Abstract
Recently, nanosized cellulose materials extraction is extensively interesting from the sources of sustainable materials. Cellulose nanofibrils (CNF) extraction through green bio-based materials featured as promising interest in the field of science. In this study, dimethyl sulfoxide (DMSO) was applied to examine its effectiveness in pretreating the Ficus natalensis barkcloth cellulose (FNBC) for CNF production before 2,2,6,6,-tetramethylpiperidine-1-oxyl (TEMPO) oxidation. The pretreatment performance of DMSO was evaluated based on the structural and morphological changes. DMSO pretreated FNBC attained the most dramatic morphological changes as compared to untreated cellulose samples. The results of the scanning electron microscope (SEM) and transmission electron microscope (TEM) shows that there is an extensive structural disruption of FNBC during the pretreatment process, which could be because of outstanding ability to eliminate non-cellulosic materials and amorphous regions from the FNBC, confirmed by the X-ray diffractometry (XRD) showing higher crystallinity values, as well as higher thermal stabilities values of pretreated FNBC samples, were also noted. Overall, this study revealed a tremendously effective and pioneer pretreatment method for fractionating FNBC, to stimulate the successive extraction of cellulose nanofibrils. Furthermore, based on the cellulose and CNF characterizations, this study showed that F. natalensis barkcloth could be considered as an alternative source of cellulose for potential value-added industrial applications such as the food industry, paper making, and biomedicines.
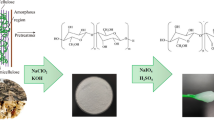
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulosic materials evaluating less than 100 nm are denoted as nanocellulose, because of biocompatibility, biodegradability, excellent durability, and tremendous tunability, nanocellulose is considered as an ultimate material for a new biopolymer composites industry [1]. With the extensive increase of sustainable and renewable energy demand, extraction of cellulose materials from various natural green sources has dramatically increased over the last decades. In recent years, the identification and assessment of new renewable sources for the production of biodegradable nanomaterials have been increased [2, 3]. Therefore, the extraction of CNF from wide biomass sources has been extensively increased, mainly due to the substantial decline in petroleum-based resources, certainly expanding prices of crude oil-based materials and rising environmental issues [4]. However, among various attempts with a huge variety of natural sources, Ficus natalensis may hold an outstanding perspective based on its excellent characteristics like easy availability, sustainability, low price, and massive productivity.
Recently, wood pulps and cotton linters were treated with 2,2,6,6,-tetramethylpiperidine-1-oxyl (TEMPO) radical for the extraction of CNF, which oxidized the primary alcohol groups in aqueous media [5]. Generally, CNF yield (over 80%) could be obtained from the TEMPO-oxidization collective with the mechanical breakdown of cellulose [6]. Irrespective of the method applied for extraction, pretreatment and disintegration process of the raw material are essential to get the desired type of cellulose nanofibrils. For the extraction of CNF, removal of non-cellulosic materials is required to overwhelm the resistance and to enable successive processing [7]. However, pretreatment by mechanical process consumes an extensive quantity of energy during treatment of bio-based cellulosic materials due to cell wall recalcitrance, which gives an excellent rigid and crystalline form to cellulose. Generally, cellulose nanofibrils extraction needs two successive phases. The first phase is the treatment of cellulosic raw materials with various chemicals to separate individual intact fibers and the fractional removal of non-cellulosic materials such as lignin, hemicellulose, and pectins [8]. The second phase is a conversion of cellulosic materials into cellulose nanofibrils using various techniques such as chemical pretreatments, mechanical disintegration, or biological pretreatments [9]. But, the direct usage of bleaching and pulping chemical pretreatments can have drawbacks including the lower production of cellulose nanofibrils as some of the noncellulosic material could exist during the first phase purification process [10], so to improve the yield, proper dimensions and structural properties of cellulose nanofibrils pretreatment must be carried before the second phase of CNF extraction.
The extraction of CNF can be accomplished by implementing pretreatment techniques before TEMPO oxidation. CNF properties and yield can be increased by applying chemical pretreatments to enhance the defibrillation prior to TEMPO oxidation process. The proposed chemical pretreatment mechanism exert the dissolution of amorphous regions within the cellulose chain such as lignin and hemicellulose, hence reducing the required energy for fiber disintegration in CNF extraction [11]. Numerous pre-treatment techniques have been proposed from many years to extract or modify fiber components.
For that reason, a chemical ought to be chosen that can assure polymeric structural breakdown of non-cellulosic configuration in lignocellulose while retaining the cellulosic portion for the production of cellulose nanofibrils. Considering the importance of pretreatment effectiveness, dimethyl sulfoxide (DMSO) can be measured as one of the potential pretreatment agents. Ebringerova and Heinze [12] reported that DMSO or blend of DMSO and water are more suitable hemicellulose solvents and numereous hemicellulose purification practices by using DMSO as a solvent have been accomplished [13, 14]. DMSO is proved to swell cellulose microfibrils by disruption of inter and intra-molecular H-bonds of lignocellulose materials and is regarded as the safest, cheapest, and innocuous reagent [15]. Additionally, DMSO is an effective neutral solvent for non cellulosic impurities dissolution and no chemical changes has been reported during DMSO pretreatment [16]. So using DMSO pretreatment protocol can helpful in disruption of cellulose by eliminating remaining noncellulosic materials facilitating the TEMPO oxidation process for cellulose nanofirbils extraction.
Thus in this study, CNF extraction through Ficus natalensis barkcloth using DMSO pretreatment with minimum steps and an eco-friendly manner would create a supportable development in nanotechnology. Ficus natalensis is a tree having 20 m height and is part of the Moraceae family, generally, locals named it Natal fig, or Mutuba [17]. Ficus natalensis barkcloth (FNB) is found to be a kind of naturally biodegradable substance from the tree bark of Ficus natalensis. Ficus natalensis is extensively scattered in tropical and subtropical regions of Zambia, Malawi, South Africa, Zimbabwe, Kenya, Mozambique, and surroundings [18]. Through harvesting, the internal bark is openly unwrapped from the stem of the tree. Generally, the Ficus tree takes 8 months to recreate a fresh bark for the next harvest as well as it can be debarked annually for up to 40 years, yielding up to 200 m2 of barkcloth individually [19, 20].
In earlier studies, genus Ficus (Moraceae) species are detailed to be used in various ethnomedical remedies in the world like gastric problems, anti-diabetic medication, anthelmintic, hypotensive, infectious disorders, sexual disorders, mild laxative, digestive and anti-dysentery drugs [21]. Biodegradable FNB has been used in automotive instrument panel composites as a reinforced material [22, 23]. Similarly, the non-woven natural fabric structure of barkcloth was also evaluated by acoustic and thermal behavior [24]. Rwawiire et al. contributed to evaluating the morphology and thermal behavior of Ficus natalensis barkcloth by enzyme and plasma treatment effects [25]. Similarly, the physical, mechanical, and thermal properties of Ficus natalensis barkcloth have also been evaluated [26]. Cellulose nanocrystals and cellulose nanofibrils extraction from Ficus natalensis barkcloth and characterization has also been reported [27, 28].
Although the preliminary studies about characterization and applications of the leaf, fruits, bark, and barkcloth of Ficus natalensis are efficiently reported in the literature, however according to our knowledge till now no report has been devoted to the chemical pretreatment effectiveness of DMSO in extraction and characterization of CNF from FNBC by TEMPO-oxidation. In the present study, the method of CNF extracting using DMSO pretreatment with minimum steps and an eco-friendly manner would create a supportable development in nanotechnology. The present study describes the isolation and detailed characterization of CNF from Ficus natalensis barkcloth.
Experimental Work
Materials
Barkcloth fabric procured from Ficus natalensis tree after specific extraction techniques [29], was purchased from Uganda (district Mpigi). Obtained lignocellulosic material was thoroughly washed with the distilled water, sun-dried for 3 days to remove the maximum moisture, and packed in polyethylene bags. Dimethyl sulfoxide (DMSO) and 2,2,6,6,-tetramethyl piperidine-1-oxyl (TEMPO, 98%) and were obtained from Aladdin Chemistry Co. Ltd., Shanghai, China. The other chemicals, sodium hydroxide (NaOH, 98%), sodium chlorite (NaClO2, 80%), sodium hypochlorite (NaClO, 13%), hydrochloric acid (HCl, 37%) and sodium bromide (NaBr, 99%) used were of analytical grade and attained from Sino-pharm Chemical Reagent Co., Ltd., Shanghai, China.
Method
Extraction of Cellulose
FNB was chopped into 2–4 cm in lengths and then milled into powder form using a grinding machine (DJ-04, Shanghai Dianjiu traditional machinery manufacturing Co., Ltd, Shanghai China) to pass through a 60-mesh sieve, labeled as NF-R. The powder NF-R was subjected to an alkali treatment to remove the soluble polysaccharides [30]. The dried samples were treated with 1.0 M NaOH solution (2 h at 80 °C) by taking 50 g/L residual barkcloth fibers powder. The samples were completely washed with deionized water and then dried in an oven at 40 °C for 6 h. The residual mass was then heated with sodium chlorite (2.5% w/v, 90 °C, pH 4.5, 1 h, material to liquor ratio 1:20) as reported in [31]. After that, the obtained samples were filtered out and washed with distilled water until neutral pH was obtained and dried in an oven for 4 h at 40 °C. Obtained samples were cellulose and labeled as NF-N.
Pretreatment of Cellulose with DMSO
Half of the NF-N were treated with DMSO at 60 °C for 5 h, stirring at 600 rpm with powders to liquid ratio of 1:30. The suspension was repeatedly washed with deionized water using suction filter machine SHZ-D (Tianjin Huaxin Instrument factory, Tianjin, China). The wet samples were dried in a vacuum drying oven at 50 °C for 6 h and labeled as NF-D. The other half of the sample was directly used for TEMPO-oxidation.
Preparation of CNF
Cellulose samples were oxidized using TEMPO and NaBr followed by NaClO by maintaining pH between 10 and 10.5 according to the report [32]. The whole process is schematically described in Fig. 1. The samples NF-D (DMSO treated) and NF-N (without DMSO treatment) were weighted as 2 g individually and dispersed in 150 ml water solutions separately for 6 h. Then a fixed amount of TEMPO (0.025 g) and NaBr (0.25 g) was dissolved in each suspension, separately. After that 20 ml/gcellulose of 13% NaClO solution was added drop-wise at room temperature by stirring at 600 rpm. With a 0.5 M NaOH solution, the pH value was kept between 10 and 10.5 for both solutions and monitored with a pH meter for 4 h. The reaction was stopped with the addition of 0.5 M HCl. The final solution was filtered through the suction filter machine (Tianjin Huaxin Instrument factory, Tianjin, China) using filter papers (0.45 μm). The obtained cellulose nanofibrils were washed 5 times with deionized water by successive centrifugations with an H1650 super centrifuge (Hunan Xiangyi Laboratory Instrument Development Co., Ltd, Changsha, China) at 6000 rpm for 10 min. The obtained colloidal suspension was homogenized using an IKA T25 ultra Turrax machine for 10 min. Each sample was dried in a vacuum oven at 90 °C for 24 h and the obtained cellulose nanofibrils were named as NF–Dt (DMSO pretreated) and NF–Nt (directly TEMPO-oxidized).
Characterization
Chemical Composition Analysis
The chemical composition of extracted FNB was determined through the standard gravimetric method [33]. In short, three samples were taken from barkcloth fiber powder (NF–R) to get an average value. Sample NF-R (2.5 g) was placed in a cellulose thimble and treated with acetone (150 mL) to eliminate the extractives for 4 h at 70 °C. After the extraction process, the residue was first air-dried and then oven-dried (105 °C, 20 min) to get a constant weight. The extractives weight (%w/w) was evaluated as a difference in weight before and after the reaction. Hemicellulose weight percentage was carried out by boiling 1 g extractives with 150 mL of 500 mol/m3 NaOH using an Erlenmeyer flask (250 mL) for 3.5 h. Then the residual was cooled and washed with distilled water and desiccated in the convection oven at 105 °C for 20 min. The resultant group was the hemicellulose content (%w/w) of dried biomass (the difference between the sample weight before and after the reaction process). Lignin percentage was determined by mixing dried extracted mass (0.3 g) with 72% H2SO4 solution and kept at room temperature (2 h) for initial hydrolysis, then adding distilled water (84 mL). For the second hydrolysis, the residual mass was autoclaved at 121 °C for 1 h. The residual solution was cooled at room temperature, and the hydrolytes were separated from the solution by filtration. Insoluble lignin was determined after drying the filtrates at 105 °C. The acid-soluble lignin was measured by taking absorbance of the acid hydrolyzed specimen at 320 nm. So the lignin percentage was determined by summation of acid-soluble and acid-insoluble lignin. Similarly, the ash percentage was carried out by burning the samples at 575 °C using a Muffle furnace. Finally, the cellulose content (%w/w) was determined by difference, assuming that extractives, hemicellulose, lignin, ash, and cellulose were the only components of the entire biomass.
Fourier-Transformed Infrared Spectroscopy (FTIR)
FTIR spectra were noted in transmission mode (T%). After grinding and mixing with potassium bromide (KBr) every sample was compressed into pellet form. The Fourier transform infrared (FTIR) spectrograms were recorded of all the models from 400 to 4000 cm−1, with 32 scans at a resolution of about 8 cm−1 on Nicolet 8700 FTIR spectrometer (Thermo Fisher Scientific Co., Ltd, Waltham, MA, USA).
Morphological Analysis
The morphology of all the samples was analyzed by using SEM and TEM devices named JSM-6360LA, instrument (Jeol, Japan), and JEM-2100 TEM (JEOL Ltd, Tokyo, Japan) respectively. Barkcloth fabric and powder were mounted on the stabs separately and coated with a thin film of gold by a sputtering method to examine the morphology of the samples by SEM instrument operating at an accelerating voltage of 30 kV. To better understand the dimensions, shape, and size, a drop of each sample with ethanol dispersion solution at a concentration of 0.5 wt% was mounted on a glow-discharged carbon-coated Cu grid and examined by TEM. An accelerating voltage of 120 kV was applied during TEM analysis.
X-ray Diffraction (XRD) Analysis
XRD pattern of dried CNFs was attained by D500 diffractometer (SIEMENS) operated at 30 kV and 15 mA, using a CuKα radiation source (k = 0.154). A diffract-AC software programmer was used for controlling the scans. Every sample was milled into powder form, placed on the sample holder, and leveled to obtain a uniform X-ray exposure. The crystallinity index was calculated with the Segal method [34] (Eq. 1) using the maximum intensity of diffraction from the 002 planes (I002), and the minimum intensity between the 002 and 110 peaks (Iam).
where I002 is the maximum intensity of the (002) lattice diffraction peak and Iam is the intensity scattered by the amorphous part of the sample. For XRD analysis, the fiber samples were prepared in powder form. The clean and tidy samples were prepared and well-combed to examine the orientation of the samples.
Thermogravimetric Analysis (TGA)
A Perkin Elmer TGA-7 thermo-gravimetric analyzer studied the thermal stability of all the samples. The specimens were kept in a platinum sample pan and heated from 30 to 600 °C at 10 °C/min. TGA of all the samples was carried out under a nitrogen gas atmosphere of 19.8 mL/min. The sample weight for each measurement was 0.6 mg.
Determination of Carboxylate Content
In this section, two types of TEMPO-oxidized samples under various conditions were examined as a yield percentage by collecting the water-insoluble fractions in the oxidized products after filtration. Similarly, characterization from several aspects for obtaining fundamental information about the surface-modified celluloses was done with various functional groups. Determination of carboxylate contents of TEMPO-oxidized cellulose nanofibrils was done as the method mentioned in [35, 36] by electric conductivity titration method. The titration was carried out three times for each sample. TEMPO-oxidized cellulose nanofibrils were examined to determine the carboxylate content by the electric conductivity titration method. Carboxylate contents were expressed in this paper as mmol per gram of the oxidized samples (mmol/g).
Results and Discussion
Chemical Composition Analysis
The contents of cellulose, hemicellulose and lignin were determined as 43.5%, 24.5%, and 19.5% respectively. Cellulose content was reported quite less as compared to the report [24], but relatively higher as compared to other specific lignocellulose resources as shown in Table 1 (Supplementary File). The structural and morphological changes during every process are shown in Fig. 2. Figure 2a shows the raw biomass of barkcloth without any treatment. FNB was chopped into small pieces (Fig. 2b), and further processed into powder form (Fig. 2c), and then directed to the purification process. In Fig. 2d, it is observed that the pretreated samples were light yellow but the extracted cellulose samples were fine and white (Fig. 2e), indicating that an enormous fraction of the initial non-cellulosic components were removed during the cellulose extraction process [37].
FTIR Analysis
Figure 3 shows the FTIR spectra of the samples. The spectra similarity indicated that cellulose was not removed during chemical purification process in all samples. The FTIR peaks observed in the samples involved a broad brand at 3306 cm−1 (–OH group stretching vibration) and a peak at 2900 cm−1 (C–H–bond vibration) [38], that are endorsed to the elemental functional groups found in lignocellulosic materials.
The absorption peak at around 1165 cm−1 corresponds to C–C and peak at 894 cm−1 is representing β-1,4-glycosidic linkages in pyranose ring of cellulose. Interestingly, the intensity of these two peaks improved steadily due to elimination of impurities during progressive chemical treatments. Besides, the broad absorption band at about 1028 cm−1 was due to C–O–C pyranose ring stretching vibration. The absorption peak at 1608 cm−1 is sharped and clear for the samples NF-Nt and NF-Dt due to sodium carboxylate groups formed by TEMPO-oxidation and assigned to the O–C = O asymmetric stretching [39], but uncleared in other samples NF-R, NF-D, and NF-N. Similarly, findings from FTIR analysis revealed that most of the amorphous region was eliminated during successive pretreatments including scouring, bleaching, and DMSO, since the peak at 1740 cm−1(COO– linkages of carbonyl groups prevalent in non cellulosic components) for barkcloth raw material NF-R is almost disappeared in NF-D and NF-N. The spectrum in the peak shape of NF–Nt fitted perfectly to the NF-Dt which showed that there was no new functional group in NF-Dt after the DMSO pretreatment. The spectra of NF-Dt and NF-Nt have almost similar absorption peaks, demonstrating that the structure of cellulose I remained during the extraction of CNFs.
Morphological Analysis
The morphology of pristine and powdered FNB, as well as extracted cellulose samples by different chemical treatments, is shown in Fig. 4. The SEM scans show a mechanism of the microstructure of different samples at various stages of CNF extraction. Figure 4a, b shows that barkcloth material consists of microfibers that are aligned and naturally bonded with each other. FNB contains non-cellulosic impurities such as hemicelluloses, lignin, as well as wax, and pectin which are known to cover the surface of naturally occurring fibers as a protective layer [24]. Conversion of barkcloth fibers into powder form after passing through a mesh sieve showed a mess structure Fig. 4c, d. Samples NF-N and NF-D exhibited the fibrous shape, demonstrating the removal of non-cellulosic components during chemical purification. The alkali-treatment contributed to remove the non-cellulose impurities, leading to reduced dispersion of micro-fibrils. The fiber separation process can be observed easily (Fig. 4e,f) because of alkali treatment and bleaching resulting in irregular fibrillar structure and an obvious reduction in fibers diameter. Similar results were also reported earlier by Nurain Johar [40]. Sample NF–N in Fig. 4e demonstrated that the cellulose samples existed as an aggregate of microfibers. Meanwhile, microfibers were separated and well-aligned in NF-D, as DSMO-treatment contributed to breaking the intermolecular hydrogen bonding, resulting in removing the non-cellulosic substances [41]. In general, it can be summarized that the DMSO pretreatment can provide broad results in CNF extraction by selective removal of lignin and hemicellulose with high preservation of cellulose.
Scanning electron microscopy (SEM) analysis of the barkcloth from Ficus natalensis in every stage of the cellulose extraction process, barkcloth material without any treatment (a, b), barkcloth fibers in powder form NF–R (c, d), cellulose fibers without DMSO treatment NF-N (e), cellulose samples after DMSO treatment NF-D (60 °C, 5 h, 600 rpm) (f)
TEM (Fig. 5) gives a clear image of NF–Nt and NF–Dt, demonstrating that the obtained structures were relatively isolated with defined shapes. Most of the nanoparticles displayed diameters in the range of 12–40 nm.
The diameter distributions of 100 samples of CNFs were taken by using image J. software. Figure 6 shows the diameter and frequency histogram, specifying that NF-Dt had a narrower distribution range as compared to NF-Nt. Figure 6a, b shows that the diameter was distributed mainly from 16 to 42 nm and 12.5 to 37.5 nm for NF-Nt and NF-Dt respectively. The significant difference observed in the morphology of CNFs was that NF-Nt demonstrated an average diameter of about 33 nm while NF-Dt showed an average diameter of 25 nm with a length of several hundred nanometers for both type samples. During TEMPO-oxidation, strong repulsive forces were required to extract the cellulose nanofibrils from cellulose. The steric hindrance effect of TEMPO was the reason that reactive ions hardly penetrate the inner part of the cellulose materials. Pretreatment of cellulosic material with DMSO before TEMPO-oxidation considerably facilitates the disintegration process of cellulose samples [42]. In the case of NF-Dt, DMSO pre-treatment enables reactive ions to penetrate the cellulose molecules during TEMPO-oxidation. So the diameters of NF–Dt samples were noted less as compared to NF-Nt. Thus, DMSO treatment before TEMPO-oxidation could affect the distribution and dimension of the obtained CNF. This study shows that FNB can be an alternative sources of CNF and detailed evaluation of chemical pretreatment revealed the fabulous results boosting to use dimethyl sulfoxide as an additional chemical in TEMPO oxidation.
X-ray Diffraction
Figure 7 shows the diffraction peaks at around 16.02°, 22.25°, and 34.68° for all the samples, corresponding to 110, 002, and 004 [43] attributing to the crystallographic plane of type I cellulose as the similar evaluation was reported in [44]. The diffraction peaks at about 22° for NF-D, NF-N, NF-Dt, and NF-Nt were sharper than in NF-R. The oxidation slightly changed the diffraction pattern of the low crystalline cellulose I and the diffraction peak position of barkcloth raw material. These slight changes may reflect the introduction of amounts of carboxylate and aldehyde groups on cellulose I crystal surfaces [45].
The crystallinity of various samples was determined as shown in Table 1. The crystallinity of NF-N (50.3%) and NF-D (56.6%) was remarkably increased as compared to NF-R (35.7%). The increase was attributed to the progressive removal of amorphous non-cellulosic substances during chemical purification. Regarding NF–D, the higher increase was due to the DMSO treatment that further dissolved residual lignin and hemicellulose as reported in the literature [39]. Thus, DMSO stimulated the hemicellulose and lignin removal, so the cellulose swelling and breaking of the inter-link hydrogen bonding were occurred [37].
The crystallinity of NF-Dt and NF-Nt was examined higher (67.3% and 58.1% respectively) as compared to NF-N and NF-D, as TEMPO-oxidized randomly the hydroxyl groups of cellulose to carboxyl groups [46]. The alkali treatment and TEMPO-oxidation removed the amorphous regions of cellulose and affected the crystallinity values of cellulose nanofibrils. Moreover, the DMSO in the case of NF-D removed more non-cellulosic substances, which endorsed the TEMPO reagents to penetrate the cellulose samples and disrupted the amorphous and crystalline regions more efficiently.
Thermal Stability Analysis
The thermal stability of samples was studied by thermo-gravimetric analysis (TGA) and derivative thermo-gram (DTG), as shown in Fig. 8a,b. It illustrated a wide temperature range according to the degradation of all the samples [47]. An initial weight loss between 50 °C and 150 °C was observed due to the loss of water and low molecular compounds in all the samples [48]. It was quite interesting to note that the samples showed a broad weight degradation phenomenon and contained various substances followed by almost identical mechanisms, with different degradation temperatures.
The presence of hemicellulose, lignin, and other non-cellulosic constituents in NF-R contributed to decompose at lower temperatures [49] help to cause early-onset degradation (265 °C), and the rate of degradation reached its higher level at 363 °C [27]. Due to the sample’s chemical purification, the non-cellulosic constituents were removed, so the onset decomposition temperature of NF-N was increased (311 °C), and degrading ends at 342 °C. The degradation of cellulose occurs between 275 and 400 ℃ [50, 51] which is close to our findings. The onset decomposition and final degrading temperature of NF-D was higher (330 °C, 380 °C, respectively) than NF-N (311 °C, 342 °C, respectively) because non-cellulosic constituents were further dissolved by DMSO pretreatment. This demonstrated that NF-D has better thermal stability compared to NF-N. The higher decomposition temperature obtained is recognized to the higher crystallinity of cellulose [50]. As indeed shown by the XRD diffraction study, the crystallinity of NF-D was more elevated, and this is accountable for the higher thermal stability of NF-D. As well as, the reorientation and rearrangement of the cellulose crystals in NF-D samples increase the onset degradation temperatures.
Conversely, in the case of CNFs samples obtained from the TEMPO-oxidation process, the decomposition temperatures have an obvious decline. CNFs were thermally degraded between 210–250 °C and 250–300 °C. The decrease in decomposition temperatures is attributed to the introduction of unstable sodium carboxylate groups [45]. The formation of sodium carboxylate groups from the C6 primary hydroxyls of CNFs surfaces by the TEMPO-mediated oxidation leads to a comparatively higher decrease in the thermal degradation point [52]. NF-Nt peak was lower than the raw material (NF-R) and original cellulose (NF-N, NF-D) as also reported in [39]. This indicates that the CNF chains decreased in their peak points using anhydroglucuronic acid units, which were thermally more unstable. The decline of NF-Nt degradation peak compared to NF-Dt is due to the pretreatment of NF-Dt with DMSO, as it dissolved the non-cellulosic components. Amorphous regions in NF-Nt due to some non-cellulosic elements caused the lower thermal stability as compared to NF-Dt having more crystalline regions [45]. From the aforementioned results, it can be seen that DMSO chemical treatment as an extra step in a conventional TEMPO oxidation process for cellulose nanofibrils extraction, show great impacts on the thermal stabilities of the samples. It could be asserted that DMSO pretreatment used in nanocellulose isolation process is providing an economical and feasible approach for improving the cellulose nanofibrils efficiency.
Figure 8b illustrated the DTG curve of all the samples. It showed that NF-R exhibited two degradation peaks: one at a lower temperature (258 °C) which possibly due to non-cellulosic substances and another at a higher temperature (315 °C) due to cellulosic compounds. Two closely and non-sharped degraded peaks occurred, indicating the presence of non-cellulosic substances in the case of NF-N. Only one degradation peak has occurred in the DTG curve for the sample of NF-D. Similarly, one sharp degradation peak for every sample (NF-Dt and NF-Nt) is observed.
Determination of Carboxylate Contents
The original fibrous forms of two types of cellulose (NF-N and NF-D) were mostly maintained as cellulose I when treated with TEMPO/NaBr/NaClO system under aqueous conditions even after the oxidation for 4 h [53]. As TEMPO-mediated oxidation of cellulose samples is a surface effect in which anionic carboxylate groups are introduced as functional groups at solid cellulose surfaces. Cellulose nanofibrils were obtained actually by the formation of the C6 carboxylate groups selectively on the surfaces of the cellulose crystallites through TEMPO-mediated oxidation that generates a strong repulsive force. Carboxylate contents were different between two types of oxidized cellulose samples examined at a different duration of the oxidation process (0 and 4 h) ranging from 0.5 to 1.5 mmol/g as shown in Table 1. (supplementary file). Carboxylate groups show similar behavior for 0 h to both of the samples. But the carboxylate content increases with the increase of oxidation time. During TEMPO oxidation of cellulose, significant amounts of C6 aldehyde are formed as intermediate structures during the oxidation process at pH 10.5 during the oxidation [54] as evidenced from the FTIR spectra. The higher value of carboxylate content for the sample NF-Dt as compared to NF-Nt is a possibility due to the extra chemical treatment which may cause the external surfaces and internal cellulose microfibrils to be more oxidized or degraded into water-soluble fractions, and cellulose microfibers collapse into pieces which helps to enhance the oxidation efficiency and more excellent depolymerization of cellulose occurs [55]. So the DMSO pretreatment appeared to increase the oxidation efficiency and speed up the TEMPO oxidation process with anionic carboxylate groups on the microfibril surfaces.
Conclusion
In this study, cellulose nanofibrils have been produced from the novel material Ficus natalensis barkcloth fibers via the TEMPO-oxidation method, and the effects of dimethyl sulfoxide (DMSO) were evaluated. Chemical composition investigation demonstrated that barkcloth fiber materials contained 34.5% cellulose, 23.5% hemicellulose, and 19.5% lignin. SEM analysis showed that significant changes have been observed in the surface morphology of barkcloth fibers pretreated with DMSO compared to untreated samples. As a result of TEM image analysis, the cellulose nanofibrils diameter was found in the range of 12–40 nm, while the average diameter of samples treated with DMSO is lower (25 nm) than that of untreated ones (33 nm). XRD consequences verified all the samples attributed to the type I cellulose crystallographic plane although the crystallinity was remarkably increased from 35.7% (NF-R) to 50.3% (NF-N) during chemical purification. The NF-D crystallinity was higher (56.6%) since residual lignin and hemicellulose were dissolved with DMSO. Similarly, excellent crystallinity value as 67.3% of samples NF-Dt was noted as compared to untreated CNF samples i.e. 58.1% (NF-Nt). DMSO affected the onset decomposition temperature of NF-D (330 ℃) as compared to NF-R (265 ℃) and NF-N (310 ℃). Thus, the chemical pretreatment process through DMSO appeared to be appropriate and effective for the extraction of cellulose nanofibrils and then to develop various applications including energy storage, food packaging, and biomedical applications.
References
P. Huang, Y. Zhao, S. Kuga, M. Wu, and Y. J. N. Huang, "A versatile method for producing functionalized cellulose nanofibers and their application," vol. 8, no. 6, pp. 3753–3759, 2016.
Frone AN, Chiulan I, Panaitescu DM, Nicolae CA, Ghiurea M, Galan A-MJML (2017) Isolation of cellulose nanocrystals from plum seed shells, structural and morphological characterization. Matteer Lett. 194:160–163
K. Ramanaiah, A. R. Prasad, K. H. C. J. M. Reddy, and Design, "Mechanical, thermophysical and fire properties of sansevieria fiber-reinforced polyester composites," Mater Design. 49, 986–991, 2013.
E. A. Hassan, M. L. Hassan, R. E. Abou-Zeid, N. A. J. I. C. El-Wakil, and Products, Novel nanofibrillated cellulose/chitosan nanoparticles nanocomposites films and their use for paper coating. Ind Crops Prod. 93, 219–226, 2016.
Fukuzumi H, Saito T, Iwata T, Kumamoto Y, Isogai A (2008) Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromol 10(1):162–165
Saito T, Shibata I, Isogai A, Suguri N, Sumikawa N (2005) Distribution of carboxylate groups introduced into cotton linters by the TEMPO-mediated oxidation. Carbohyd Polym 61(4):414–419
Li Y et al (2016) Facile extraction of cellulose nanocrystals from wood using ethanol and peroxide solvothermal pretreatment followed by ultrasonic nanofibrillation. Green Chem 18(4):1010–1018
Rajinipriya M, Nagalakshmaiah M, Robert M, Elkoun S (2018) Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: a review. ACS Sustainable Chemistry & Engineering 6(3):2807–2828
Shak KPY, Pang YL, Mah SK (2018) Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J Nanotechnol 9(1):2479–2498
Sirviö JA, Visanko MJJOMCA (2017) "Anionic wood nanofibers produced from unbleached mechanical pulp by highly efficient chemical modification. Carbohydrate Poly 5:21828–21835
Tabar IB, Zhang X, Youngblood JP, Mosier NSJCP (2017) Production of cellulose nanofibers using phenolic enhanced surface oxidation. Carbohydrate Poly 174:120–127
Ebringerová A, Heinze TJMRC (2000) Xylan and xylan derivatives–biopolymers with valuable properties, 1 Naturally occurring xylans structures, isolation procedures and properties. Macromol Rapid Commun 21:542–556
F. Xu et al., "Fractional separation of hemicelluloses and lignin in high yield and purity from mild ball-milled Periploca sepium," vol. 43, no. 11–12, pp. 3351–3375, 2008.
Sun R, Tomkinson JJIJOPA (2003) Fractional isolation and spectroscopic characterization of sago starch. Int J Poly Anal Char. 8:29–46
Pramanik MM, Rastogi N (2016) Visible light catalyzed methylsulfoxidation of (het) aryl diazonium salts using DMSO. Chem Commun 52(55):8557–8560
Höije A, Gröndahl M, Tømmeraas K, Gatenholm PJCP (2005) Isolation and characterization of physicochemical and material properties of arabinoxylans from barley husks. Carbohydrate Poly 61:266–275
Singh D, Singh B, Goel RK (2011) Traditional uses, phytochemistry and pharmacology of Ficus religiosa: A review. J Ethnopharmacol 134(3):565–583
Serrato A, Ibarra-Manríquez G, Oyama K (2004) Biogeography and conservation of the genus Ficus (Moraceae) in Mexico. J Biogeogr 31(3):475–485
Awolola GV, Chenia H, Baijnath H, Koorbanally NA (2017) Anti-adhesion potential of non-polar compounds and extracts from Ficus natalensis. Rev Bras 27(5):599–602
Y. Kim and V. Chalivendra, "Handbook of Natural Fibres," Natural fibre composites (NFCs) for construction and automotive industries, pp. 469–498, 2020.
A. Hutchings, Zulu medicinal plants: An inventory. University of Natal press, 1996.
Rwawiire S, Tomkova B, Militky J, Jabbar A, Kale BM (2015) Development of a biocomposite based on green epoxy polymer and natural cellulose fabric (bark cloth) for automotive instrument panel applications. Compos B Eng 81:149–157
D. Cousins and M. A. J. A. S. M. Huffman (2002) Medicinal properties in the diet of gorillas: an ethno-pharmacological evaluation, 23, 65–89.
Rwawiire S, Tomkova B, Militky J, Hes L, Kale BM (2017) Acoustic and thermal properties of a cellulose nonwoven natural fabric (barkcloth). Appl Acoust 116:177–183
Rwawiire S, Tomkova B, Wiener J, Militky J (2016) Effect of enzyme and plasma treatments of bark cloth from Ficus natalensis: morphology and thermal behavior. J Textile Institute 107(5):663–671
Rwawiire S, Luggya GW, Tomkova B (2013) Morphology, thermal, and mechanical characterization of bark cloth from Ficus natalensis. ISRN Textiles 2013:1–8
Mugaanire IT, Wang H, Sun J (2019) Fibrous microcrystalline cellulose from Ficus natalensis barkcloth. European J Wood Wood Products 77(3):483–486
A. Farooq, S. Jiang, A. Farooq, M. A. Naeem, A. Ahmad, and L. Liu (2019) Structure and properties of high quality natural cellulose nano fibrils from a novel material Ficus natalensis barkcloth. Journal of Industrial Textiles, p. 1528083719887533.
S. Rwawiire, N. Catherine, K. S. Baker, and G. Davis (2012) Processing of natural fiber textile from Ficus natalensis and Antiaris toxicaria," in Proceedings of the 2nd International Symposium on Sustainable Development through Research in Natural Textile Fibers, Textile Products, Trade and Marketing.
L. Costa, A. F. Fonseca, F. V. Pereira, and J. I. J. C. C. T. Druzian (2015) Extraction and characterization of cellulose nanocrystals from corn stover. 49, 127–133.
Shaheen TI, Emam HE (2018) Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using acid hydrolysis. Int J Biol Macromol 107:1599–1606
Isogai A, Kato Y (1998) Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 5(3):153–164
Adeeyo O, Oresegun OM, Oladimeji TE (2015) Compositional analysis of lignocellulosic materials: Evaluation of an economically viable method suitable for woody and non-woody biomass. Am J Eng Res (AJER) 4(4):14–19
Segal L, Creely J, Martin A Jr, Conrad C (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29(10):786–794
Saito T, Isogai A (2004) TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromol 5(5):1983–1989
Saito T, Isogai A (2005) Ion-exchange behavior of carboxylate groups in fibrous cellulose oxidized by the TEMPO-mediated system. Carbohyd Polym 61(2):183–190
Das K et al (2010) Physicomechanical and thermal properties of jute-nanofiber-reinforced biocopolyester composites. Ind Eng Chem Res 49(6):2775–2782
Penjumras P, Rahman RBA, Talib RA, Abdan KJA, Procedia AS (2014) Extraction and characterization of cellulose from durian rind. Agri Agri Sci Procedia. 2:237–243
Okita Y, Fujisawa S, Saito T, Isogai A (2010) TEMPO-oxidized cellulose nanofibrils dispersed in organic solvents. Biomacromol 12(2):518–522
Johar N, Ahmad I, Dufresne A (2012) Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind Crops Prod 37(1):93–99
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3(1):71–85
Benhamou K, Dufresne A, Magnin A, Mortha G, Kaddami H (2014) Control of size and viscoelastic properties of nanofibrillated cellulose from palm tree by varying the TEMPO-mediated oxidation time. Carbohyd Polym 99:74–83
Miao X, Lin J, Tian F, Li X, Bian F, Wang J (2016) Cellulose nanofibrils extracted from the byproduct of cotton plant. Carbohyd Polym 136:841–850
Haafiz MM, Hassan A, Zakaria Z, Inuwa I (2014) Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohyd Polym 103:119–125
Fukuzumi H, Saito T, Okita Y, Isogai A (2010) Thermal stabilization of TEMPO-oxidized cellulose. Polym Degrad Stab 95(9):1502–1508
Zhai L, Kim HC, Kim JW, Choi ES, Kim J (2018) Cellulose nanofibers isolated by TEMPO-oxidation and aqueous counter collision methods. Carbohyd Polym 191:65–70
Kim H-S, Kim S, Kim H-J, Yang H-S (2006) Thermal properties of bio-flour-filled polyolefin composites with different compatibilizing agent type and content. Thermochim Acta 451(1–2):181–188
Spinella S et al (2016) Concurrent cellulose hydrolysis and esterification to prepare a surface-modified cellulose nanocrystal decorated with carboxylic acid moieties. ACS Sustainable Chemistry & Engineering 4(3):1538–1550
Mandal A, Chakrabarty D (2011) Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohyd Polym 86(3):1291–1299
B. Deepa et al., "Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion," vol. 102, no. 2, pp. 1988–1997, 2011.
Ouajai S, Shanks RJPD (2005) Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Poly Degradation Stability. 89:327–335
Fukuzumi H, Saito T, Iwata T, Kumamoto Y, Isogai AJB (2009) Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromol 10:162–165
Jakob H, Fratzl P, Tschegg S (1994) Size and arrangement of elementary cellulose fibrils in wood cells: a small-angle X-ray scattering study of Picea abies. J Struct Biol 113(1):13–22
De Nooy A, Besemer AC, Van Bekkum H, Van Dijk J, Smit J (1996) TEMPO-mediated oxidation of pullulan and influence of ionic strength and linear charge density on the dimensions of the obtained polyelectrolyte chains. Macromolecules 29(20):6541–6547
Ye W, Liu L, Wang Z, Yu J, Fan Y (2019) Investigation of pretreatment methods for improving TEMPO-mediated oxidation and nanofibrillation efficiency of α-chitin. ACS Sustain Chem Eng 7(24):19463–19473
Acknowledgements
The authors would like to mention the financial support for the research, authorship, and/or publication of this article
Funding
The work was supported by “National Key R&D Program of China (2018YFC2000900)” and “Suzhou Science and Technology Project (ZXL2018134)”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Farooq, A., Li, M., Alasood, A. et al. Novel Pretreatment Performance Evaluation for Cellulose Nanofibrils Extraction from Ficus natalensis Barkcloth. J Polym Environ 30, 1547–1559 (2022). https://doi.org/10.1007/s10924-021-02297-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02297-x