Abstract
Adsorptive removal of toxic metals from water using materials with high removal capability and sufficient environmental degradability is of great importance. In this study, a novel nanocomposite adsorbent was synthesized by reinforcing poly(vinyl alcohol)/alginate (PVA/Alg) hydrogel using cysteine-modified bentonite (Cys-BNT). The synthesized composite was characterized using XRD, FTIR, and TEM techniques and subsequently used to remove Pb from aqueous solutions. The factors that affect the Pb adsorption of the composite, including solution pH, Pb concentration, and time were also evaluated. The XRD and TEM results revealed the coexistence of exfoliated and intercalated BNT layers in the polymer matrix. The Pb removal was pH-dependent with the highest Pb removal efficiency at pH 5.0. The incorporation of Cys-BNT significantly (P < 0.05) enhanced the maximum Pb adsorption capacity of PVA/Alg hydrogel from 480 to 995 μmol g−1. The Elovich model was the best to fit the Pb adsorption kinetic data, and the adsorption equilibrium time was 4 h. The Pb removal rate was also significantly (P < 0.05) increased through embedding the Cys-BNT into the PVA/Alg hybrid. Overall, the prepared Cys-BNT/PVA/Alg nanocomposite exhibits a great adsorption efficiency and a rapid adsorption behavior, making it a suitable material to remove Pb from polluted waters.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution is a worldwide crisis caused by various chemicals released from agricultural, industrial, and municipal activities, leading to enormous economic, environmental, and human health problems [1]. Lead (Pb) is among the most hazardous metals commonly found in polluted waters, affecting human health worldwide by inducing detrimental health disorders such as damage to the nervous, renal, and reproductive systems [2, 3]. According to the US Environmental Protection Agency (EPA), the maximum permissible limit of Pb in drinking water is 0.015 mg/L [4]. Lead concentrations in industrial wastewaters approach 200–500 mg/L; this concentration is very high in water quality standards, and the Pb concentration of wastewaters must be reduced to a level of 0.05–0.10 mg/L before discharging to waterways or sewage systems [5]. Hence, developing efficient purification technologies to remove Pb from water is critically required [6].
Several separation technologies such as membrane process, distillation, electro-dialysis, flotation, photo-catalysis, and chemical coagulation have been applied to remove Pb and other toxic metals from water [7]. Nevertheless, most of these methods show some disadvantages, including excessive operation and maintenance costs, low selectivity, insufficient removal capacity, creating secondary sludge, and persistent requisition for chemicals [8]. Therefore, it is crucial to develop efficient, eco-friendly, and inexpensive treatment methods to remove metals from water. As a highly efficient, easily operated, and affordable technique, adsorption has been extensively used for removing Pb from aqueous solutions [9,10,11]. Some extensively used adsorbents for the Pb removal include activated carbons [12], clay minerals [13], biomass and lignocellulosic materials [14], industrial byproducts [11], and polymers [15].
Natural and synthetic polymers, containing numerous coordinating groups on their backbones and side-chains, are effective adsorbents for metal removal from water [15]. Polyvinyl alcohol (PVA) is a hydroxyl-rich, non-hazardous, and biodegradable synthetic polymer that strongly interacts with metals [16]. However, pure hydrogels made from PVA are mechanically unstable and should be blended with other polymers or clay fillers to form more mechanically robust hydrogel networks [17]. Alginate (Alg) is a natural, highly hydrophilic biopolymer containing 1,4-linked β-D-mannuronic and α-L-guluronic acid residues [18]. Alginate contains abundant carboxyl groups which can potentially react with polyvalent cations through unidentate or bidentate coordination [19, 20], making it a suitable material for water purification purposes. Nevertheless, the application of Alg-based hydrogels in water treatment practices is limited by their low mechanical strength and high solubility in water [21]. Combination of Alg with PVA forms hydrogels [22] which have been successfully applied for cell immobilization and controlled release of agrochemicals and pharmaceuticals [23,24,25,26]. However, Alg/PVA hydrogels have been scarcely used for the adsorptive removal of metal ions from polluted waters. High dissolution rate and weak mechanical strength are potential limitations of PVA/Alg hydrogels for use in water treatment practices. Hence, these hydrogels are often reinforced by various fillers like expandable clays to form nanocomposites [27, 28].
The expandable clay minerals, especially montmorillonite, are usually considered fillers in nanocomposite preparation because of their excellent structural stability, great surface area, non-toxicity, and low cost. Bentonite (BNT) is a geological material that mainly consists of montmorillonite (MMT) clay. The crystal units (layers) of MMT are constituted by an octahedral sheet sandwiched between two tetrahedral sheets. Isomorphic substitution in the octahedral sheet (Mg2+ for Al3+) results in an overall negative charge, which is counterbalanced by interlayer cations such as Na+, Ca2+, etc. [29]. BNT has been extensively used to produce clay-polymer nanocomposites, given its outstanding physicochemical properties [30,31,32,33]. Strong interfacial interaction between the clay filler and polymer matrix is crucial to obtain the required dispersion of the filler. Hence, surface modification of the clay particles using coupling agents, such as amino acids, is usually performed to introduce functional groups on the clay surfaces, enhancing the interaction between the phases [15].
Recently, nanocomposites containing PVA and BNT exhibit promising results in metal and metalloid removals from water. For example, Wang et al. [33] prepared a chitosan–poly (vinyl alcohol)/bentonite nanocomposite showing a high adsorption capacity for Hg as 460 mg g−1. Baigorria et al. [34] used PVA/Alg hydrogel beads containing natural BNT for As removal from polluted water and showed that clay incorporation played a crucial role in the As removal efficiency. Sanchez et al. [35] also developed novel eco-friendly hydrogel adsorbents based on PVA and an acid-treated BNT. They reported that the presence of acid BNT was beneficial for improving the removal capacity of PVA-based hydrogels. Although considerable studies have been carried out on the different aspects of PVA-clay nanocomposites, there is still a necessity to develop new nanocomposites that possess high adsorption potentials for toxic metals.
In this paper, we prepared and characterized a novel polymer/clay nanocomposite by dispersing a cysteine-modified BNT in a PVA/Alg matrix. The cysteine amino acid modifier was used to functionalize the BNT surface and make it more interactive with PVA and Alg polymers. The synthesized nanocomposite was further utilized for the removal of Pb from aqueous solutions. To the best of our knowledge, no previous research has been conducted using this starting materials’ combination to synthesize adsorbent for Pb removal from aqueous solutions.
Materials and Methods
Materials
All applied chemicals were analytical grade, and distilled water was used in all experimental processes. The PVA with a polymerization degree of 1500 (molecular weight: 66,000 g mol−1) and l-cysteine (99%, Mw = 121.15 g mol−1) were supplied by Dae-Jung Chemicals and Metals Co., Korea. Sodium alginate (Alg) was obtained from Sigma–Aldrich with a mannuronate/guluronate ratio of 39/61 and a molecular weight of 120–190 kDa. The natural BNT from the Mehredjan mine (33° 36′ 7″ N, 55° 10′ 4″ E) was applied in the study. The elemental analysis of the BNT sample was performed using X-ray fluorescence spectrometry (XRF) and is represented in Table 1. The cation exchange capacity (CEC) of the BNT was 66.0 cmol kg−1 as measured by the ammonium acetate method [36]. A stock solution containing 1000 mg Pb L−1 was produced by dissolving PbNO3 (> 99%, Merck) in distilled water and subsequently used for preparing the dilutions.
Surface Modification of BNT with Cysteine
Bentonite (3.0 g., dry basis) was added to 75 mL distilled water and agitated for 2 h at 60 °C. Subsequently, 30 mL of 0.2 M l-cysteine was slowly added to the suspension and stirred for another 3 h. The suspension was centrifuged, and the resulting modified clay (Cys-BNT) was separated using filter paper [37]. Finally, the Cys-BNT was freeze-dried and stored at room temperature.
Synthesis of Cys-BNT/PVA/Alg Nanocomposites
Three grams of PVA and 0.3 g of Na–Alg were mixed with 100 mL distilled water and stirred for 3 h with a magnetic stirrer at 60 °C. After 2 h, 10 mL boric acid 6% was added to the solution for developing chemical bonds between the two polymers. Subsequently, the final solution was undergone a freezing-melting period (24 h under − 20 °C and then 4 h under room temperature) to create a physical bond between these components. Subsequently, a 10% Cys-BNT suspension (10 mL) was added to the PVA/Alg solution and stirred for 2 h. The mixture was then placed at room temperature for 42 h to cause more interaction between the various components. Finally, the produced Cys-BNT/PVA/Alg composite was sediment by adding 100 mL of acetone. The nanocomposite was washed with distilled water, dried at 100 °C for 12 h, and powdered in liquid nitrogen. The same procedure was carried out to prepare the BNT/PVA/Alg composite except that BNT instead of Cys-BNT was used.
Characterization
X-ray diffraction (XRD) analysis was performed using a Philips PW1730, (Philips, Eindhoven, Netherlands) X-ray diffractometer with Cu-Kα radiation (λ = 1.54056 Å) operated at 40 kV and 30 mA. The data were collected in the 2θ range of 2°–80° with a step size of 0.05° and a counting time per step of 1 s.
Fourier transform infrared (FT-IR) spectra were obtained between 4000 and 400 cm−1 on an IR spectrometer (JASCO-680, Tokyo, Japan) using the standard KBr pellet disc technique. Each IR spectrum was an accumulation of 32 scans at a resolution of 4 cm−1.
The morphology of the nanocomposites was observed using a transmission electron microscope (TEM) (Philips, CM120, Eindhoven, Netherland) with an accelerating voltage of 100 kV.
Lead Adsorption Kinetics
To determine the equilibrium time and rate of Pb adsorption on the composites, kinetic experiments were performed. Samples (0.02 g) of the adsorbents were weighed in polyethylene containers in triplicates. Subsequently, 10 mL of a solution containing a Pb concentration of 100 μmol L−1 was added to each container. The suspensions were shaken in an incubator shaker (30 °C, 180 rpm) for different time intervals of 0.25 to 9 h. Then, the suspensions were centrifuged for 10 min at 3500 rpm, and the resulting supernatants were analyzed for Pb concentration by atomic absorption spectroscopy (AAS) performed with a Perkin-Elmer AAnalyst 200 model.
The adsorbed Pb per mass unit of the sorbents was calculated according to the following formula:
where qt is the amount of Pb adsorbed on the adsorbent at time t (μmol g−1), C0 and Ct are Pb concentrations (μmol L−1) at times 0 and t, respectively, V is the volume of the solution (L), and W is the mass of the adsorbent used (g).
Finally, the pseudo-first-order, pseudo-second-order, Power function, Elovich, and parabolic diffusion models (Table 2) were used to describe the experimental data.
Lead Adsorption Isotherms
Lead adsorption isotherms by PVA/Alg, BNT/PVA/Alg, and Cys-BNT/PVA/Alg composites were determined using the batch method. Samples (0.02 g) of each composite were added to 15 mL of solutions containing Pb with concentrations ranging from 25 to 1200 μmol L−1. The mixtures were shaken for 4 h, as determined in the preliminary kinetic test, in an incubator shaker (30 °C, 180 rpm). A control sample without adsorbent was also considered for each Pb concentration. After equilibration, the suspensions were centrifuged for 10 min at 4500×g and the supernatants were separated and analyzed for Pb concentration by the AAS. The adsorbed amount of metal in the systems was calculated according to Eq. 2:
where qe is the Pb adsorbed at equilibrium (μmol g−1), C0 and Ce are Pb concentrations (μmol L−1) at initial and equilibrium conditions, respectively, V is the solution volume (L), and W is the adsorbent mass (g).
The Langmuir and Freundlich models were fitted to the Pb adsorption equilibrium data. The parameters of the isothermal models are described in Table 3.
Effect of pH on Pb Removal
The Pb adsorption experiments were carried out at different pH values to determine the optimum range for Pb adsorption. For this purpose, 15 mL of Pb solution (97 μmol L−1) and 0.02 g adsorbent were added to each container. Afterward, the pH values of the suspensions were adjusted from 2 to 7 either by 0.1 M HCl or 0.1 M NaOH before they were shaken in an incubator-shaker for 4 h. The suspensions were then centrifuged for 10 min at 3500 rpm and passed through a filter paper. Finally, the Pb concentration in the solution was measured by the AAS.
The PHREEQC geochemical model (version 2.18.00) was applied to estimate the dominant Pb species in solutions. The saturation index (SI) was also calculated according to Eq. (3), to determine if the solutions were supersaturated with respect to the Pb minerals:
where IAP is the ion activity product and Ksp is the solubility product constant of the mineral. A negative SI value indicates under-saturation, while a positive one represents super-saturation, with respect to a given mineral.
Model Fitting and Statistical Analysis
Kinetic and isothermal models were fitted to the Pb adsorption data using the nonlinear regression method in Graphpad Prism 8.4.2 software (GraphPad Software, Inc., CA, USA). The goodness-of-fit of the models for describing the Pb adsorption data was assessed using the determination coefficients (R2), and the standard errors of estimate (SEE) were calculated as follows:
where q and q' are measured and predicted Pb adsorbed values, respectively, and n is the number of measurements.
Statistical comparison between the model parameters was performed using the extra sum-of-squares F test in Graphpad Prism 8.4.2 (GraphPad Software, Inc., CA, USA).
Results and Discussion
X-ray Diffraction Patterns
Figure 1 represents the XRD diffractograms of the composites prepared in this study. The XRD pattern of the PVA/Alg composite exhibited only one broad peak at 2θ = 19.5°. This peak corresponds to the semicrystalline PVA structure, consistent with previous reports [38,39,40].
Figure 1 also shows that the raw BNT sample mainly consisted of montmorillonite (MMT), quartz (QTZ), and cristobalite (CST). The MMT characteristic peak was observed at 2θ = 7.25°, attributed to basal spacing (d 001) of 1.21 nm, which is consistent with a Na-MMT. The diffraction peak of MMT shifted to a lower 2θ value of 6.09° as a result of the clay treatment with Cys (Fig. 1), which indicates that the basal spacing of MMT was increased to 1.45 nm due to the intercalation of Cys molecules into the MMT layers. This weakens attractive forces and lowers the energy barrier needed to exfoliate the clay lamellae into the polymer matrix. The results are consistent with that reported by Öztürk et al. [37], showing that the Cys molecules diffused into the BNT interlayers spaces as the d001 peak shifted from 1.27 nm to 1.40 nm after Cys intercalation. Ahmad and Mirza [30] also reported that l-methionine amino acid successfully penetrated the MMT internal spaces and increased the distance between the clay layers, as revealed by an XRD analysis.
The XRD analysis was used to investigate the exfoliation/intercalation of the clay in the polymer matrix. Based on the wide angle (Fig. 1) and the low angle (Fig. 2) XRD diffractograms, the d001 diffraction peak on the XRD pattern corresponding to basal spacing of MMT disappeared for the Cys-BNT/PVA/Alg nanocomposite, suggesting that the parallel arrangement of the clay layers was not maintained due to the exfoliation of clay layers within the PVA/Alg matrix. Jose et al. [41] reported that BNT nanoclays were intercalated in the PVA matrix at lower concentrations and exfoliated at higher concentrations. Exfoliation of BNT in kappa-carrageenan/PVA and chitosan/PVA composites have also been reported by Hosseinzadeh et al. [42] and Wang et al. [33], respectively. Nevertheless, Morgan and Gilman [43] stated that the absence of a peak is not proof of exfoliation, and the configuration of the clay within the polymer should be confirmed using TEM techniques.
The PVA characteristic peak at 2θ = 19.5° was broader in the Cys-BNT/PVA/Alg diffractogram than in the PVA/Alg diffractogram, indicating the crystallinity of the PVA polymer reduced with the clay addition.
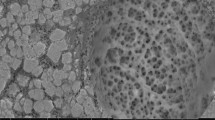
TEM Images
TEM analysis was adopted to further validate and confirm the structure, which was presumed through XRD measurements. The TEM images of the Cys-BNT/PVA/Alg nanocomposite is shown in Fig. 3. The images showed regions where intercalated tactoids of clay present. However, in some parts, the bentonite layers are discrete and dispersed within the polymer matrix accompanied by the formation of exfoliated structure, which was confirmed by the XRD analysis. Hence, a nanocomposite with a mixture of intercalated and exfoliated microstructures was formed. Using XRD and TEM techniques, Hosseinzadeh et al. [42] also showed that both intercalated and exfoliated structures of κ-carrageenan/poly(vinyl alcohol) nanocomposite hydrogel were produced after montmorillonite incorporation. Generally, a completely exfoliated structure on a polymer–clay nanocomposite is scarce and difficult to obtain, and the majority of the nanocomposites have mixed structures [44].
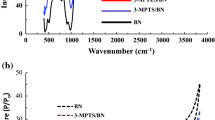
FT-IR Spectra
The FT-IR spectra of BNT, Cys-BNT, PVA/Alg, BNT/PVA/Alg, and Cys-BNT/PVA/Alg are shown in Fig. 4. The peaks at 3434 cm−1 and 3630 cm−1 were assigned to asymmetric stretching vibrations of O–H in the Si–OH and Al–OH groups of the MMT, and water molecules, respectively [45,46,47]. The peak at 1638 cm−1 can be related to the bending of OH groups in water molecules [48]. The bands at 1087 cm−1 and 1041 cm−1 are related to Si–O–Si or Si–O stretching in the bentonite structure. The characteristic bands at 795 cm−1, 621 cm−1, 518 cm−1, and 469 cm−1 are related to Si–O in quartz and silica structures, Al–O and Si–O out-of-plane vibration, Si–O–Al bending, and Si–O–Si bending, respectively [41].
The modification of BNT with cysteine noticeably changed the FT-IR spectrum of BNT (Fig. 4). For instance, several new bands in the 2900–3420 cm−1 range correspond to the –NH2 group, 1550–1600 cm−1 range, and 1382 cm−1 related to –COO− group, 1294 cm−1 associated with C–H bending, and 600–800 cm−1 range attributed to C–S groups [49, 50] appeared on FT-IR spectrum of BNT after Cy’s adsorption. The existing IR absorption bands of –COO− and –NH2 in the Cys-BNT spectrum confirm that the surface modification of the BNT by Cys was successfully achieved.
The FT-IR spectrum of the PVA/Alg composite shows the characteristic peak of –OH in the 1350–3550 cm−1, which is attributed to the OH stretching vibration of the intermolecular and intramolecular hydrogen bindings [51]. The band at 2924 cm−1 is related to the symmetric, and asymmetric stretching vibrations of the CH2 group, and the one at 1735 cm−1 is associated with the acetate groups from polyvinyl acetate [52]. The peak at 1628 cm−1 can be attributed to symmetrical vibration or deformation of H–OH. The 1429 and 1383 cm−1 bands are attributed to the vibrations of the C–H groups. The symmetric stretching vibration of –COOH has also been reported at this wavenumber [53]. The band centered at 1258 cm−1 is related to the symmetric C–C or C–O stretching as a part of the polymer chain [52, 54]. The peak at 1093 cm−1 is due to, C–O and O–H stretching and C–H deformation in PVA/Alg composite [55]. The band at 831 cm−1 is related to –CH3 vibration, and that at 603 cm−1 is assigned to C–C–O and C–C–H groups. The peak of 415 cm−1 can be attributed to the vibration of the C–OH bond.
When BNT was added to the PVA/Alg composite, some changes occurred in the IR spectrum. For example, the 1258 cm−1 peak shifted to 1262 cm−1, the 415 cm−1 peaks moved to 423 cm−1, and the 831 and 603 cm−1 peaks disappeared, reflecting the interaction between PVA/Alg and clay particles.
When Cys-BNT was added to the PVA/Alg composite, the peak at 3420 cm−1 was shifted to 3434 cm−1, probably due to increased hydrogen bonding in the Cys-BNT/PVA/Alg in the presence of the –NH groups of the Cys amino acid. Incorporation of Cys–BNT eliminated the peak at 1429 cm−1 in the PVA/Alg spectrum related to vibration of the C–H bands. The peak at 1429 cm−1 in the PVA/Alg spectrum related to vibration of the C–H bands was eliminated after the incorporation of Cys–BNT. Instead, a band at 1458 cm−1 appeared in the Cys-BNT/PVA/Alg spectrum, which may be related to the NH-induced vibration caused by the presence of Cys-BNT. Moreover, the peak at 1903 cm−1 in the PVA/Alg composite shifted to 1120 cm−1 in the presence of Cys-BNT, and its intensity decreased consequently (Fig. 4). This peak may be due to the presence of C–N, indicating a proper interaction of Cys-BNT and PVA/Alg. Also, the peak at 831 cm−1 was shifted to 849 cm−1, and its intensity decreased, which can be attributed to the –CH3 and C–S band vibrations [56]. Moreover, in the spectrum of this nanocomposite, the peak of 603 cm−1 disappeared.
Lead Adsorption Isotherms
The Langmuir and Freundlich isotherm constants, determination coefficients, and standard errors of estimates are listed in Table 4. The results indicate that both models adequately explained the experimental data, but the Langmuir model fits the equilibrium data better (Table 4, Fig. 5). Shooto et al. [57] and Ren et al. [58] also successfully used the Langmuir model to describe Pb adsorption on poly (vinyl alcohol) hydrogel strengthened with nanofibers and alginate/carboxymethyl cellulose gel, respectively. Similarly, adsorption of heavy metals by poly (vinyl alcohol)/carboxymethyl starch- grafted poly(vinyl imidazole) composite beads obeyed the Langmuir equation [59].
Lead adsorption isotherms of the poly (vinyl alcohol)/alginate (PVA/Alg), bentonite/poly (vinyl alcohol)/alginate (BNT/PVA/Alg), and cysteine-bentonite/poly (vinyl alcohol)/alginate (Cys-BNT/PVA/A) composites fitted by the Longmuir (a) and Freundlich (b) models. (T: 30 °C; sorbent dosage, 1.33 g L−1; agitation speed: 180 rpm; contact time: 4 h; C0: 25 to 1200 µmol Pb L−1)
The maximum Pb adsorption capacity (qmax) of PVA/Alg, BNT/PVA/Alg, and Cys-BNT/PVA/Alg composites were 480, 724, and 995 μmol g−1, respectively, indicating a significant improvement in Pb adsorption potential of PVA/Alg hydrogel after modification by BNT and particularly Cys-BNT. The Freundlich KF parameter, an adsorption capacity factor, also increased from 578 for the PVA/Alg to 979 and 1395 for the BNT/PVA/Alg and Cys-BNT/PVA/Alg, respectively (Table 4). The increased Pb sorption capacity of PVA/Alg after BNT addition is probably due to the development of new sorption surfaces as a result of the expansion or exfoliation of the MMT layers. The more significant role of Cys-BNT than BNT in enhancing the sorption capacity of PVA/Alg hydrogel might be due to the higher surface functional groups of Cys-BNT, as confirmed by FT-IR analysis. Faghihian and Nejati-Yazdinejad [60] also found that the Pb adsorption capacity of Cys-BNT was higher than that of natural BNT. Table 5 compares the Pb sorption capacities of various polymer-based composites reported in previous articles with those of the Cys-BNT/PVA/Alg and BNT/PVA/Alg composites attained in this study. Even though it is somewhat rough to compare the sorption capacities obtained under dissimilar experimental setups, the Cys-BNT/PVA/Alg synthesized in the current study has a relatively high Pb sorption capacity.
The Langmuir KL constant was 20.85, 16.50, and 9.76 L mmol−1 for the PVA/Alg, BNT/PVA/Alg, and Cys-BNT/PVA/Alg samples, respectively, indicating that the overall affinity of the PVA/Alg active sites for Pb binding is higher than those of the BNT/PVA/Alg, and Cys-BNT/PVA/Alg. The value of the Freundlich N parameter was 0.36 for PVA/Alg, which increased to 0.42 and 0.50 after the incorporation of BNT and Cys-BNT (Table 4), confirming that PVA/Alg functional groups form stronger bonds with Pb ions as the smaller Freundlich N parameter suggests the formation of stronger bonds [61].
Effect of pH on Pb Removal
The solution pH is a significant factor regulating the adsorption of heavy metals. The impact of solution pH on Pb adsorption was explored by ranging the pH value of the metal solution from 2.0 to 7.0. The results showed that the adsorption was maximum at pH 5.0 and decreased as the solution pH was raised or lowered (Fig. 6). The maximum removal efficiency of Pb ions by the PVA/Alg, BNT/PVA/Alg, and Cys-BNT/PVA/Alg composites at the optimum pH value were about 50%, 61%, and 75%, respectively. Free Pb2+ ions were the dominated Pb species in solutions in the pH range of 2–7 (Table 6). At the low pH values, the H+ concentration is far higher than that of the Pb ions; therefore H+ ions compete with the Pb ions for chemisorption on the surface functional groups [62, 63]. Moreover, the protonation of amino groups on the composite surfaces produces positive charges, preventing the metal cations from moving toward the adsorbent surface through repulsive forces [64]. Formation of hydrolyzed species Pb(OH)+ and Pb precipitate (Pb(OH)2), as revealed from speciation calculations (Table 6), could be possible factors reducing the Pb removal efficiency past the optimum pH value [65]. Anitha et al. (2015) also found that the maximum removal of Pb by poly (vinyl alcohol)/chitosan was obtained at pH 5. The same pH values were also reported by Jamnongkan et al. [66] as the optimum pH value for Cu removal by poly (vinyl alcohol) hydrogels.
Kinetics of Pb Removal
The Pb adsorption process by the composites was rapid and reached equilibrium in almost 4 h (Fig. 7). The fast adsorption might be due to plenty of high-energy empty sites accessible for binding Pb ions, which give rise to immediate Pb adsorption. Over 60% of the Pb adsorption occurred within the first hour, indicating a high binding affinity between Pb ions and active sites on the composite surfaces. The available active sites on the composites were gradually occupied over time, and the adsorption rate gradually decreased. Abraham et al. [67] reported the equilibrium time of 8 h for Pb adsorption by Poly (vinyl alcohol)‐based MWCNT hydrogel.
Time-dependent Pb adsorption data of the poly (vinyl alcohol)/alginate (PVA/Alg), bentonite/poly (vinyl alcohol)/alginate (BNT/PVA/Alg), and cysteine-bentonite/Poly (vinyl alcohol)/alginate (Cys-BNT/PVA/Alg) composites fitted by the pseudo-first-order (A), pseudo-second-order (B), Elovich (C), power function (D), and parabolic diffusion (E) models. (T: 30 °C; sorbent dosage, 2 g L−1; agitation speed: 180 rpm; contact time: 0.25 to 9 h; C0: 100 μmol Pb L−1)
The time-dependent Pb adsorption onto the composites significantly (P < 0.01) fitted by the pseudo-first-order, pseudo-second-order, Elovich, power function, and intraparticle diffusion models. The Elovich model was superior in describing the kinetic data according to the R2 values presented in Table 7. Based on the kinetic results, incorporation of Cys-BNT significantly (P < 0.05) increased the amount and rate of Pb adsorption by the PVA/Alg hydrogel, while the increases induced by BNT addition were not statistically significant at P < 0.05 (Table 7).
The determination coefficients for the first-order kinetic model were 0.69–0.90 (Table 7). The calculated qe1 values were 29.7, 30.3, and 33.5 mmol kg−1 for the PVA/Alg, BNT/PVA/Alg, and Cys-BNT/PVA/Alg composites, respectively, which agreed well with the experimentally measured Pb adsorption at equilibrium. The Pb adsorption rate constant k1 was found to be 0.917, 1.088, and 1.552 h−1 for the PVA/Alg, BNT/PVA/Alg, and Cys-BNT/PVA/Alg, respectively (Table 7), indicating the shortest Pb adsorption reaction time on Cys-BNT/PVA/Alg if a fixed amount of Pb is present in the system.
The pseudo-second-order parameters, qe2 and k2, obtained from the pseudo-second-order plots are presented in Table 7. The R2 values (0.84–0.95) were higher compared to those of the pseudo-first-order model. The estimated qe2 values (34.0, 34.2, and 36.9 mmol kg−1 for PVA/Alg, BNT/PVA/Alg, and Cys-BNT/PVA/Alg, respectively) also agree with the results obtained from the pseudo-second-order kinetics. The k2 value increased from 0.035 for PVA/Alg to 0.044 and 0.062 g μmol−1 h−1 for the BNT/PVA/Alg and Cys-BNT/PVA/Alg, respectively. The calculated initial Pb adsorption rates (ρ) for the PVA/Alg were 41.0 μmol g −1 h−1 which increased to 51.1 and 83.9 μmol g −1 h−1 after incorporation of BNT and Cys-BNT, respectively.
The Elovich model fitted the Pb adsorption better than the pseudo-first-order, and the pseudo-second-order models with R2 values ranged from 0.91 to 0.95 (Table 7). The Elovich α and β constants, related to the initial adsorption rate and surface coverage, respectively [68], were found to increase with the addition of BNT and Cys-BNT to the PVA/Alg. Therefore, the incorporation of BNT and Cys-BNT increased the rate of Pb adsorption as well as the available adsorption surface for Pb ions (Table 7). However, only the enhancing effects of Cys-BNT were statistically significant (P < 0.05).
The power function model was also able to describe the adsorption kinetics of Pb by all adsorbents and was the best-fitted model for BNT/PVA/Alg nanocomposite Pb adsorption kinetic data (Table 7). The addition of BNT and Cys-BNT to the PVA/Alg composite significantly increased the specific Pb adsorption rate at the unit time (ab).
Finally, the kinetic data were adequately (R2 = 0.86–0.91) described by the Weber and Morris intraparticle diffusion model (Table 7). The calculated intraparticle diffusion coefficient (kdif) and intercept (C) constants for the Pb sorption on the composites are listed in Table 7. The C constant, which reflects the boundary layer effect, significantly (P < 0.05) increased from 9.7 for the PVA/Alg to 11.5 and 16.6 for the BNT/PVA/Alg and Cys-BNT/PVA/Alg, respectively, suggesting a higher contribution of surface adsorption reactions in the rate-controlling step in Pb retention after modification of the PVA/Alg by BNT and Cys-BNT [69].
Conclusions
A poly(vinyl alcohol) (PVA)/alginate (Alg) hydrogel containing cysteine-functionalized bentonite (Cys-BNT) was developed, characterized, and evaluated as a potential device for Pb removal from aqueous solutions. Morphological studies using TEM revealed the excellent dispersion of the clay particles into the polymer matrix, producing both exfoliated and intercalated regions. These results agree well with those obtained in the XRD analysis.
The synthesized nanocomposite was successfully used for Pb removal from aqueous solutions. The incorporation of Cys-BNT caused a significant increase in both the capacity and the rate of Pb adsorption by the PVA/Alg hydrogel. The Cys-BNT/PVA/Alg nanocomposite showed a high Pb adsorption capacity of 995 μmol g−1 in a short equilibrium time. The estimated maximum adsorption capacity of the nanocomposite was considerably higher than those of many polymer-based composite adsorbents reported in the literature. Therefore, the synthesized Cys-BNT/PVA/Alg nanocomposite can be effectively used as a suitable adsorbent material for effective and fast removal of Pb from aqueous solutions.
Data Availability
The data are available from the corresponding author on request.
Code Availability
No special code was applied in the study.
References
Jayaswal K, Sahu V, Gurjar BR (2018) Water pollution, human health and remediation. In: Bhattacharya S, Gupta AB, Gupta A, Pandey A (eds) Water remediation. Springer, Singapore, pp 11–27
RoyChowdhury A, Datta R, Sarkar D (2018) Heavy metal pollution and remediation. In: Török B, Dransfield T (eds) Green chemistry. Elsevier, Amsterdam, pp 359–373
Schweitzer L, Noblet J (2018) Chapter - water contamination and pollution. In: Török B, Dransfield T (eds) Green chemistry. Elsevier, Amsterdam, pp 261–290
EPA U (2003) Environmental Protection Agency: National primary and secondary drinking water standard. Office of Water (4606M), EPA 816-F-03
Arbabi M, Hemati S, Amiri M (2015) Removal of lead ions from industrial wastewater: A review of Removal methods. Int J Epidemiol Res 2:105–109
Pereira CP, Goldenstein JPN, Bassin JP (2022) Industrial wastewater contaminants and their hazardous impacts. In: Selvasembian R, Singh P (eds) Biosorption for wastewater contaminants. Wiley, Pondicherry, pp 1–22
Bolisetty S, Peydayesh M, Mezzenga R (2019) Sustainable technologies for water purification from heavy metals: review and analysis. Chem Soc Rev 48:463–487
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq 290:111197
Gupta VK, Sharma S (2003) Removal of zinc from aqueous solutions using bagasse fly ash: a low cost adsorbent. Ind Eng Chem Res 42:6619–6624
Joseph L, Jun B-M, Flora JRV, Park CM, Yoon Y (2019) Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere 229:142–159
Singh NB, Nagpal G, Rachna SA (2018) Water purification by using adsorbents: a review. Environ Technol Innov 11:187–240
Periyasamy S, Kumar IA, Viswanathan N (2020) Activated carbon from different waste materials for the removal of toxic metals. In: Naushad M, Lichtfouse E (eds) Green materials for wastewater treatment. Springer International Publishing, Cham, pp 47–68
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462
Salman M, Athar M, Farooq U (2015) Biosorption of heavy metals from aqueous solutions using indigenous and modified lignocellulosic materials. Rev Environ Sci Biotechnol 14:211–228
Rivas BL, Sánchez J, Urbano BF (2016) Polymers and nanocomposites: synthesis and metal ion pollutant uptake. Polym Int 65:255–267
Wang L-Y, Wang M-J (2016) Removal of heavy metal ions by poly(vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze-thaw method. ACS Sustain Chem Eng 4:2830–2837
Kumar A, Han SS (2017) PVA-based hydrogels for tissue engineering: A review. Int J Polym Mater Polym Biomater 66:159–182
Wang B, Wan Y, Zheng Y, Lee X, Liu T, Yu Z, Huang J, Ok YS, Chen J, Gao B (2019) Alginate-based composites for environmental applications: a critical review. Crit Rev Environ Sci Technol 49:318–356
Attar K, Demey H, Bouazza D, Sastre MA (2019) Sorption and desorption studies of Pb(II) and Ni(II) from aqueous solutions by a new composite based on alginate and magadiite materials. Polymers 11:340
Papageorgiou SK, Kouvelos EP, Favvas EP, Sapalidis AA, Romanos GE, Katsaros FK (2010) Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr Res 345:469–473
Hajiali H, Heredia-Guerrero JA, Liakos I, Athanassiou A, Mele E (2015) Alginate nanofibrous mats with adjustable degradation rate for regenerative medicine. Biomacromol 16:936–943
Shen W, Hsieh Y-L (2014) Biocompatible sodium alginate fibers by aqueous processing and physical crosslinking. Carbohydr Polym 102:893–900
Flórez-Castillo JM, Ropero-Vega JL, Perullini M, Jobbágy M (2019) Biopolymeric pellets of polyvinyl alcohol and alginate for the encapsulation of Ib-M6 peptide and its antimicrobial activity against E. coli. Heliyon 5:01872
Kamoun EA, Kenawy E-RS, Tamer TM, El-Meligy MA, Mohy Eldin MS (2015) Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: characterization and bio-evaluation. Arab J Chem 8:38–47
Russo R, Giuliani A, Immirzi B, Malinconico M, Romano G (2004) Alginate/Polyvinylalcohol Blends for Agricultural Applications: Structure-Properties Correlation, Mechanical Properties and Greenhouse Effect Evaluation. Macromol Symp 218:241–250
Şanlı O, Ay N, Işıklan N (2007) Release characteristics of diclofenac sodium from poly(vinyl alcohol)/sodium alginate and poly(vinyl alcohol)-grafted-poly(acrylamide)/sodium alginate blend beads. Eur J Pharm Biopharm 65:204–214
Ali MHM, Kahder MM, Al-Saad KA, Al-Meer S (2013) Properties of nanoclay PVA composites materials. QSci connect 2013:1–9
Karimi A, Wan Daud WMA (2017) Materials, preparation, and characterization of PVA/MMT nanocomposite hydrogels: a review. Polym Compos 38:1086–1102
Zhou C, Tong D, Yu W (2019) Smectite nanomaterials: preparation, properties, and functional applications. In: Wang A, Wang W (eds) Nanomaterials from clay minerals. Elsevier, Amsterdam, pp 335–364
Ahmad R, Mirza A (2015) Sequestration of heavy metal ions by Methionine modified bentonite/Alginate (Meth-bent/Alg): a bionanocomposite. Groundw Sustain Dev 1:50–58
He YF, Zhang L, Yan DZ, Liu SL, Wang H, Li HR, Wang RM (2012) Poly(acrylic acid) modifying bentonite with in-situ polymerization for removing lead ions. Water Sci Technol 65:1383–1391
Rijith S, Anirudhan TS, Sumi VS, Anitha PK, Abhilash S, Shibli SMA (2015) Carboxylate functionalized Chitosan/Bentonite composite matrix as a cation exchanger for the removal of Pb(II) from aqueous media: Kinetic and equilibrium studies. Orient J Chem 31:1113–1120
Wang X, Yang L, Zhang J, Wang C, Li Q (2014) Preparation and characterization of chitosan–poly (vinyl alcohol)/bentonite nanocomposites for adsorption of Hg (II) ions. Chem Eng J 251:404–412
Baigorria E, Cano LA, Sanchez LM, Alvarez VA, Ollier RP (2020) Bentonite-composite polyvinyl alcohol/alginate hydrogel beads: Preparation, characterization and their use as arsenic removal devices. Environ Nanotechnol Monit Manag 14:100364
Sanchez LM, Alvarez VA, Ollier RP (2019) Acid-treated bentonite as filler in the development of novel composite PVA hydrogels. J Appl Polym Sci 136:47663
Rhoades J (1983) Cation exchange capacity. In: Page C (ed) Methods of soil analysis: Part 2 chemical and microbiological properties. ASA Press, Madison, pp 149–157
Öztürk N, Tabak A, Akgöl S, Denizli A (2008) Reversible immobilization of catalase by using a novel bentonite–cysteine (Bent–Cys) microcomposite affinity sorbents. Colloids Surf A 322:148–154
Fan L, Du Y, Wang X, Huang R, Zhang L, Hu L (2005) Preparation and characterization of alginate/poly (vinyl alcohol) blend fibers. J Macromol Sci A 42:41–50
Golafshan N, Rezahasani R, Tarkesh Esfahani M, Kharaziha M, Khorasani SN (2017) Nanohybrid hydrogels of laponite: PVA-alginate as a potential wound healing material. Carbohydr Polym 176:392–401
Hema M, Selvasekarapandian S, Arunkumar D, Sakunthala A, Nithya H (2009) FTIR, XRD and ac impedance spectroscopic study on PVA based polymer electrolyte doped with NH4X (X=Cl, Br, I). J Non-Cryst Solids 355:84–90
Jose T, George SC, Maria HJ, Wilson R, Thomas S (2014) Effect of bentonite clay on the mechanical, thermal, and pervaporation performance of the poly (vinyl alcohol) nanocomposite membranes. Ind Eng Chem Res 53:16820–16831
Hosseinzadeh H, Zoroufi S, Mahdavinia GR (2015) Study on adsorption of cationic dye on novel kappa-carrageenan/poly (vinyl alcohol)/montmorillonite nanocomposite hydrogels. Polym Bull 72:1339–1363
Morgan AB, Gilman JW (2003) Characterization of polymer-layered silicate (clay) nanocomposites by transmission electron microscopy and X-ray diffraction: a comparative study. J Appl Polym Sci 87:1329–1338
Ismail NHC, Akil HM (2018) Effects of organomodified muscovite on the properties of acrylonitrile–butadiene–styrene nanocomposites. J Appl Polym Sci 135:46827
Castellini E, Malferrari D, Bernini F, Brigatti MF, Castro GR, Medici L, Mucci A, Borsari M (2017) Baseline studies of the clay minerals society source clay montmorillonite stx-1b. Clays Clay Miner 65:220–233
Madejová J (2003) FTIR techniques in clay mineral studies. Vib Spectrosc 31:1–10
Slaný M, Jankovič Ľ, Madejová J (2019) Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl Clay Sci 176:11–20
Che C, Glotch TD, Bish DL, Michalski JR, Xu W (2011) Spectroscopic study of the dehydration and/or dehydroxylation of phyllosilicate and zeolite minerals. J Geophys Res Planets 116:1–23
Panhwar S, Hassan SS, Mahar RB, Canlier A, Sirajuddin AM (2018) Synthesis of l-cysteine capped silver nanoparticles in acidic media at room temperature and detailed characterization. J Inorg Organomet Polym Mater 28:863–870
Parsons JG, Dokken KM, McClure J, Gardea-Torresdey JL (2013) FTIR, XAS, and XRD study of cadmium complexes with l-cysteine. Polyhedron 56:237–242
Wu J, Yu H-Q (2007) Biosorption of 2, 4-dichlorophenol by immobilized white-rot fungus Phanerochaete chrysosporium from aqueous solutions. Bioresour Technol 98:253–259
Mansur HS, Sadahira CM, Souza AN, Mansur AA (2008) FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater Sci Eng C 28:539–548
Dong Y, Zhang L, Shen J, Song M, Chen H (2006) Preparation of poly (vinyl alcohol)-sodium alginate hollow-fiber composite membranes and pervaporation dehydration characterization of aqueous alcohol mixtures. Desalination 193:202–210
Mansur HS, Oréfice RL, Mansur AA (2004) Characterization of poly (vinyl alcohol)/poly (ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 45:7193–7202
Awadhia A, Agrawal S (2007) Structural, thermal and electrical characterizations of PVA: DMSO: NH4SCN gel electrolytes. Solid State Ion 178:951–958
Abd El-Latif M, El-Kady M, Ibrahim AM, Ossman M (2010) Alginate/polyvinyl alcohol-kaolin composite for removal of methylene blue from aqueous solution in a batch stirred tank reactor. J Am Sci 6:280–292
Shooto ND, Wankasi D, Sikhwivhilu LM, Dikio ED (2016) Modified electro-spun polyvinyl alcohol nanofibers used as super adsorbing material for lead ions in aqueous solution. J Residuals Sci Technol 13:233–242
Ren H, Gao Z, Wu D, Jiang J, Sun Y, Luo C (2016) Efficient Pb (II) removal using sodium alginate–carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr Polym 137:402–409
Pour ZS, Ghaemy M (2015) Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly (vinyl alcohol)/carboxymethyl starch-g-poly (vinyl imidazole). RSC Adv 5:64106–64118
Faghihian H, Nejati-Yazdinejad M (2009) Sorption performance of cysteine-modified bentonite in heavy metals uptake. J Serbian Chem Soc 74:189
USEPA (2009) Drinking water treatability database, GAC Isotherm, US Environmental Protection Agency, Cincinnati
Bulut Y, Aydın H (2006) A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194:259–267
Yang H, Wang JH, Cheng KP, Yan MT, Qiao CZ (2013) Adsorption of Pb (II) on Hydroxyapatite/(Poly Vinyl Alcohol) Blend Material. Adv Mater Res 781:2253–2256
Hameed B, Mahmoud D, Ahmad A (2008) Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste. J Hazard Mater 158:65–72
Igberase E, Osifo P, Ofomaja A (2018) Adsorption of metal ions by microwave assisted grafting of cross-linked chitosan beads: equilibrium, isotherm, thermodynamic and desorption studies. Appl Organomet Chem 32:4131
Jamnongkan T, Kantarot K, Niemtang K, Pansila PP, Wattanakornsiri A (2014) Kinetics and mechanism of adsorptive removal of copper from aqueous solution with poly (vinyl alcohol) hydrogel. Trans Nonfer Met Soc China 24:3386–3393
Abraham TN, Kumar R, Misra R, Jain S (2012) Poly (vinyl alcohol)-based MWCNT hydrogel for lead ion removal from contaminated water. J Appl Polym Sci 125:E670–E674
Teng H, Hsieh C-T (1999) Activation energy for oxygen chemisorption on carbon at low temperatures. Ind Eng Chem Res 38:292–297
Metwally SS, Rizk HE (2014) Preparation and characterization of nano-sized iron–titanium mixed oxide for removal of some lanthanides from aqueous solution. Sep Sci Technol 49:2426–2436
Sahmetlioglu E, Yilmaz E, Aktas E, Soylak M (2014) Polypyrrole/multi-walled carbon nanotube composite for the solid phase extraction of lead(II) in water samples. Talanta 119:447–451
Lim CW, Song K, Kim SH (2012) Synthesis of PPy/silica nanocomposites with cratered surfaces and their application in heavy metal extraction. J Ind Eng Chem 18:24–28
Nyairo WN, Eker YR, Kowenje C, Akin I, Bingol H, Tor A, Ongeri DM (2018) Efficient adsorption of lead (II) and copper (II) from aqueous phase using oxidized multiwalled carbon nanotubes/polypyrrole composite. Sep Sci Technol 53:1498–1510
Badruddoza AZM, Shawon ZBZ, Tay WJD, Hidajat K, Uddin MS (2013) Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr Polym 91:322–332
Jiang N, Xu Y, Dai Y, Luo W, Dai L (2012) Polyaniline nanofibers assembled on alginate microsphere for Cu2+ and Pb2+ uptake. J Hazard Mater 215–216:17–24
Ghoul M, Bacquet M, Morcellet M (2003) Uptake of heavy metals from synthetic aqueous solutions using modified PEI—silica gels. Water Res 37:729–734
Nazarzadeh Zare E, Mansour Lakouraj M, Ramezani A (2016) Efficient sorption of Pb(ii) from an aqueous solution using a poly(aniline-co-3-aminobenzoic acid)-based magnetic core–shell nanocomposite. New J Chem 40:2521–2529
Ermolenko A, Vikulova M, Shevelev A, Mastalygina E, Ogbuna Offor P, Konyukhov Y, Razinov A, Gorokhovsky A, Burmistrov I (2020) Sorbent based on polyvinyl butyral and potassium polytitanate for purifying wastewater from heavy metal ions. Processes 8:690
Khalek M, Mahmoud GA, El-Kelesh NA (2012) Synthesis and characterization of poly-methacrylic acid grafted chitosan-bentonite composite and its application for heavy metals recovery. Chem Mater Res 2:16
Funding
This research was funded by the Isfahan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to Participate
This research did not involve human participants, identifiable human data, or human tissue.
Consent for Publication
This research did not involve human participants, identifiable human data, or human tissue.
Ethical Approval
No animals or human participants, their data, or biological materials were included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salehi, T., Shirvani, M., Dinari, M. et al. Adsorptive Removal of Lead from Water Using a Novel Cysteine-Bentonite/Poly(vinyl alcohol)/Alginate Nanocomposite. J Polym Environ 30, 4463–4478 (2022). https://doi.org/10.1007/s10924-022-02530-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02530-1