Abstract
Recently, polyfurfuryl alcohol (PFA) based material has been gaining attention. Despite its use as an intermediate in various industries, the degradation process of PFA has rarely been reported. In this study, neat PFA (PF) and polylactic acid (PLA) incorporated PFA (PF-PL) based thermoset biopolymers were prepared by casting method. The degradation of the prepared biopolymer specimens was carried out under environmental conditions via soil-burial test and photo-degradation method for 21-months. The extent of degradation of PF and PF-PL was assessed by evaluating weight loss, variation in mechanical properties and change in complex viscosity. Structural and morphological changes of degraded PF and PF-PL samples were evaluated by Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM), respectively. Weight loss percentage in case of photo-degraded samples was found to be much higher compared to soil buried specimens. SEM micrographs showed a blistered surface and visible cracks on the surface of soil buried and photo-degraded samples, respectively. FTIR spectra of photo-degraded samples showed a new peak at 673 cm−1 indicating the furan ring opening during the degradation process. Significant variation in mechanical properties of PF and PF-PL specimens after soil-burial test also indicated biodegradable nature of the biopolymers. Approximately 45% and 63% of loss in tensile strength was obtained in PF and PF-PL soil buried specimens, respectively. Complex viscosity was lowest for PF and PF-PL samples after 21 months of degradation compared to undegraded specimens thus indirectly indicating the decrease in molecular weight after degradation. All the obtained data revealed the fragmentation of biopolymers, hence supporting the biodegradable nature of PFA-based biopolymer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The invention of synthetic polymers has introduced humankind to a new paradigm. They gained popularity instantly because of their durability and low cost. As a result, by 2015, plastic production had reached 381 million tonnes compared to 1950 when only about 2 million tonnes of plastics were produced per year, increasing almost 200 times [1].
But, because of the recalcitrant nature, their disposal has become the biggest threat [2]. Every minute, about 1 million plastic drinking bottles are consumed worldwide, and, every year, around 5 trillion single-use plastic bags are utilized. For the last 60 years (starting in 1950), an estimated 8.3 billion tonnes of plastic have been produced. 60% of those produced plastics have been dumped into the natural environment [1]. The primary source of plastic production is non-renewable sources e.g., oil, natural gas and, coal. If this trend continues, plastic production could consume almost 20% of the total oil production in the next few decades [1]. In the last two decades, the world has become more aware and concerned about the environment. It is a well-known fact that the surplus use of synthetic polymers has adverse effects on our ecosystem. Hence, the focus has been shifted from using synthetic to natural polymers [2].
Polyfurfuryl alcohol (PFA), a green thermoset polymer, has the potential to meet the requirements [3]. Furfuryl alcohol (FA) is the building (monomeric) unit of PFA biopolymer which can be obtained from wastes of sugarcane i.e., bagasse, corn cobs, and other agricultural wastes containing pentoses. The uncontrolled exothermic reactions during FA polymerization prevent the commercialization of PFA. Kumar and Anandjiwala in 2011 reported an acid catalyzed polymerization of FA with step-wise curing of FA to PFA and hence eliminating the risk of explosion [4]. Thus, PFA-based polymers can now be explored to prepare eco-friendly products [5]. FA-based thermoset resins have a vast array of applications as wood adhesives, binder, polymer concretes, solvent, corrosion-resistant coatings, etc. [6]. Due to heat resistant nature and good mechanical properties, PFA has high demand in foundry industries, metal casting cores, and molds. PFA also plays a key role in the production of carbonaceous products and bio-based nanocomposites [6, 7]. Nowadays, PFA is also being employed in wood stabilization, PFA- silica materials and sol-gel based nanocomposites [8].
The most alluring aspect of PFA- based biopolymers is the capacity of FA to degrade by micro-organisms in general and enzymes in particular [9]. In order to assess the potential of any biopolymer as a commodity material, it is vital to study its degradation aspect in environmental conditions [10]. Intrinsic degradation property can ultimately aid to plan the safe disposal method for used biopolymers. Also, the extent of stability of biopolymer can help to target the end-use applications of the products. In addition to this, any kind of modification (e.g. addition of additives or stabilizers) can also be apprehended for preparing an improved biopolymer with required properties [11]. Many physical and chemical ways for degradation of phenolic resins containing aromatic ring have been reported; but these methods are energy consuming and uneconomical. Hence, alternative methods like soil-burial test and photo-degradation method are being focused on degradation of both neat and modified polymer containing aromatic rings.
According to few reports, soil-burial test is considered as a viable way to study the degradation process of biopolymers [12]. In this method, polymers may get attacked by various micro-organisms (bacteria/fungi), which release enzymes capable of degrading chemical bonds of polymer chains leading to the depolymerization step. It was reported by Wang et al. that specific enzymes cause primary cleavage in polymer chain, and microbes utilize the resulting residual fragments as carbon source [13]. Photo-degradation of polymers is an advanced oxidation process capable for degrading aromatic compounds [13]. According to various reports, UV radiation is one of the most destructive environmental factors. Literature states that UV radiation causes photooxidative reaction leading to polymer chain breakage with the release of free radicals. This depolymerization results in decrease in the weight and mechanical properties of the polymer. The present study attempts to degrade neat PFA (PF) and PLA incorporated PFA (PF-PL) through soil-burial method and photo-degradation. Following the work of Sharib et al., PLA has been used to prepare incorporated PFA biopolymers. PLA is one of the biodegradable polymers produced prominently at industrial scale [14]. It is mainly obtained from renewable resources such as corn, starch, sugar etc. It is well reported that PLA degrades spontaneously in the environment due to a hydrolysis mechanism in which water molecules break the ester bonds that make up the polymer backbone. However, PLA stent or fibers when exposed to in-vivo conditions do not begin to degrade till 12 months approximately [15]. PLA degradation has been studied in animal and human bodies for medical applications like implants, surgical sutures, and drug delivery materials [16]. Considering the biodegradable nature of PLA, its incorporation into PFA based biopolymer may increase its biodegradability. Due to its environmental credit and good material properties, PLA and its copolymer are being used to manufacture medical devices, plastic film, bottles etc. [17].
Degradation was monitored structurally and morphologically by FTIR and SEM, in addition, to change in weight, complex viscosity and mechanical properties. The prime concern is to design such polymers which retain their strength while in use and can be biodegradable afterward. This study will provide essential information about the extents of degradability and stability of biopolymer specimens during the degradation process so that appropriate use can be planned.
Materials and Methods
Materials
Furfuryl alcohol (FA) from Sigma Aldrich, Germany, was used as a monomer for the preparation of biopolymer specimens (PFA). P-toluene sulphonic acid (PTSA) supplied by Sigma Aldrich, Germany, was used as a catalyst in FA polymerization. PLA fibre was purchased from Dalian Impex International trading, China. Citric acid, sodium hydroxide, and chloroform were procured from Merck, India. Silicon mold was used for the preparation of biopolymer specimens. For biodegradation of biopolymers in soil, the soil sample was aseptically collected from industrial effluent contaminated areas near the Indian Institute of Carpet Technology (IICT), Bhadoi (Uttar Pradesh), India.
Preparation of Neat and PLA Incorporated PFA Specimen
For assessing the biodegradability of FA-based biopolymers, degradation of both PF and PF-PL biopolymers has been studied. As reported by Kumar and Anandjiwala, the process of PFA formation involves condensation reaction of FA in the presence of PTSA as an acid catalyst [4]. For preparing PF samples, 10 ml of PTSA solution (0.3% (w/v) wrt FA) was added in 50 ml of FA. The acid-catalyzed FA solution was poured in a silicon mould at room temperature and kept undisturbed for about 4 h. Then, the mould containing acid-catalyzed FA was kept in an oven at 50 °C for 96 h to harden (curing). Finally, the casted samples were kept for 1 h at 100 °C and at 150 °C for 30 min to get completely cured neat PFA biopolymeric samples [14]. To prepare PF-PL bioplastic samples, 0.5% (w/v) of PLA fabric was added in FA. The resulting mixture was magnetically stirred for about 45 min to ensure complete mixing of PLA fabric into FA. Then, the above process was followed for the preparation of PLA incorporated PFA samples also, but as reported by Sharib et al. 1st step for curing samples was carried out in an oven at 50 °C for just 22 h [14]. PF and PF-PL based biopolymeric samples have been successfully prepared for degradation study.
Degradability of PF and PF-PL Biopolymers by Different Methods
Two processes, viz. soil-burial and photo-degradation test have been performed to assess the degradation potential of PF and PF-PL biopolymers in environmental conditions. The prepared biopolymer specimens were buried 30 cm deep under the soil surface (Fig. 1). Garden soil was mixed with soil sample collected from effluent, mainly aromatic compounds exposed area of Indian Institute of Carpet Technology (IICT) Bhadoi (Uttar Pradesh), India, for this purpose. The collected soil sample was first homogenized and then mixed with garden soil. The test was planned to be carried out for 21 months, and samples (in triplicates) were taken out every 3 months initially and then after 6 months of the incubation period for the studies related to the extent of degradation. PF and PF-PL based biopolymers after 0, 3, 6, 9, 15, and 21 months of soil-burial degradation have been designated as PF-0 M to PF-21 M, and PF-PL-0 M to PF-PL-21 M, respectively.
a Photograph showing prepared biopolymers, b homogenization of soil sample collected from IICT, Bhadoi, India c biopolymer samples tied in wire boxes and buried 30 cm below the soil surface, d setup showing biopolymer specimens inside a transparent box to be kept under direct sunlight. In the Figure, mode I and II represent soil burial degradation process and photo-degradation method
After the designated time, the degraded PF and PF-PL based biopolymers were taken out one after another, rinsed carefully with water, treated with 0.1 M NaOH, and then dried in a hot air oven at 60 °C for 24 h for the weight loss measurements after biodegradation [18]. Thus, initially degradation was evaluated by analysing change in weight of the samples (before and after degradation) and changes in the mechanical properties and complex viscosity.
Similarly, to find out the extent of photo-degradation of PF and PF-PL based biopolymers, samples were kept under direct sunlight for 21 months. Again, the samples were taken out at every 3 months initially and then after 6 months of the incubation period for the degradation study. Prior to keeping the samples in under sunlight (UV), samples were uniformly cut and weighed [18]. PF and PF-PL based biopolymers after 0, 3, 6, 9, 15, and 21 months of photo-degradation are designated as Ph-PF-0 M to Ph-PF-21 M, and Ph-PF-PL-0 M to Ph-PF-PL-21 M, respectively.
Analysis of the Extent of Degradation PF and PF-PL Based Biopolymer
Weight Loss
Initial weights of the prepared samples were taken in order to compare the degraded and undegraded samples. The weight of solid samples was measured after an interval of three months or six months and is designated as Mt. The obtained weight was compared to the initial weight (M0) [19]. Percentage degree of degradation (D) was then calculated as.
where, M0 = weight of undegraded sample; Mt = weight of degraded samples after t months.
Mechanical Properties
Any mechanical change in a polymer can be easily assessed by monitoring variation in its mechanical properties [20]. Tensile strength, elongation at break and Young’s modulus of the prepared dumbbell shaped biopolymer samples (PF and PF-PL) before and after degradation were determined using the tensile testing machine (Lloyd, LR 100 K, USA) following ASTM D638 at IIT Patna, India. The test rate was maintained between 1 and 3 mm min−1. Dynamic mechanical analysis (DMA) was also performed to measure the complex viscosity (indirectly for analyzing the change in molecular weight) and storage modulus of degraded and undegraded PFA based biopolymers at varying temperature. It was done using a dynamic mechanical analyzer (DMA8000, PerkinElmer, Buckinghamshire, UK) with dual cantilever at a frequency of 1 Hz. Neat and incorporated PFA biopolymer with dimensions 40 mm ×10 mm (length × width) were used for testing at the temperature ranging from room temperature (35 °C) to 190 °C. The heating rate was maintained at 2 °C min−1.
Any mechanical change in a polymer can be easily assessed by monitoring variation in its mechanical properties [20]. Tensile strength, elongation at break and Young’s modulus of the prepared dumbbell shaped biopolymer samples (PF and PF-PL) before and after degradation were determined using the tensile testing machine (Lloyd, LR 100 K, USA) following ASTM D638 at IIT Patna, India. The test rate was maintained between 1 and 3 mm min−1. Dynamic mechanical analysis (DMA) was also performed to measure the complex viscosity (indirectly for analyzing the change in molecular weight) and storage modulus of degraded and undegraded PFA based biopolymers at varying temperature. It was done using a dynamic mechanical analyzer (DMA8000, PerkinElmer, Buckinghamshire, UK) with dual cantilever at a frequency of 1 Hz. Neat and incorporated PFA biopolymer with dimensions 40 mm ×10 mm (length × width) were used for testing at the temperature ranging from room temperature (35 °C) to 190 °C. The heating rate was maintained at 2oC min−1.
Water Uptake
The capacity of any biopolymer to absorb water from the environment may lead to change the basic properties of the polymer. Thus, it is crucial to find out water uptake nature of PF and PF-PL before and after degradation. Water uptake of the biopolymer specimens was analyzed using the standard protocol of ASTM D570-81 [21]. The prepared biopolymer specimens were first weighed and then immersed in distilled water for 24 h at 24 ± 1 °C. After 24 h, excess water was removed, and weight of the specimens after 24 h was noted [22]. Water uptake was calculated in terms of percentage of increase in weight as.
where, W0 = weight of biopolymer specimen; W1 = weight of specimen after immersion in water for 24 h.
Structural and Morphological Changes
Variation at the molecular level and morphological changes have been characterized by FTIR and SEM. FTIR studies of biopolymer samples (PF and PF-PL) before and after degradation were conducted using Perkin- Elmer at IIT Patna (40 scans at a resolution of 4 cm−1). FTIR spectra were recorded in order to locate change in any functional group after degradation either by soil-burial test or photo-degradation method. Spectra were taken between 400 and 4000 cm−1 (wave number). Further, to detect any change on surface morphology of the biopolymer specimens after degradation, SEM was performed. Surface morphology of undegraded and degraded samples in the soil buried well as photo-degraded biopolymeric samples were carried out on EVO-SEM 15/18 (Carl Zeiss Microscopy, Ltd) at an accelerating voltage of 20 kV. The samples were coated with gold prior to subjecting it for the SEM analysis.
Results and Discussion
Evaluating Degradation Depending Upon Weight Change
The extent of biodegradation (percentage) in terms of weight change obtained for PF and PF-PL biopolymeric specimens are given in Table 1. There was less than 1% degradation of the biopolymers in the soil-burial method for both neat as well as incorporated samples. In the case of photo-degradation, there was around 2.7% degradation for PF and about 3.6% degradation in PF-PL biopolymer samples. Thus, degradation in terms of weight loss is less for the soil-burial test as compared to photo-degradation, and the differences in the weight loss could be related to the catalytic effect of UV radiation on the degradation process of biopolymer.
Mechanical Properties and Water Uptake
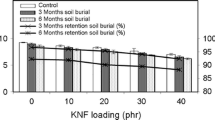
Biodegradation of biopolymers under soil-burial test was monitored through change in mechanical properties. Obtained results (Figs. 2 and 3) depict the decrease in tensile properties like tensile strength (TS) and elongation at break (%E) with the increase in degradation time (incubation time). The reduction in TS properties increases the brittleness of biopolymeric samples which, thus, can be related to the degradable nature of PFA with time.
After comparing the maximum and lowest values obtained for TS, it was found that the decrease in TS for PF specimens was around 45%, while for PF-PL based biopolymeric samples, it was around 63% after 21 months of the soil-burial test (Table 2). A similar trend of tensile properties was reported with PLA incorporated PFA samples by Sharib et al. while working with different concentrations of PLA in PF-PL specimens [14]. It can thus be assumed that the incorporation of PLA enhances the decrease in TS of PF-PL biopolymeric samples. No significant changes in the weight of the specimens were obtained after immersing the degraded samples for 24 h in distilled water. Negligible water uptake by PF and PF-PL biopolymeric samples indicates the hydrophobic nature of PFA even after 21 months of degradation.
Measuring the viscosity of polymer solutions is another technique to figure out the polymer’s size, hence chain length and molecular weight. Figure 4a shows the complex viscosity of degraded PFA based biopolymeric specimens after different time intervals with respect to temperature. It is evident from Fig. 4 that the complex viscosity and storage modulus of PF specimens decrease with an increase in the degradation time. It is lowest after 21 months of soil burial degradation. Similarly, Fig. 5a shows the complex viscosity of degraded PLA incorporated PFA based biopolymeric specimens after different time intervals with respect to temperature. Low complex viscosity, as observed in Figs. 4a and 5a, indicates less drag and intermolecular attraction in the degraded PF and PF-PL specimens. Storage modulus decreased with the increase in degradation time for PF-PL samples (Fig. 5b) indicating the same trend as observed for degraded PF samples (Fig. 4b). The decrease in intermolecular bonding is caused due to degradation during the soil burial processes that have resulted in lower storage modulus for the degraded biopolymeric specimens.
As explained by Lucas et al. degradation of any polymeric material cannot always end up in carbon recycling and mineralization. The process of degradation is a very complex event and can end up at any step [23]. So, the change in mechanical properties and complex viscosity of the PF or PF-PL biopolymeric specimens can be due to the fragmentation of biopolymers. Based on the obtained results, we propose the degradation pathways for PF and PF-PL samples after soil-burial tests in Fig. 6. Before degradation, the PF and PF-PL specimens were long chains of crosslinked polymer (black and red lines). After biodegradation (soil-burial test), the long chain polymer, breaks into several short-chain polymers resulting in decrease in TS as observed in Figs. 2 and 3, in addition to weight loss as shown in Table 1. The minor change in weight and reasonably good TS after the soil burial method indicates the stability of PFA-based biopolymer samples supporting its use in various sectors yet showing its biodegradable nature.
Morphological Aspects
Figures 7, 8, 9 and 10 show the morphology of undegraded and degraded PF and PF-PL samples. Figures 7b, c and 8b, c show the surface morphology of PF and PF-PL samples, respectively after 6 months and 21 months of soil-burial tests. Small holes were found on the surface of the specimens after 6 months of the soil burial process. Images of the specimens after 21 months indicated a comparatively rough and weathered surface with a large number of holes (Figs. 7c and 8c), indicating the degradation of PFA samples which is confirmed by low weight loss as well as decrease in TS and storage modulus. Similar results in form of small gaps and blisters were obtained in the case of PF-PL based biopolymeric samples (Fig. 8b, c).
On the contrary to the soil-burial test, significant changes in the form of cracks on the surface of both neat as well as incorporated samples (Figs. 9b and 10b) were obtained in photo-degraded specimens after 6 months. SEM micrographs after 21 months showed that the biopolymer surface had been degraded into various parts as the cracks must have been increased as well as deepened with time (Figs. 9c and 10c). This could be due to exposure to UV light which may have led to the removal of formaldehyde from the PFA polymeric backbone resulting in PFA with only methylene linkages (discussed later in the pathway). These fissures and gaps observed in SEM micrographs can be correlated with the higher weight loss as obtained after photo-degradation as compared to soil-burial degradation method (Table 1). It is also important to mention that if we compare micrographs of PF and PF-PL samples, it is clearly seen that extent of weathering or biofragmentation is more in PF-PL samples after the degradation.
FTIR Studies
FTIR spectra of PF and PF-PL based biopolymeric samples after the soil-burial test are given in Fig. 11. The identifying peaks of PFA are as follows: 596 cm−1 is attributed to the furan rings of PFA, 788 cm−1 and 876 cm−1 are related to the in-plane deformation mode of the ring and out of plane deformation of CH bonds (Fig. 11a). The peak at 1546 cm−1 is related to –C=C– stretching in 2-5 di-substituted furanic ring [24]. In addition to these fingerprint regions, PLA incorporated in PFA shows a peak at 1745, which is attributed to the C=O stretching of the ester bond (Fig. 11b) [14]. FTIR spectrum obtained after the soil-burial test (Fig. 11a, b) shows shifting and changes in the shape of the peaks at 596 cm−1 and 788 cm−1. The shifting in peaks to lower frequency from 1745 cm−1 to 1743 cm−1 for PF-PL based biopolymeric samples can also be observed, which may be attributed to depolymerization of the PFA-based polymer, which indicates the start of degradation of polymers. The depolymerization of PFA-based biopolymeric specimens will result in decreased mechanical properties of the degraded samples, which are confirmed from Figs. 2 and 3.
Figure 12a, b show the FTIR spectra for photo-degraded PF and PF-PL samples. In photo-degraded samples, major peaks were almost similar, as observed in Fig. 11a, b for PF and PF-PL samples. However, the differences in few peaks, which are characteristic of photo-degradation of PF and PF-PL samples, are highlighted here. Results of photo-degradation of both types of specimen show the appearance of a new peak at 673 cm−1. This peak attributes to mono or di-substituted benzene ring [25]. During acid-catalyzed polymerization, the color of PFA changes from brown to black after polymerization; hence, the role of a chromophoric group can also be contemplated. Recently, Tondi et al. in 2019 has given pathways for the development of chromophoric group in PFA [26, 27]. Thus, we may assume that during photo-degradation of PF and PF-PL samples, photo-oxidation leads to furanic ring- opening resulting in Diels-Alder rearrangements that form benzene ring. Figure 13b pathway shows the formation of benzene ring after Diels alder reaction [28]. However, this is possible only if the acid catalyzed reaction follows a pathway where PFA is joined by only a methylene linkage that is obtained after the removal of formaldehyde from PFA samples during photo-degradation Fig. 13a.
Conclusions
In-depth knowledge of the degradability nature of biopolymer is crucial for developing efficient PFA biopolymer and that will help to achieve the way it has been targeted for end-use. Change in weight, decrease in tensile properties, storage modulus and complex viscosity as well as shifting of FTIR peaks of soil buried specimens, can be considered as a positive indication of the degradable nature of the biopolymer. SEM images clearly showed the modification on the surface of the biopolymers in the form of holes after the soil-burial degradation process. It is noteworthy to mention that these results also help to predict the stability of biopolymer when used in various products. In photo-degraded PFA or PLA incorporated PFA, there is a higher magnitude of weight change. SEM images of photo-degraded samples clearly showed various cracks, which can be due to the emission of formaldehyde. The appearance of a new peak at 673 cm−1 in case of photo-degraded samples indicates the aromatization of PFA ring. Thus, it may be stated that the PFA samples degrade slowly under soil-burial degradation as compared to the photo-degradation process. However, further study is needed to unfold the steps required for the processing of PFA-based polymers to be utilized in different sectors. Like the addition of stabilizers or antioxidants to prevent the crack in PFA based biopolymers upon exposure to sunlight. Moreover, for degradation of the biopolymers combination of both photo-degradation and soil-burial may give more efficient degradation results. PFA-based biopolymers can be first exposed to direct sunlight, which will catalyze the depolymerization process and then subjecting it for microbial degradation via the soil-burial method.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Geyer R, Jambeck JR, Law KL (2017) Sci Adv 3:25–29
Okada M, Okada Y, Tao A, Aoi K (1996) J Appl Polym Sci 62:2257–2265
Singh P, Kumar R (2019) J Polym Environ 27:901–906
Kumar R, Kumar R, Anandjiwala R (2012) Plast Rubber Compos 41:1–7
Spange S (2000) Prog Polym Sci 25:781–849
Guigo N, Mija A, Vincent L, Sbirrazzuoli N (2010) Eur Polym J 46:1016–1023
Toriz G, Arvidsson R, Westin M, Gatenholm P (2003) J Appl Polym Sci 88:337–345
Grund S, Kempe P, Baumann G, Seifert A, Spange S (2007) Angew Chem—Int Ed 46:628–632
Kumar R, Rashmi D (2018) World J Microbiol Biotechnol 34:1–9
Pischedda A, Tosin M, Degli-Innocenti F (2019) Polym Degrad Stab 170:109017
Kumar AP, Depan D, Singh Tomer N, Singh RP (2009) Prog Polym Sci 34:479–515
Obasi HC, Igwe IO, Madufor IC (2013) Adv Mater Sci Eng 2013:326538
Wang H, Wei D, Zheng A, Xiao H (2015) Polym Degrad Stab 116:14–22
Sharib M, Kumar R, Kumar KD (2018) J Therm Anal Calorim 132:1593–1600
Bergstrom JS, Hayman D (2016) Ann Biomed Eng 44:330–340
Hamad K, Kaseem M, Yang HW, Deri F, Ko YG (2015) Express Polym Lett 9:435–455
Bhushan B, Kumar R (2019) Plasma treated and untreated thermoplastic biopolymer/biocomposites in tissue engineering and biodegradable implants. In: Grumezescu A, Holban A-M (eds) Materials for biomedical engineering: hydrogels and polymer-based scaffolds. Elsevier, United States, pp 339–369
Irin SC, Begila DS (2014) Int J Chem Stud 2:46–54
Radu ER, Panaitescu DM, Nicolae CA, Gabori RA, Rădiţoiu V, Stoian S, Alexandrescu V, Fierăscu R, Chiulan I (2021) J Polym Environ 29:2310–2320
Kausch HH (2005) Macromol Symp 225:165–178
Kazemi NS, Mostafazadeh MM, Chaharmahali M (2010) J Polym Environ 18:720–726
Riyajan SA, Sukhlaaied W (2019) J Polym Environ 27:1918–1936
Lucas N, Bienaime C, Belloy C, Queneudec M, Silvestre F, Saucedo JE (2008) Chemosphere 73:429–442
Yao J, Wang H, Liu J, YuChan K, Zhang L, Xu N (2005) Carbon 43:1709–1715
Moazzen K, Zohuriaan-Mehr MJ, Jahanmardi R, Kabiri K (2018) J Appl Polym Sci 135:45921
Tondi G, Cefarin N, Sepperer T, D’Amico F, Berger RJF, Musso M, Birarda G, Reyer A, Schnabel T, Vaccari L (2019) Polymers 11:1–15
Tondi G, Link M, Oo CW, Petutschnigg A (2015) J Spectrosc 3:1–8
Kumar R (2012) Recent Patents Catal 1:35–42
Funding
Author Ms. Priyaragini Singh is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for financial assistance in the form of Senior Research fellowship Grant (Sanction Letter No. 09/1144(0002)/2018-EMR-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work cited in this paper.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Only subscription based.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, P., Kumar, K.D. & Kumar, R. Degradation of Polyfurfuryl Alcohol-Based Biopolymer by Soil-Burial and Photo-Degradation Methods. J Polym Environ 30, 1920–1931 (2022). https://doi.org/10.1007/s10924-021-02330-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02330-z