; Lotter and Bigler 2000) to modern assemblages that are still dominated by these fragilarioid taxa, but with increased abundances of other benthic taxa with more complex environmental preferences (e.g. naviculoid taxa, Nitzschia; Figs. 3, 5). In many shallow lakes and ponds, warmer temperatures and extended growing seasons have been found to increase the availability and complexity of the benthic environment (e.g. increased abundance and diversity of macrophytes), favouring the development of diatom assemblages with complex and diverse habitat requirements (e.g. periphytic taxa; Douglas et al. 1994; Rühland et al. 2014; Summers et al. 2017). The transitions can be subtle given the generalist nature of the small, benthic fragilarioid taxa (Lotter and Bigler 2000). As such, the diatom assemblage transitions we recorded in the majority of our study lakes are subtle, but such changes are expected responses to a warming climate given the shallow depths of the sites (Rühland et al. 2014; Summers et al. 2017).
Although the majority of our study lakes demonstrated similar assemblage changes among benthic taxa (i.e. shifts from benthic, fragilarioid taxa to specialized benthic taxa), several sites showed increased abundances of planktonic taxa in modern assemblages. We found substantial assemblage shifts from a high dominance of benthic fragilarioid taxa to modern assemblages dominated by small, planktonic Discostella stelligera in some of the relatively deeper, but still shallow, sites in our set of lakes (2014-Z, 3.2 m; Pushup, 5.1 m; Fig. 3). We also observed changes in RAMP 268 (1.8 m) from a benthic fragilarioid-dominated (~ 40%) pre-disturbance assemblage to a modern assemblage comprised of > 20% Fragilaria tenera, which is a pennate, planktonic species. These assemblage changes are consistent with expected responses to changes in lake water properties and associated changes in resource availability that result from warming and longer ice-free periods in deeper lakes (Rühland et al. 2015). For example, top–bottom studies from across the Northern Hemisphere (Rühland et al. 2008), including lakes from the central Canadian subarctic (Rühland et al. 2003), northwestern Ontario (Enache et al. 2011), and New Brunswick (Harris et al. 2006), attributed high modern abundances of small, cyclotelloid taxa, including D. stelligera, to decreases in vertical mixing and increases in thermal stability and associated changes in light and internal nutrient distributions. Although our study sites are likely too shallow to thermally stratify, warmer conditions and a longer open-water period may have reduced the duration and strength of vertical mixing and increased water column stability, enabling planktonic diatoms to thrive in some of our sites.
The diatom assemblage changes in our study lakes are mainly subtle, but the nature of these changes are consistent with more substantial increases in whole-lake primary production that were found in all lakes of this study and are documented in Summers et al. (2016). The increasing primary production trends in the Summers et al. (2016) study were linked to climate warming and the associated effects on the region’s lakes, while no clear link to a fertilization effect from local industry was detected (Summers et al. 2016). The clear increases in sediment-inferred whole-lake primary production corroborate the changes in the diatom assemblages (although they are subtle) in our 18 study sites, and support the conclusion that the region’s changing climate likely played an important role in structuring the biological communities in shallow AOSR lakes.
The differences in assemblage responses in our shallow lakes (i.e. most lakes transitioned to more complex benthic assemblages, whereas three lakes shifted to planktonic assemblages) are likely explained by site-specific conditions, and underscore the importance of the limnological context of a lake prior to a shift (Rühland et al. 2015). For example, 2014-Z (3.2 m) and Pushup (5.1 m), the two lakes that demonstrate a large assemblage turnover from benthic fragilarioid taxa to Discostella stelligera, are comparable in depth to RAMP 464 (4.6 m) and Kearl (3.5 m). Rather than shifting to assemblages dominated by planktonic taxa (as recorded in 2014-Z and Pushup), RAMP 464 and Kearl change to a more complex benthic assemblage, as is common in the majority of our study sites. Diatom assemblages from different lakes can have different responses to a widespread stressor such as climate change. Such different responses have been documented in the AOSR (Laird et al. 2017) and may be accounted for by differences in lake characteristics, such as morphologic and littoral characteristics (e.g. basin shape, shoreline substrate), that provide a variety of different benthic habitats for diatoms to exploit. Although we observed lake-specific responses, broad interpretation of the bottom-to-top changes in the assemblages of our 18 study lakes, spanning a range of industrial airborne contamination, provide a valuable assessment of the region’s lakes and suggest relative resistance in the face of substantial environmental stress. Detailed, downcore analyses of the diatom assemblages in lakes that have high inputs of industrial airborne pollution and/or show large taxonomic changes between pre-disturbance and modern assemblages will further elucidate the effects of ongoing multiple stressors on the lakes in the AOSR.
Conclusions
Overall, the diatom assemblages in our shallow AOSR study lakes record only minor changes since ~ 1850. The muted responses may be a consequence of naturally high nutrient concentrations in the lakes buffering the response to contaminant and nutrient deposition from nearby industry. In addition, the benthic diatom taxa that dominate the assemblages of these shallow lakes in most top and bottom samples are considered environmental generalists and would therefore tolerate stress and a wide range of environmental conditions. Although three study sites showed marked diatom community change between bottom and top sediment sections, no sites demonstrated changes clearly linked to deposition of industrial pollution. Instead, the majority of diatom assemblage responses are broadly consistent with indirect changes linked to climate warming. The differences in the nature and magnitude of the assemblage responses among seemingly comparable sites suggest that individual lake changes are modified by site-specific characteristics. Our research corroborates the findings of other paleolimnological studies in the AOSR, and farther downwind, that find aquatic community responses primarily determined by climate change, rather than industrial contaminant and nitrogen deposition (Hazewinkel et al. 2008; Curtis et al. 2010; Kurek et al. 2013a; Laird et al. 2013, 2017; Summers et al. 2016). The findings of this study highlight the relative biological stability of primary producers found in the region’s lakes despite ongoing environmental stress.
References
Alberta Energy Regulator (2015) ST98-2015: Alberta’s energy reserves 2014 and supply/demand outlook 2015–2024. Calgary, Canada
Appleby PG (2001) Chronostratigraphic techniques in recent sediments. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments, vol 1. Kluwer Academic Publishers. Dordrecht, Netherlands, pp 171–203
Battarbee R, Jones VJ, Flower R et al (2001) Diatoms. In: Smol J, Birks H, Last W (eds) Tracking environmental change using lake sediments: terrestrial, algal, and siliceous indicators, 3rd edn. Kluwer Academic Publishers, Dordrecht, pp 155–202
Bayley SE, Creed IF, Sass GZ, Wong AS (2007) Frequent regime shifts in trophic states in shallow lakes on the Boreal Plain: alternative “unstable” states? Limnol Oceanogr 52:2002–2012. https://doi.org/10.4319/lo.2007.52.5.2002
Birks HJB (2007) Estimating the amount of compositional change in late-Quaternary pollen-stratigraphical data. Veg Hist Archaeobot 16:197–202. https://doi.org/10.1007/s00334-006-0079-1
Birks HJB (2012) Introduction and overview of part II. In: Birks HJB, Lotter AF, Juggins S, Smol JP (eds) Tracking environmental change using lake sediments: data handling and numerical techniques. Springer, Dordrecht, pp 101–121
Blais JM, Duff KE, Schindler DW et al (2000) Recent eutrophication histories in Lac Ste. Anne and Lake Isle, Alberta, Canada, inferred using paleolimnological methods. Lake Reserv Manag 16:292–304. https://doi.org/10.1080/07438140009354237
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, New York
Camburn K, Charles D (2000) Diatoms of low alkalinity lakes in the northern United States. The Academy of Natural Sciences of Philadelphia, Philadelphia
Canadian Association of Petroleum Producers (2015) Crude oil forecast. Markets and Transportation, Calgary
Canadian Society of Petroleum Geologists (1973) Guide to the Athabasca oil sands area. Edmonton, Canada
Curtis CJ, Flower R, Rose N et al (2010) Palaeolimnological assessment of lake acidification and environmental change in the Athabasca Oil Sands Region, Alberta. J Limnol 69:92–104. https://doi.org/10.3274/JL10-69-S1-10
Douglas MSV, Smol JP, Blake WJ (1994) Marked post-18th century environmental change in high-Arctic ecosystems. Science 266:416–419
Dowdeswell L, Dillon P, Ghoshal S et al (2010) A foundation for the future: building an environmental monitoring system for the oil sands. Ottawa, Canada
Downing JA, McCauley E (1992) The nitrogen:phosphorus relationship in lakes. Limnol Oceanogr 37:936–945. https://doi.org/10.4319/lo.1992.37.5.0936
Enache MD, Paterson AM, Cumming BF (2011) Changes in diatom assemblages since pre-industrial times in 40 reference lakes from the Experimental Lakes Area (northwestern Ontario, Canada). J Paleolimnol 46:1–15. https://doi.org/10.1007/s10933-011-9504-2
Government of Alberta (2014) Alberta oil sands industry: quarterly update, Spring 2014
Guéguen C, Cuss C, Cho S (2016) Snowpack deposition of trace elements in the Athabasca Oil Sands Region, Canada. Chemosphere 153:447–454. https://doi.org/10.1016/j.chemosphere.2016.03.020
Harrell FEJ (2017) Hmisc: harrell miscellaneous. R package version 4.0-3
Harris MA, Cumming BF, Smol JP (2006) Assessment of recent environmental changes in New Brunswick (Canada) lakes based on paleolimnological shifts in diatom species assemblages. Can J Bot 84:151–163. https://doi.org/10.1139/b05-157
Hazewinkel RRO, Wolfe AP, Pla S et al (2008) Have atmospheric emissions from the Athabasca Oil Sands impacted lakes in northeastern Alberta, Canada? Can J Fish Aquat Sci 1567:1554–1567. https://doi.org/10.1139/F08-074
Hill MO, Gauch HGJ (1980) Detrended correspondence analysis: an improved ordination technique. Vegetation 42:47–58. https://doi.org/10.1007/BF00048870
Jautzy J, Ahad JME, Gobeil C, Savard MM (2013) Century-long source apportionment of PAHs in Athabasca Oil Sands Region lakes using diagnostic ratios and compound-specific carbon isotope signatures. Environ Sci Technol 47:6155–6163. https://doi.org/10.1021/es400642e
Juggins S (2015) Rioja: analysis of quaternary science data. R package version 0.9-5
Kelly EN, Short JW, Schindler DW et al (2009) Oil sands development contributes polycyclic aromatic compounds to the Athabasca River and its tributaries. Proc Natl Acad Sci USA 106:22346–22351. https://doi.org/10.1073/pnas.0912050106
Kelly EN, Schindler DW, Hodson PV et al (2010) Oil sands development contributes elements toxic at low concentrations to the Athabasca River and its tributaries. Proc Natl Acad Sci USA 107:16178–16183. https://doi.org/10.1073/pnas.1008754107
Kerkhoven E, Gan TY (2011) Differences and sensitivities in potential hydrologic impact of climate change to regional-scale Athabasca and Fraser River basins of the leeward and windward sides of the Canadian Rocky Mountains respectively. Clim Change 106:583–607. https://doi.org/10.1007/s10584-010-9958-7
Kilham SS, Kilham P (1975) Melosira granulata (EHR.) RALFS: morphology and ecology of a cosmopolitan freshwater diatom. Verh Int Verein Theor Angew Limnol 19:2716–2721
Kirk JL, Muir DCG, Gleason A et al (2014) Atmospheric deposition of mercury and methylmercury to landscapes and waterbodies of the Athabasca Oil Sands Region. Environ Sci Technol 48:7374–7383. https://doi.org/10.1021/es500986r
Korosi JB, Cooke CA, Eickmeyer DC et al (2016) In-situ bitumen extraction associated with increased petrogenic polycyclic aromatic compounds in lake sediments from the Cold Lake heavy oil fields (Alberta, Canada). Environ Pollut 218:915–922. https://doi.org/10.1016/j.envpol.2016.08.032
Krammer K, Lange-Bertalot H (1986–1991) Bacillariophyceae, parts 1–4. In: Subwasserflora von Mitteleuropa. Fischer Verlag, Stuttgart
Kurek J, Kirk JL, Muir DCG et al (2013a) Legacy of a half century of Athabasca oil sands development recorded by lake ecosystems. Proc Natl Acad Sci 110:1761–1766. https://doi.org/10.1073/pnas.1217675110
Kurek J, Kirk JL, Muir DCG et al (2013b) Reply to Hrudey: tracking the extent of oil sands airborne pollution. Proc Natl Acad Sci 110:E2749. https://doi.org/10.1073/pnas.1307696110
Laird KR, Das B, Kingsbury M et al (2013) Paleolimnological assessment of limnological change in 10 lakes from northwest Saskatchewan downwind of the Athabasca oil sands based on analysis of siliceous algae and trace metals in sediment cores. Hydrobiologia 720:55–73. https://doi.org/10.1007/s10750-013-1623-5
Laird KR, Das B, Hasjdal B et al (2017) Paleolimnological assessment of nutrient enrichment on diatom assemblages in a priori defined nitrogen- and phosphorus-limited lakes downwind of the Athabasca Oil Sands. J Limnol, Canada. https://doi.org/10.4081/jlimnol.2017
Landis MS, Pancras JP, Graney JR et al (2012) Chapter 18—Receptor modeling of epiphytic lichens to elucidate the sources and spatial distribution of inorganic air pollution in the Athabasca Oil Sands Region. In: Percy KE (ed) Alberta oil sands. Elsevier Press, Oxford, pp 427–467
Landis MS, Pancras JP, Graney JR et al (2017) Source apportionment of ambient fine and coarse particulate matter at the Fort McKay community site, in the Athabasca Oil Sands Region, Alberta, Canada. Sci Total Environ 484–485:105–117. https://doi.org/10.1016/j.scitotenv.2017.01.110
Leong DNS, Donner SD (2015) Climate change impacts on streamflow availability for the Athabasca Oil Sands. Clim Change 133:651–663. https://doi.org/10.1007/s10584-015-1479-y
Lotter AF, Bigler C (2000) Do diatoms in the Swiss Alps reflect the length of ice-cover? Aquat Sci 62:125–141. https://doi.org/10.1007/s000270050002
Manzano CA, Marvin C, Muir D et al (2017) Heterocyclic aromatics in petroleum coke, snow, lake sediments, and air samples from the Athabasca Oil Sands Region. Environ Sci Technol 51:5445–5453. https://doi.org/10.1021/acs.est.7b01345
Mushet GR, Laird KR, Das B et al (2017) Regional climate changes drive increased scaled-chrysophyte abundance in lakes downwind of Athabasca Oil Sands nitrogen emissions. J Paleolimnol 58:419–435. https://doi.org/10.1007/s10933-017-9987-6
Natural Regions Committee (2006) Natural regions and subregions of alberta. Government of Alberta, Edmonton
Oksanen J, Blanchet FG, Kindt R, et al (2012) Vegan: community ecology package. R package version 2.0-4
Perren BB, Douglas MSV, Anderson NJ (2008) Diatoms reveal complex spatial and temporal patterns of recent limnological change in West Greenland. J Paleolimnol 42:233–247. https://doi.org/10.1007/s10933-008-9273-8
Quinlan R, Paterson AM, Hall RI et al (2003) A landscape approach to examining spatial patterns of limnological variables and long-term environmental change in a southern Canadian lake district. Freshw Biol 48:1676–1697. https://doi.org/10.1046/j.1365-2427.2003.01105.x
R Development Core Team (2015) R: a language and environment for statistical computing
Rooney RC, Bayley SE, Schindler DW (2012) Oil sands mining and reclamation cause massive loss of peatland and stored carbon. Proc Natl Acad Sci 109:4933–4937. https://doi.org/10.1073/pnas.1117693108
Rühland K, Priesnitz A, Smol JP (2003) Paleolimnological evidence from diatoms for recent environmental changes in 50 lakes across Canadian Arctic treeline. Arct Antarct Alp Res 35:110–123. https://doi.org/10.1657/1523-0430(2003)035%5b0110:PEFDFR%5d2.0.CO;2
Rühland K, Paterson AM, Smol JP (2008) Hemispheric-scale patterns of climate-related shifts in planktonic diatoms from North American and European lakes. Glob Chang Biol 14:2740–2754. https://doi.org/10.1111/j.1365-2486.2008.01670.x
Rühland KM, Hargan KE, Jeziorski A et al (2014) A multi-trophic exploratory survey of recent environmental changes using lake sediments in the Hudson Bay Lowlands, Ontario, Canada. Arct Antarct Alp Res 46:139–158. https://doi.org/10.1657/1938-4246-46.1.139
Rühland KM, Paterson AM, Smol JP (2015) Lake diatom responses to warming: reviewing the evidence. J Paleolimnol 54:1–35. https://doi.org/10.1007/s10933-015-9837-3
Sauchyn DJ, St-Jacques J, Luckman BH (2015) Long-term reliability of the Athabasca River (Alberta, Canada) as the water source for oil sands mining. Proc Natl Acad Sci USA 112:12621–12626. https://doi.org/10.1073/pnas.1509726112
Schindler DW, Donahue WF (2006) An impending water crisis in Canada’s western prairie provinces. Proc Natl Acad Sci USA 103:7210–7216. https://doi.org/10.1073/pnas.0601568103
Simpson GL, Oksanen J (2016) Analogue: analogue and weighted averaging methods for paleoecology. R package version 0.17-0
Smol JP (1981) Problems associated with the use of “species diversity” in paleolimnological studies. Quat Res 15:209–212
Smol JP (2008) Pollution of lakes and rivers: a paleoenvironmental perspective. Blackwell, Oxford
Smol JP, Stoermer EF (eds) (2010) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, New York
Stancliffe R, van der Kooij M (2001) The use of satellite-based radar interferometry to monitor production activity at the Cold Lake heavy oil field, Alberta Canada. Am Assoc Pet Geol 85:781–793
Summers JC, Kurek J, Kirk JL et al (2016) Recent warming, rather than industrial emissions of bioavailable nutrients, is the dominant driver of lake primary production shifts across the Athabasca Oil Sands Region. PLoS ONE 1–20:e0153987. https://doi.org/10.1371/journal.pone.0153987
Summers JC, Kurek J, Rühland KM et al (2017) Assessment of multi-trophic changes in a shallow boreal lake simultaneously exposed to climate change and aerial deposition of contaminants from the Athabasca Oil Sands Region, Canada. Sci Total Environ 592:573–583. https://doi.org/10.1016/j.scitotenv.2017.03.079
Sweetman JN, LaFace E, Rühland KM et al (2008) Evaluating the response of Cladocera to recent environmental changes in lakes from the central Canadian arctic treeline region. Arct Antarct Alp Res 40:584–591. https://doi.org/10.1657/1523-0430(06-118)
ter Braak CJF, Šmilauer P (2012) CANOCO Reference manual and user’s guide. 496
Zhang Y, Shotyk W, Zaccone C et al (2016) Airborne petcoke dust is a major source of polycyclic aromatic hydrocarbons in the Athabasca Oil Sands Region. Environ Sci Technol 50:1711–1720. https://doi.org/10.1021/acs.est.5b05092
Acknowledgements
Thanks to D. Muir, J. Kirk, X. Wang, J. Keating, A. Gleason, J. Wiklund, C. Cooke, and personnel from Environment and Climate Change Canada’s Centre for Inland Waters for their contributions in the field and the lab. Additionally, thanks to colleagues from Queen’s University’s Paleoecological Environmental Assessment and Research Laboratory. This study was supported by the Canada-Alberta Joint Oil Sands Monitoring Program (www.jointoilsandsmonitoring.ca; 2012–2014) and the Natural Sciences and Engineering Research Council of Canada (www.nserc-crsng.gc.ca; Grant Nos. 2360-2009 to Smol).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. S1
Radionuclide activity versus depth and date versus depth for cores from the 23 lakes that were included and/or considered for inclusion in the study. Profiles are arranged by the year the sites were cored (indicated in parentheses after the site name). Decay curves of total 210Po activity, which was used as proxy for total 210Pb activity, are shown by black circles and the associated error bars (± 1 SD). Background 210Pb activity, also known as supported 210Pb, was measured using 226Ra as a proxy and is plotted on the same scale as total 210Po activity (black dashed line). Dates calculated using the constant rate of supply (CRS) model are shown by grey circles and the associated error bars (± 2 SD) (TIF 101909 kb)
ESM Fig. S2
Detrended correspondence analysis (DCA) plot including species scores from the first two ordination axes. Taxa are indicated by numbers that are listed in ESM Table S3. Assemblage tops were included actively in the DCA whereas bottoms were included passively (TIF 101909 kb)
ESM Table S1
Summary of environmental variables included in the principal components analysis, the sources of the measurements, and the transformations applied to achieve approximately normal distributions (XLSX 11 kb)
ESM Table S2
Summary of geographic lake characteristics and core information for each of the 18 lakes (XLSX 10 kb)
ESM Table S3
Taxa represented by numbers in the detrended correspondence analysis (DCA) plot, including species scores from the first two ordination axes (XLSX 11 kb)
ESM Table S4
Taxa included in the complexes and summed groups shown in the histogram of diatom relative abundances (Fig. 3) (XLSX 12 kb)
Rights and permissions
About this article
Cite this article
Summers, J.C., Rühland, K.M., Kurek, J. et al. A diatom-based paleolimnological survey of environmental changes since ~ 1850 in 18 shallow lakes of the Athabasca Oil Sands Region, Canada. J Paleolimnol 61, 147–163 (2019). https://doi.org/10.1007/s10933-018-0050-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-018-0050-z