Abstract
Decomposition of used poly(ethylene terephthalate) (PET) bottles was accomplished by methanolytic degradation using bamboo leaf ash (BLA) as a green and highly efficient heterogeneous catalytic system. The reaction at 200 °C in an autoclave reactor gave dimethyl terephthalate (DMT) and ethylene glycol (EG) in 78% and 76% yields, respectively. The chemical and physical characteristics of the prepared catalyst were studied using X-ray fluorescence (XRF), Fourier-transform infrared spectroscopy (FTIR), X-ray diffractograms (XRD), Energy dispersive X-ray spectrometry (EDX), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Thermal Gravimetric Analysis (TGA) and Brunauer–Emmett–Teller (BET) analyses. The conversion of PET to DMT and EG was completed within 2 h, and the crude as well as the recrystallized products were characterized using HPLC, NMR and IR. The catalyst, owing to its excellent thermal stability, mesoporous nature, bio-compatibility, cost free, easy preparation, recyclability and efficacy make it an attractive alternative and greener solid catalyst for PET depolymerization.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics have become unescapable in our daily life owing to their low cost of production, durability, high tensile strength and an unabated growth of usage in different sectors. The annual production of polymeric materials was 381 million tons in 2015 [1], and poly(ethylene terephthalate) (PET) shared 13% of the global plastic output [2, 3]. The thermal and mechanical features are outstanding [4, 5], for which PET is widely used as food and beverage packing materials to the electronics and textile industry [6,7,8]. The capability to produce a variety of various quality of different molecular weights in a single operation is the primary reason for the extensive application of PET in the pharmaceutical fields [9, 10]. However, extensive use of such polymeric materials, often limited to their single-used coupled with their non-biodegradability has generated a huge problem with respect to its disposal, with volume and range used increases dramatically in recent years [11, 12]. In addition, when it comes to total solid trash generated globally, PET waste contributes 8 percent and 12 percent by weight and volume of the total garbage produced [13, 14]. It was also estimated that India alone has generated 62 million tons of municipal solid waste every year, which is foreseen to expand at a rate of 1–2% annually, of which plastic wastes account for 5–6% of the total solid waste generated [15, 16]. With these continuous increase in the productions of PET waste and its ability to withstand biological and atmospheric agents, plastic waste has put forward a huge environmental as well as economic problems and there is a high demand for its proper recycling [10, 17,18,19]. With the exception to its motive towards ecological incentive, recycling of PET waste highly entertained savings of raw petrochemical products and energy [20,21,22,23]. Notably, when compared with products obtained from virgin grade resin, the same products obtained from recycled plastic waste results in 50–60% energy saving [3].
Currently, primary recycling, secondary or mechanical recycling, tertiary or chemical recycling, and plastic’s energy content retrieval through incineration (quaternary recycling) are the pragmatic accessible recycling processes of PET [24,25,26]. Primary recycling involves reprocessing of uncontaminated low grade industrial waste generated by the manufacturing industry that is deprived of the desired standard [25]. Mechanical recycling involves segregation of PET materials from its contaminants which are then re-utilized by melt extrusion into granules [27]. Chemical recycling, which is referred to as a well adopted and sustainable recycling method transformed PET back to its monomer or secondary value-added products [25, 28, 29]. There have been many reports on chemical recycling methods that use various catalysts. These include aminolysis, ammonolysis, glycolysis, hydrolysis, and methanolysis. [11, 30, 31]. The energy recovery process through incineration of PET waste encounters strong societal opposition due to its high environmental impacts [16, 24]. Only the chemical recycling method, which results in the regeneration of monomers from which polymers are built, was found to meet the criteria of sustainable development among the recycling procedures discussed above [32].
Methanolysis of PET is the breakdown of PET polyesters in the presence of methanol at an elevated temperature ranging from 180 to 280 ˚C and pressure of 20 to 40 atmosphere, resulting in the production of the desired dimethyl terephthalate monomer and ethylene glycol [33,34,35,36]. The main advantages of this method are: i) the quality of the obtained DMT is identical to that of the virgin DMT, ii) the ease of purification of DMT, and iii) easy recovery of EG and methanol. In addition, this method presents a relatively high tolerance to contaminants and hence can treat low quality feedstocks [35, 36]. Different techniques such as liquid methanolysis, vapor methanolysis and supercritical methanolysis were explored [35, 37,38,39,40]. As with other depolymerization method, liquid methanolysis in the presence of catalyst proceeds effortlessly. Thus, some divalent metal catalysts including zinc acetate, cobalt acetate, manganese salts, magnesium acetate, calcium phosphate, sodium silicate, lead dioxide, alkali earth metal hydroxides, lead acetate and aluminium triisopropoxide were used as an effective catalyst [24, 25, 35, 40, 41]. Even though the number of research for recycling of PET polyester has increased dramatically, high cost of reaction procedures has become the limiting factor for this method at present [2, 24, 25].
The use of biomass-derived solid catalysts as a substitute to typical heterogeneous catalysts to promote different chemical processes was strongly encouraged in the perspective of environmental and sustainability [42, 43]. As a result, several examples regarding the utilization of carbon-based solid catalysts from biomass waste by various research groups were available recently [42, 44,45,46,47,48,49,50]. They are primarily employed as a catalyst in the manufacture of biodiesel and the formation of C–C bonds in organic reactions [42, 51]. Our most recent work has included describing for the first time, a glycolysis technique for old PET that makes use of orange peel ash (OPA) and bamboo leaf ash (BLA) as economically feasible and reusable heterogeneous catalysts [52, 53]. As a result, in addition to our inclination toward the development of more environmentally friendly methods for PET depolymerization [52,53,54], the present study demonstrated the exploitation of biomass-waste bamboo leaf ash (BLA), which is inexpensive, readily recoverable, and environmentally friendly, as a heterogeneous catalyst for methanolytic conversion of PET to its DMT monomer.
The centerpiece of our research work, “bamboo” is accounted as one among the fast-growing diversity of plants in major parts of the world. The tall, tree-like grasses of the family Poaceae were profusely used for a variety of purposes, especially in East and Southeast Asian countries [55]. Mostly, its stems and fibers are a natural resource, widely used in housing construction, home appliances, and handiwork applications [56]. However, the bamboo leaf which has high silica content (30–50%) is considered waste and its applications are almost negligible [57]. Recent studies describe the application of ash obtained from bamboo leaves to cement composites for the generation of pozzolanic materials [58]. Recently, Fatimah and co-workers reported the application of bamboo leaf ash (BLA) supported by ZrO2 in the microwave-promoted production of biodiesel [56]. Considering the potency, recyclability and renewability, the present communication deals with the application of BLA for depolymerization of poly(ethylene terephthalate) waste bottles through methanolysis, which may perhaps offer an alternative greener route for PET recycling.
Experimental
Materials
Post consumed PET bottles were procured from the market and all the labels along with the caps were removed, washed with distilled water, shredded into 1 mm × 1 mm square chip and dried in an oven. Bamboo (Melocanna baccifera) leaves were gathered from Aizawl, Mizoram, India. Methanol, chloroform, commercial ethylene glycol (EG), 2-hydroxyethyl methyl terephthalate (HEMT) and bis-(2-hydroxyethyl) terephthalate (BHET) were purchased from Sigma Aldrich, and no further purification was performed prior to use.
Preparation of BLA Catalyst
The collected bamboo leaf was cleaned thoroughly with distilled water, parched at 80 °C for 8 h and then burnt to ashes. Calcination of the ash was carried out at 700 °C for 4 h, and the greyish color bamboo leaf ash (BLA) was collected, crushed to a fine powder, sieved and then stored in a sample vial at room temperature.
Characterization of PET Waste
The thermal analysis of the post consumed PET was carried out using PerkinElmer DSC 6000 under an inert atmosphere with nitrogen flow of 20 ml min−1. PET samples (2–4 mg) were then heated from 50 to 300 °C at a rate of 20 °C min−1 using indium (In) as a standard for measuring enthalpy of fusion (ΔHf). Degree of Crystallinity of PET was calculated from the enthalpy change obtained from DSC analysis by taking 140 J g−1 as ΔHf of pure crystalline PET using the following equation [59]:
Characterization of the Catalyst
To analyze the morphological structure, crystallinity, d chemical content of the prepared BLA catalysts FTIR, XRF, XRD, TEM, SEM, EDX, BET and TGA analyses were performed. FT-IR spectra of the prepared catalyst was obtained using KBr disks on Spectrum BX FT-IR, Perkin Elmer (υmax in cm−1). SEM images and determination of the elemental composition was performed on JSM-6360, Jeol and ESEM EDAX XL-30, respectively. TEM images were obtained using JEM-2100, 200 kV instrument. X-ray fluorescence (XRF) of the catalysts were screened through PANalytical, Axios mAX. For the investigation of crystalline properties of the catalyst, Powder X-ray diffraction (XRD) patterns were obtained from Philips, Xpert MPD. The N2 adsorption–desorption isotherm of the prepared BLA was documented using Quanta chrome Nova-1000 surface area and porosity analyzer. The thermogravimetric analysis (TGA) was recorded on Perkin Elmer TGA 4000 and the thermal stability of the catalyst was analyzed at a temperature ranging from 30 to 999 °C.
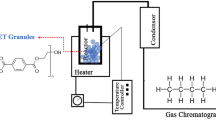
Methanolysis of PET Waste
PET depolymerization was conducted using Shilpent Teflon Lined Hydrothermal Autoclave (50 mL) charged with 480 mg of PET flakes (2.50 mmol), 100 mg of BLA catalyst and 5 mL of methanol. The autoclave was then plunged to an oil bath at 200 °C temperature. As soon as the autoclave was immersed, the temperature of the oil bath dropped significantly and it was then gradually heated to the predetermined temperature within 20 min. When the fixed temperature had been reached, the reaction was kept constant for 2 h. After the reaction had ended, the BLA catalyst was isolated by way of filtration and then washed with 40 ml of heated MeOH. The filtrate was enabled to cool down to room temperature and then placed in a refrigerator at 2 °C for 4 h, where a crystallized DMT separates out, which was filtered, dried and weighed (78%). The filtrate was distilled under reduced pressure to recover methanol and ethylene glycol. The white crystalline residue was collected and analyzed using HPLC and NMR. The percentage conversionnd yield recrystallized DMT were calculated using the equations given below:
Product Characterization
Nuclear Magnetic Resonance Spectroscopy (1H NMR and 13CNMR) were recorded on Bruker, 400 MHz Avance III spectrometer in DMSO-d6. FT-IR spectra were recorded on a Spectrum BX FT-IR, Perkin Elmer in the range of 4000–500 cm−1. HPLC (High Pressure Liquid chromatography) was recorded on Waters 1525 binary pump and Waters UV detector 2489 using Spherisorb ODS2 5 μm, 4.6 × 250 mm analytical column at 254 nm. The solution of methanol and water at 70/30 volume fraction at a flow rate of 1 mL/minute was used as a mobile phase. The UV–Vis spectra were recorded on Labtronics UV–Vis Spectrophotometers Model-LT-2202, and the samples were dissolved in chloroform (5 mg/mL).
Results and Discussion
Post-consumer PET Characterization
Physical properties of PET such as glass transition temperature, heat of fusion and melting temperature were studied and is highlighted in Table 1. From the analysis, the glass transition temperature Tg of post consumed PET was found to be in between that of amorphous and crystalline [60], with clear PET having the highest Tg of 79 °C, followed by light yellow color PET with 78 °C Tg, while green color PET has the lowest Tg with 75 °C. Moreover, enthalpy of fusion (ΔHf) of post consumed PET was determined by heating it to a temperature well above its melting point (300 °C), and cooling it to a lower temperature (50 °C), which is then again reheated to 300 °C temperature using Indium as a standard reference for calibration. It was observed that green color PET has the highest ΔHf with 60.0128 J g−1, while clear and light-yellow color PET have 55.1033 J g−1 and 54.5185 J g−1 respectively. As aforementioned, enthalpy of fusion (ΔHf) obtained was employed for calculating the degree of crystallinity, which is a measure of the orderliness of the polymer chain for each sample. As a result, green color PET having the highest enthalpy of fusion has the highest degree of crystallinity with 42.86%, next to clear PET with 39.35 crystallinity %, while light yellow color has the lowest crystalline component with 38.94%. In general, the rate of decomposition of PET material was known to be inversely related to their degree of crystallinity, i.e., rate of degradation becomes sluggish with an increase in degree of crystallinity [61]. However, crystallinity studies of different colored PET bottles showed that the percentage crystallinity are almost the same, thus, explaining a uniform depolymerization time for each colored PET bottle.
Catalyst Characterization
XRF analysis was performed to evaluate the compositions of the prepared catalyst and the findings are shown in Table 2. The composition was found to be of 76.026% silicon dioxide (SiO2), 6.892% sulphur trioxide (SO3), 4.783% calcium oxide (CaO), 2.876% potassium oxide (K2O), 2.341% ferric oxide (Fe2O3), 1.888% manganese oxide (MnO) and 1.277% magnesium oxide (MgO) as the major constituent. Apart from these, oxides of phosphorus, aluminium, sodium, chromium, titanium, etc., were found to be present in minor quantities for the catalytic activation.
X-ray Photoelectron Spectroscopy (XPS) evaluation was performed out to decode the elemental configuration of the fresh BLA catalyst (Fig. 1). After extensive study of the wide-scan spectrum, the availability of Si, O, C, Ca, and K as the major elements in the external region can be confirmed. On deconvolution, the C1s spectra comprise four distinct peaks. The peaks at 285.38 and 293.78 eV may be assigned to the presence of C–C and C = O of metal carbonates on the ash particle. The deconvoluted O1s spectra shows one hump, positioned at binding energy of 529.94 eV, which may be attributed to the presence of metal oxides. Moreover, on deconvolution, the K2p spectra present two peaks at 293.84 and 291.12 binding energies, which was assigned to K2O and K2CO3. Likewise, the Ca2p spectra also shows two peaks at 348.63 and 344.56 eV, which confirms the presence of CaCO3 and CaO respectively. Furthermore, a single peak located at binding energy of 100.68 eV within the Si2p spectra credited the presence of an abundant SiO2 compound of the catalyst.
The presence of crystalline complex in BLA was identified by XRD analysis wherein compounds in the form of their carbonates and oxides were determined (Fig. 2a). It can be seen that strong characteristic diffraction peaks observed at 2θ = 20.817, 26.566, 50.088, 29.405, 45.688 corresponding to (100), (011), (003), (104), and (221) planes confirm the presence of SiO2 and CaCO3 (JCPDS reference file No: 89–1961, 89–8936, 89–8945, 86–2334, 87–1863). Typical spectra of the catalyst profusely demonstrate silica as the dominant phase, and other diffraction peaks at 47.422°, 54.755°, and 59.822° correlated to (113), (141), and (060) planes (JCPDS reference no: 86–0294, 86–0305,73–1539) were due to the presence of Na2CO3 and MnO2. Beside these, the occurrence of Fe2O3, SO3, K2O, K2CO3, CaO, and MgO were observed at 35.750°, 36.840°, 39.422°, 40.222°, 42.355°, and 43.089° which corresponds to (110), (112), (220), (221), (422), and (200) planes, matching well with the existing library JCPDS reference no: 89-8104, 73-2169, 47-1701, 70-0292, 02-1088, 87-0653, 88-0107, respectively. In continuation, the results of XRF and XRD data were strongly supported by EDX analysis showing O (48.01%), Si (32.27%), Ca (8.98%), K (4.61%) as the primary constituent elements with Mg (1.59%), Mn (1.25%), Al (1.12%), P (0.83%), S (0.49%), and Cl (0.32%) as the less abundant elements in the ash sample (see Supporting Information Fig. S11).
Functional group analysis (FTIR) of the catalyst was performed as shown in Fig. 2b. The peaks at 1082 cm−1 and 796 cm−1 highlights the asymmetric stretching and bending vibrations of Si–O bond. The peak at 3442 cm−1 is associated with water molecule absorbed by the catalyst. The appearance of peaks at 1641 and 1446 cm−1 can be attributed to the C–O vibrations of metal carbonates in the catalyst, respectively.
Brunauer–Emmett–Teller (BET) method was used to evaluate the surface area of the catalyst, and Barrett-Joyner-Halenda (BJH) model was employed to detect the average pore size distribution. These methods elaborate the surface area and pore size of the ash catalyst, determining the total specific area in m2/g unit at standard temperature and pressure, thereby providing an important information regarding the effects of external porosity of the catalyst. The surface area, pore diameter and pore volume of the catalyst were found to be 21.847 m2/g, 136.84 Å, and 0.127 cm3/g, respectively. The N2 adsorption–desorption isotherm (Fig. 2c) showed a typical type-IV isotherm, which is the characteristics of mesoporous materials [62]. The higher BET external area and mesoporous characteristics of the material may contribute to the higher catalytic activity of the ash catalysts towards transesterification reaction.
Thermal Gravimetric Analysis (TGA) of the prepared BLA was presented to investigate its thermal inertness with temperature (Fig. 2d). Results showed that 5% of the initial weight was lost between 30 and 200 °C, corresponding to the removal of water content from the catalyst. Moreover, additional dropping in the weight of the ash material typically corresponds to oxygenation of carbon moiety of the ash material [63].
SEM and TEM images delineated the external outline of the ash samples. The SEM images (Fig. 3a, b) illustrates that the outline of bamboo leaf ash particles is rough and spongy with an agglomerated assembly of microporous and mesoporous structures. Besides, TEM images (Fig. 3c, d) also show aggregated irregular globular surface of the catalyst with different size distributions, having an average spherical diameter ranging from 10 to 50 nm approximately (Fig. 4).
Analysis of Solid Products
It was reported earlier that methanolytic decomposition of PET results in DMT production as the main component with the generation of several byproducts such as 2-Hydroxyethylmethyl terephthalate (HEMT), bis-(2-hydroxyethyl) terephthalate (BHET), BHET dimer, DMT dimer and oligomers in small amount [40]. In our case, analysis of the final crude product by HPLC revealed the presence of EG, BHET, HEMT and DMT at a retention time of 2.177, 3.259, 4.273 and 7.354, respectively. This was confirmed by evaluation of HPLC studies of commercially available EG, BHET, HEMT and recrystallized DMT monomer (see Supporting Information Figs. S1–S5).
Purification of the crude reactions was then performed based on the method highlighted in the experimental section to obtain pure DMT monomer, and it was characterized by using NMR and IR spectroscopy. In its 1H NMR spectra, singlets at δ = 3.948 (–OCH3) and 8.099 (Ar–H) ppm (Fig. 5) confirm the formation of the desired product which was further substantiated by the resonances at δ = 166.85, 134.48, 130.13 and 53.01 ppm in its 13C NMR spectra (see Supporting Information Fig. S7). The FT-IR spectra of recrystallized DMT shows an absorption band at 1703 cm −1, indicating the existence of esters functionality (see Supporting Information Fig. S8). The strong absorption bands observed at 1262 and 1087 cm−1 may be allocated to the stretching vibrations of methyl and C–O bond, correspondingly. Existence of the methyl group was confirmed by the vibrational band at 2952 cm −1.
Proposed Reaction Pathway for Methanolysis of PET Waste
The framework of the possible reaction mechanism for methanolysis of PET BLA as a catalyst is given in Fig. 6. It is generally accepted that for any reaction involving exchange of ester functionality, the solid-based catalyst should exhibit several basic sites, high surface area as well as large pore diameter to provide an effective transition from start to finish [64,65,66]. In this commentary, based on the data available from characterization of the catalyst and experimental results, we consider that the occurrence of several elements like Ca, K, Fe, Mg etc., available in order of their oxides and carbonates have introduced an effective strategy for the degradation of PET waste granules. Earlier studies have also reported that decomposition of PET waste through transesterification reaction was subjected to Lewis acid–base synergistic effect [67]. Hence, for each successful conversion, a nucleophilic methoxide ion was generated with the abstraction of a proton from -OH group of methanol by the anionic sites (such as O2−, CO32−) present within the catalyst. Subsequently, the methoxide ion interacts with the cationic sites (Mn2+, K2+, Ca2+, Mg2+ etc.,) of the catalyst, which initially assembled nucleophilic attack on electron deficient carbonyl of esters group to form a tetrahedral intermediate. As the reaction proceeds further, a rearrangement reaction takes place where the esters bond gets cleaved, and the polymeric substrates experienced a new C–O bond formation between the carbonyl carbon of PET polyester and oxygen from methanol. As a result, the PET chain cleaved, decreasing the chain length to form oligomers and finally into a dimethyl terephthalate (DMT) monomer as the major product. Besides DMT, coupling of the acyl –OCH2CH2– group with proton adsorbed by the catalyst results in the formation of ethylene glycol in the reaction mixture.
Effect of Reaction Parameters on Depolymerization of PET Waste
The impact of catalyst loading on PET to DMT conversion at 200 °C with 5 ml of methanol is summarized in Fig. 7a. We experienced that the degree of polymerization and DMT yield increases exponentially with every rise in BLA loading. As such, when PET degradation reaction was carried out with the exclusion of our catalyst, even after 48 h of time, only 71% PET transformation and 0.8% DMT yield were obtained. The maximum yield of DMT monomer was obtained using 100 mg of the catalyst, furnishing 73% yield within 2 h of reaction time. However, we also observed that a further increase in the catalysts loading above 100 mg does not result in any significant enhancement of the yield. HPLC analysis of the crude product with 120 mg of BLA loading indicates an escalated formation of BHET, HEMT and other byproducts which otherwise could not be isolated under normal recrystallization procedure (see Supporting Information Fig. S13). Hence, we envisage that after reaching the optimal catalyst concentration, the depolymerization occurs hastily to form DMT, which is then repolymerized to form oligomers and other byproducts, thereby creating a chemical equilibrium [41].
Reactions were performed to analyze the influence of time of reaction and temperature of reaction on the breakdown of PET waste to its monomeric DMT units. At a varying reaction time ranging from 1 to 4 h, depolymerization of 2.50 mmol of PET waste was set up while keeping the catalyst loading and methanol concentration constant at 20 wt% and 5 ml, respectively. The level of PET decomposition and the formation of DMT monomer escalated sharply when the reaction period was increased from 1 to 2 h (Fig. 7b). But beyond that, there is a slight decrease in the overall yield of DMT which may be due to an increase in the formation of other depolymerized products as shown by HPLC analysis (see Supporting information Fig. S14). The initial increase and then subsequent decrease in the DMT formation can be explained on the grounds of their equilibration process. Generally, degradation of PET by methanolysis is believed to start from the chip surface and occurs at random places in the polymeric chain, producing the oligomers, and finally, generating DMT and EG [41]. As the reaction advanced with time, the monomer was then repolymerized to form the dimers and/or oligomers, thus reducing the overall DMT yield.
We also observed that a 180 °C reaction condition did not seem to attain completion even after a prolonged reaction time of about 4 h. It was seen that rising of the reaction temperature greatly influenced the rate of depolymerization as shown in Fig. 8a. The best result was obtained at 200 °C, where complete degradation with the highest DMT yield was achieved within 2 h of reaction time. A sharp decrease in DMT yield was seen at 220 °C with an increase in oligomers production.
The impact of methanol concentration on the degradation of post-consumer PET was also investigated and is charted in Fig. 8b. The result indicates that the rate of PET degradation and the DMT obtained upsurge rapidly with every rise in methanol loading from 2 to 10 mL. But after reaching its optimal concentration of 10 mL of MeOH, the DMT yield decreases even with a further rise in methanol concentration, and an augmented formation of the oligomers and other byproducts were again detected in the HPLC data. This indicates that an optimal methanol concentration is required for the whole effectiveness of the protocol when other reaction parameters are kept constant.
Methanolysis of Color PET Bottles
Under the optimized condition, methanolysis of green, light-yellow and red PET bottles were also investigated (see Supporting information Table S6). The reactions were completed within 2 h giving good selectivity for DMT monomer. Most of the color observed separates out during recrystallization process, and any persisting pigments can be deleted by adding activated charcoal upon recrystallization [28, 52]. The samples were dissolved in chloroform (5 mg/ml) and the absorbance of DMT obtained from colored as well as clear bottle was recorded on UV–Vis spectrophotometer at 350–800 nm wavelength range (Fig. 9a).
Catalyst Reusability
The feasibility of catalyst reusability has become an important aspect for most chemists considering the industrial and environmental applicability of the proposed process. Therefore, after separating the catalyst from the reaction mixture, it was washed with methanol 2–3 times to remove all organic impurities and then put in a hot air oven at 80 °C for 5 h. We observed a weight loss of about 5–10% catalyst, and this was recompensed with fresh catalyst. The reusability of the recovered catalyst was then tested for four catalytic series with our optimized condition. It can be seen that DMT obtained and reaction time decreased gradually with each successive catalytic cycle. This may be due to the fact that during the catalytic reaction and subsequent purification process, some of the catalyst’s active components leached into the reaction medium, thereby reducing the catalytic activity. Subsequently, the regenerated BLA after fourth successive run was subjected to SEM, TEM and EDX analysis.
For the recovered catalyst, SEM and TEM images reveal the retention of agglomerated structure with microporous and mesoporous nature identical to the virgin catalyst. EDX studies of the fresh and recovered BLA catalyst disclose a substantial loss of Fe, Mg, Mn, K, and Ca concentration in the recovered catalyst, while Si remains almost the same. These remarkable changes in the concentration may affect the percentage yield of the depolymerization product with the recovered catalyst.
Several methodologies have been employed for methanolysis of PET, and are listed in Table 3. The demand of high temperature, long reaction time, tedious purification process, presence of co-solvent for better achievements as well as high catalyst loading are some of the major disadvantages of the prior reported procedures. In order to control and conquer these problems, experimental design was demonstrated to reduce the overall cost of PET recycling using inexpensive, eco-friendly and waste origin biomass derived BLA as a heterogeneous catalyst. The ease of catalyst separation and recovery, ease of preparation and handling, excellent reusable property and high selectivity towards DMT formation are some of the advantages of the prepared BLA catalyst.
Kinetic Studies of PET Methanolysis
Random scission and specific scission are said to be involved in the degradation of PET, with the former being the more common type [38]. Random scission occurs at the beginning of the process, while specific scission occurs at the end of the process. Meanwhile, it was discovered that the initial stage of the process takes a longer time than expected and is assumed to be the step that determines the rate of the reaction. Table 4 contains the results of the calculated different kinetic parameters for various kinetic models as proposed by Basumatary et al. [70], based on the experimental findings.
These data were used to generate Arrhenius plots of ln k against the reciprocal of absolute temperature (1/T), which are depicted in Figs. S15, S16, and S17 (see Supporting information Figs. S15–S17). When the plots are fitted with a linear function and the graphical interpretation and evaluation are performed, the correlation coefficient (R2) values for first order, pseudo first order, and second order kinetics are 0.582, 0.875, and 0.115, respectively, for the three types of kinetics. Figure S16 recommended that the reaction occur in this order owing to its greatest R2 value. Additionally, the activation energy (Ea) of the BLA catalyzed PET Methanolysis was determined to be 59.943 kJ/mol as compared to 95.31 kJ/mol [71], 66.5 kJ/mol [67] and 56 kJ/mol [72] for some reported literatures.
Conclusion
In conclusion, unknown to prior technique, we have investigated and described methanolysis of PET waste bottles using BLA as a transesterification catalyst to regenerate the DMT monomer in excellent yield. The catalyst being a waste origin, hence free, was actively used for rapid and complete decomposition of PET. Its easy preparation and handling, environmentally friendliness and high efficacy are some notable advantages of this solid catalyst. The degree of conversion and selectivity for the desired monomer was greatly influenced by the methanol concentration, catalyst loading, reaction temperature and time period. Despite the presence of ample amount of SiO2 (~ 76%) in the catalyst, the availability of other basic sites (such as CaO, K2O etc.) was observed to promote the depolymerization reaction. Thus, with its high efficacy and heterogeneous active surface, the BLA is found to be an efficient, environmentally benign catalyst to offer a greener protocol to chemically treat post-consumer PET bottles.
References
Ritchie H, Roser M (2018) Plastic pollution. Our World in Data. References—Scientific Research Publishing. https://scirp.org/reference/referencespapers.aspx?referenceid=3053948. Accessed 25 Sept 2021
Jehanno C, Pérez-Madrigal MM, Demarteau J et al (2019) Organocatalysis for depolymerisation. Polym Chem 10:172–186. https://doi.org/10.1039/C8PY01284A
Karayannidis GP, Achilias DS (2007) Chemical recycling of poly(ethylene terephthalate). Macromol Mater Eng 292:128–146. https://doi.org/10.1002/mame.200600341
López-Fonseca R, Duque-Ingunza I, de Rivas B et al (2010) Chemical recycling of post-consumer PET wastes by glycolysis in the presence of metal salts. Polym Degrad Stab 95:1022–1028. https://doi.org/10.1016/j.polymdegradstab.2010.03.007
López-Fonseca R, Duque-Ingunza I, de Rivas B et al (2011) Kinetics of catalytic glycolysis of PET wastes with sodium carbonate. Chem Eng J 168:312–320. https://doi.org/10.1016/j.cej.2011.01.031
Welle F (2011) Twenty years of PET bottle to bottle recycling—an overview. Resour Conserv Recycl 55:865–875. https://doi.org/10.1016/j.resconrec.2011.04.009
Padhan RK, Sreeram A (2019) Chemical depolymerization of PET bottles via combined chemolysis methods. Recycl Polyethyl Terephthalate Bottles. https://doi.org/10.1016/B978-0-12-811361-5.00007-9
Awaja F, Pavel D (2005) Recycling of PET. Eur Polym J 41:1453–1477. https://doi.org/10.1016/J.EURPOLYMJ.2005.02.005
Nadkarni VM, Rath AK (2002) Blends of thermoplastic polyesters: sections 1–5. Handb Thermoplast Polyesters. https://doi.org/10.1002/3527601961.CH19A
Malik N, Kumar P, Shrivastava S, Ghosh SB (2017) An overview on PET waste recycling for application in packaging. Int J Plast Technol 21:1–24. https://doi.org/10.1007/s12588-016-9164-1
Sinha V, Patel MR, Patel JV (2008) Pet waste management by chemical recycling: a review. J Polym Environ 181(18):8–25. https://doi.org/10.1007/S10924-008-0106-7
Geyer B, Lorenz G, Kandelbauer A (2016) Recycling of poly(ethylene terephthalate)—a review focusing on chemical methods. Express Polym Lett 10:559–586. https://doi.org/10.3144/expresspolymlett.2016.53
Leng Z, Padhan RK, Sreeram A (2018) Production of a sustainable paving material through chemical recycling of waste PET into crumb rubber modified asphalt. J Clean Prod 180:682–688. https://doi.org/10.1016/j.jclepro.2018.01.171
Atta AM, El-Kafrawy AF, Aly MH, Abdel-Azim A-AA (2007) New epoxy resins based on recycled poly(ethylene terephthalate) as organic coatings. Prog Org Coat 58:13–22. https://doi.org/10.1016/j.porgcoat.2006.11.001
Managing Plastic Waste in India: An agenda for action. https://www.cseindia.org/managing-plastic-waste-in-india-an-agenda-for-action-10361. Accessed 25 Sept 2021
Tshifularo CA, Patnaik A (2020) Recycling of plastics into textile raw materials and products. Sustain Technol Fash Text. https://doi.org/10.1016/B978-0-08-102867-4.00013-X
Khoonkari M, Haghighi AH, Sefidbakht Y et al (2015) Chemical recycling of PET wastes with different catalysts. Int J Polym Sci. https://doi.org/10.1155/2015/124524
Al-Sabagh AM, Yehia FZ, Eshaq G et al (2016) Greener routes for recycling of polyethylene terephthalate. Egypt J Pet 25:53–64. https://doi.org/10.1016/J.EJPE.2015.03.001
Dutt K, Soni RK (2013) A review on synthesis of value added products from polyethylene terephthalate (PET) waste. Polym Sci Ser B 557(55):430–452. https://doi.org/10.1134/S1560090413070075
Neale CW, Hilyard NC, Barber P (1983) Observations on the economics of recycling industrial scrap plastic in new products. Conserv Recycl 6:91–105. https://doi.org/10.1016/0361-3658(83)90034-6
Throne JL (1987) Effect of recycle on properties and profits: algorithms. Adv Polym Technol 7:347–360. https://doi.org/10.1002/adv.1987.060070401
Yoda N (1999) Corporate management in the age of global environmental awareness: a case study of PET-bottle-recycling issues in Japan. Polym Int 48:944–951. https://doi.org/10.1002/(sici)1097-0126(199910)48:10%3c944::aid-pi252%3e3.0.co;2-y
Farahat MS, Abdel-Azim A-AA, Abdel-Raowf ME (2000) Modified unsaturated polyester resins synthesized from poly(ethylene terephthalate) waste, 1. Synthesis and curing characteristics. Macromol Mater Eng 283:1–6. https://doi.org/10.1002/1439-2054(20001101)283:1%3c1::AID-MAME1%3e3.3.CO;2-M
Siddiqui MN, Redhwi HH, Achilias DS (2012) Recycling of poly(ethylene terephthalate) waste through methanolic pyrolysis in a microwave reactor. J Anal Appl Pyrolysis 98:214–220. https://doi.org/10.1016/j.jaap.2012.09.007
Raheem AB, Noor ZZ, Hassan A et al (2019) Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: a review. J Clean Prod 225:1052–1064. https://doi.org/10.1016/J.JCLEPRO.2019.04.019
Damayanti, Wu H-S (2021) Strategic possibility routes of recycled PET. Polymers 13:1475. https://doi.org/10.3390/POLYM13091475
Shamsaei M, Aghayan I, Kazemi KA (2017) Experimental investigation of using cross-linked polyethylene waste as aggregate in roller compacted concrete pavement. J Clean Prod 165:290–297. https://doi.org/10.1016/j.jclepro.2017.07.109
Fukushima K, Coulembier O, Lecuyer JM et al (2011) Organocatalytic depolymerization of poly(ethylene terephthalate). J Polym Sci Part A 49:1273–1281. https://doi.org/10.1002/pola.24551
Lorenzetti C, Manaresi P, Berti C, Barbiroli G (2006) Chemical recovery of useful chemicals from polyester (PET) waste for resource conservation: a survey of state of the art. J Polym Environ 14:89–101. https://doi.org/10.1007/s10924-005-8711-1
López-Fonseca R, González-Marcos MP, González-Velasco JR, Gutiérrez-Ortiz JI (2009) A kinetic study of the depolymerisation of poly(ethylene terephthalate) by phase transfer catalysed alkaline hydrolysis. J Chem Technol Biotechnol 84:92–99. https://doi.org/10.1002/jctb.2011
Paszun D, Spychaj T (1997) Chemical recycling of poly(ethylene terephthalate). Ind Eng Chem Res 36:1373–1383. https://doi.org/10.1021/IE960563C
Achilias DS, Karayannidis GP (2004) The Chemical recycling of PET in the framework of sustainable development. Water Air Soil Pollut Focus 4:385–396. https://doi.org/10.1023/B:WAFO.0000044812.47185.0f
Kang MJ, Yu HJ, Jegal J et al (2020) Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem Eng J 398:125655. https://doi.org/10.1016/j.cej.2020.125655
Bartolome L, Imran M, Lee KG et al (2014) Superparamagnetic γ-Fe2O3 nanoparticles as an easily recoverable catalyst for the chemical recycling of PET. Green Chem 16:279–286. https://doi.org/10.1039/C3GC41834K
Han M (2019) Depolymerization of PET bottle via methanolysis and hydrolysis. Recycl Polyethyl Terephthalate Bottles. https://doi.org/10.1016/B978-0-12-811361-5.00005-5
Polyethylene Terephthalate Waste Recycling and Application Possibilities: a Review|Materials Science. https://matsc.ktu.lt/index.php/MatSc/article/view/26243. Accessed 25 Sept 2021
Genta M, Iwaya T, Sasaki M, Goto M (2007) Supercritical methanol for polyethylene terephthalate depolymerization: observation using simulator. Waste Manag 27:1167–1177. https://doi.org/10.1016/j.wasman.2006.06.005
Goto M, Koyamoto H, Kodama A et al (2002) Depolymerization of polyethylene terephthalate in supercritical methanol. J Phys Condens Matter 14:11427–11430. https://doi.org/10.1088/0953-8984/14/44/494
Sako T, Sugeta T, Otake K et al (1997) Depolymerization of polyethylene terephthalate to monomers with supercritical methanol. J Chem Eng Japan 30:342–346. https://doi.org/10.1252/jcej.30.342
Yang Y, Lu Y, Xiang H et al (2002) Study on methanolytic depolymerization of PET with supercritical methanol for chemical recycling. Polym Degrad Stab 75:185–191. https://doi.org/10.1016/S0141-3910(01)00217-8
Kurokawa H, Ohshima MA, Sugiyama K, Miura H (2003) Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polym Degrad Stab 79:529–533. https://doi.org/10.1016/S0141-3910(02)00370-1
Basumatary S, Nath B, Kalita P (2018) Application of agro-waste derived materials as heterogeneous base catalysts for biodiesel synthesis. J Renew Sustain Energy 10:043105. https://doi.org/10.1063/1.5043328
Abdullah SHYS, Hanapi NHM, Azid A et al (2017) A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew Sustain Energy Rev 70:1040–1051. https://doi.org/10.1016/J.RSER.2016.12.008
Lee D (2013) Preparation of a sulfonated carbonaceous material from lignosulfonate and its usefulness as an esterification catalyst. Molecules 18:8168–8180. https://doi.org/10.3390/molecules18078168
Pathak G, Das D, Rajkumari K, Rokhum L (2018) Exploiting waste: towards a sustainable production of biodiesel using: Musa acuminata peel ash as a heterogeneous catalyst. Green Chem 20:2365–2373. https://doi.org/10.1039/c8gc00071a
Nath B, Das B, Kalita P, Basumatary S (2019) Waste to value addition: utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.118112
Rajkumari K, Das D, Pathak G, Rokhum L (2019) Waste-to-useful: a biowaste-derived heterogeneous catalyst for a green and sustainable Henry reaction. New J Chem 43:2134–2140. https://doi.org/10.1039/c8nj05029e
Sarmah M, Dewan A, Mondal M et al (2016) Analysis of the water extract of waste papaya bark ash and its implications as an in situ base in the ligand-free recyclable Suzuki-Miyaura coupling reaction. RSC Adv 6:28981–28985. https://doi.org/10.1039/c6ra00454g
Gohain M, Laskar K, Paul AK et al (2020) Carica papaya stem: a source of versatile heterogeneous catalyst for biodiesel production and C-C bond formation. Renew Energy 147:541–555. https://doi.org/10.1016/j.renene.2019.09.016
Talukdar A, Deka DC (2020) Water hyacinth ash: an efficient green catalyst for the synthesis of β-amino carbonyl/nitrile compounds by aza-Michael reaction at room temperature. SN Appl Sci. https://doi.org/10.1007/s42452-020-2281-7
Changmai B, Vanlalveni C, Ingle AP et al (2020) Widely used catalysts in biodiesel production: a review. RSC Adv 10:41625–41679. https://doi.org/10.1039/D0RA07931F
Laldinpuii Z, Lalhmangaihzuala S, Pachuau Z, Vanlaldinpuia K (2021) Depolymerization of poly(ethylene terephthalate) waste with biomass-waste derived recyclable heterogeneous catalyst. Waste Manag 126:1–10. https://doi.org/10.1016/j.wasman.2021.02.056
Lalhmangaihzuala S, Laldinpuii Z, Lalmuanpuia C, Vanlaldinpuia K (2021) Glycolysis of poly(ethylene terephthalate) using biomass-waste derived recyclable heterogeneous catalyst. Polymers (Basel) 13:1–13. https://doi.org/10.3390/polym13010037
Laldinpuii TZ, Lalmuanpuia C, Lalhmangaihzuala S et al (2021) Biomass waste-derived recyclable heterogeneous catalyst for aqueous aldol reaction and depolymerization of PET waste. New J Chem. https://doi.org/10.1039/D1NJ03225A
Fei B, Gao Z, Wang J, Liu Z (2016) Biological, anatomical, and chemical characteristics of bamboo. Second Xylem Biol Orig Funct Appl. https://doi.org/10.1016/B978-0-12-802185-9.00014-0
Fatimah I, Rubiyanto D, Taushiyah A et al (2019) Use of ZrO2 supported on bamboo leaf ash as a heterogeneous catalyst in microwave-assisted biodiesel conversion. Sustain Chem Pharm 12:100129. https://doi.org/10.1016/j.scp.2019.100129
Kow K, Yusoff R, Aziz ARA, Abdullah EC (2014) From bamboo leaf to aerogel: Preparation of water glass as a precursor. J Non Cryst Solids 386:76–84. https://doi.org/10.1016/j.jnoncrysol.2013.11.041
Umoh AA, Odesola IA (2015) Characteristics of bamboo leaf ash blended cement paste and mortar. Civ Eng Dimens 17:22–28. https://doi.org/10.9744/ced.17.1.22-28
Panowicz R, Konarzewski M, Durejko T et al (2021) Properties of polyethylene terephthalate (PET) after thermo-oxidative aging. Materials 14:3833. https://doi.org/10.3390/MA14143833
Crystallization behavior of PET materials. https://acikerisim.bartin.edu.tr/handle/11772/1592. Accessed 25 Sept 2021
Jenkins MJ, Harrison KL (2008) The effect of crystalline morphology on the degradation of polycaprolactone in a solution of phosphate buffer and lipase. Polym Adv Technol 19:1901–1906. https://doi.org/10.1002/PAT.1227
Sing KSW, Williams RT (2004) Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt Sci Technol 22:773–782. https://doi.org/10.1260/0263617053499032
Chouhan APS, Sarma AK (2013) Biodiesel production from Jatropha curcas L. oil using Lemna perpusilla Torrey ash as heterogeneous catalyst. Biomass Bioenergy 55:386–389. https://doi.org/10.1016/j.biombioe.2013.02.009
Changmai B, Rano R, Vanlalveni C, Rokhum L (2021) A novel Citrus sinensis peel ash coated magnetic nanoparticles as an easily recoverable solid catalyst for biodiesel production. Fuel. https://doi.org/10.1016/j.fuel.2020.119447
Rajkumari K, Kalita J, Das D, Rokhum L (2017) Magnetic Fe3O4@silica sulfuric acid nanoparticles promoted regioselective protection/deprotection of alcohols with dihydropyran under solvent-free conditions. RSC Adv 7:56559–56565. https://doi.org/10.1039/C7RA12458A
Teo SH, Islam A, Chan ES et al (2019) Efficient biodiesel production from Jatropha curcus using CaSO4/Fe2O3-SiO2 core-shell magnetic nanoparticles. J Clean Prod 208:816–826. https://doi.org/10.1016/j.jclepro.2018.10.107
Pham DD, Cho J (2021) Low-energy catalytic methanolysis of poly(ethyleneterephthalate). Green Chem 23:511–525. https://doi.org/10.1039/d0gc03536j
Kim BK, Hwang GC, Bae SY et al (2001) Depolymerization of polyethyleneterephthalate in supercritical methanol. J Appl Polym Sci 81:2102–2108. https://doi.org/10.1002/app.1645
Nikje MMA, Nazari F (2006) Microwave-assisted depolymerization of poly(ethylene terephthalate) [PET] at atmospheric pressure. Adv Polym Technol 25:242–246. https://doi.org/10.1002/adv.20080
Basumatary B, Das B, Nath B, Basumatary S (2021) Synthesis and characterization of heterogeneous catalyst from sugarcane bagasse: Production of jatropha seed oil methyl esters. Curr Res Green Sustain Chem 4:100082. https://doi.org/10.1016/J.CRGSC.2021.100082
Mishra S, Goje AS (2003) Kinetic and thermodynamic study of methanolysis of poly(ethylene terephthalate) waste powder. Polym Int 52:337–342. https://doi.org/10.1002/PI.1147
Kint DPR, de Ilarduya AM, Bou JJ, Muñoz-Guerra S (2002) Poly(ethylene terephthalate) copolymers containing nitroterephthalic units. III. Methanolytic degradation. J Polym Sci Part A 40:2276–2285. https://doi.org/10.1002/POLA.10305
Acknowledgements
This work was supported by Science and Engineering Research Board, New Delhi, India, File no EEQ/2017/000505. ZT Laldinpuii and Samson Lalhmangaihzuala gratefully acknowledge CSIR and UGC for financial support through Junior Research Fellowship. The authors thank SICART, SAIF-NEHU, BIT-Bangalore and IIT Roorkee for sample analyses.
Author information
Authors and Affiliations
Contributions
ZTL: formal analysis, investigation, writing–review and editing. VK: formal analysis, investigation, writing—reviewing and editing. SL: software, data curation, writing—original draft preparation. CL: visualization, software, writing—reviewing and editing. ZP: supervision, writing—reviewing and editing. C. Lalhriatpuia: supervision, validation, writing—reviewing and editing. KV: conceptualization, methodology, validation, resources, project administration, supervision, funding acquisition, writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Laldinpuii, Z.T., Khiangte, V., Lalhmangaihzuala, S. et al. Methanolysis of PET Waste Using Heterogeneous Catalyst of Bio-waste Origin. J Polym Environ 30, 1600–1614 (2022). https://doi.org/10.1007/s10924-021-02305-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02305-0