Abstract
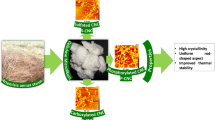
In this study, microcrystalline cellulose (MCC) at an overall yield of 30.9% was prepared from Ficus natalensis bark cloth via acid hydrolysis. MCC exhibited a high crystallinity index of 75.8% and an initial thermal degradation temperature of 314.6 °C. Morphological studies revealed fibrous rod-like particles of MCC with somewhat rough surfaces whose average length and diameter were approximately 90 ± 25 μm and 17.08 ± 3.3 μm, respectively. Thus, the thermally stable fibrous MCC prepared from this study expands the scope of applications for the barkcloth as fillers in biocomposites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Barkcloth is a natural biodegradable material made from the inner bark of Ficus natalensis—a famous tree which popularly grows in most parts of Uganda and southern Africa. During harvesting, the inner bark is freely peeled from the trunk of Ficus natalensis and it takes about 8 months for the fresh bark to regenerate until the next harvest. Besides its application in making fashionable cultural outfits, recent studies have indicated that barkcloth exhibits a rich chemical composition with approximately 68.69% cellulose, 15.07% lignin and 15.24% hemicellulose (Rwawiire et al. 2017). Therefore, this study focuses on utilisation of the high cellulose content in Ficus natalensis barkcloth to prepare microcrystalline cellulose whose global market is anticipated at USD 1.08 billion by 2020 due to its increasing demand in food and pharmaceutical industries (Nsor-Atindana et al. 2017). Characterisation techniques such as scanning electron microscopy (SEM), Fourier transform infrared (FTIR), X-ray diffraction (XRD), and thermogravimetric analysis (TGA) were employed to examine the morphology, thermal and chemical properties of extracted microcrystalline cellulose from the barkcloth.
2 Experimental procedures
2.1 Materials
Barkcloth from Ficus natalensis was provided by traditional manufacturers in Masaka—Uganda. Sodium hydroxide pellets, hydrochloric acid (37% w/w) and hydrogen peroxide (30% w/v) were supplied from Tianjin Fuchen Chemicals Reagent Factory, Tianjin—China. Triton X-100 (t-Octylphenoxypolyethoxyethanol) at a purity > 98% was purchased from Phygene Life Sciences Co. Ltd, Shanghai and was used as surfactant during scouring. All chemicals were of analytical grade and used without further purification.
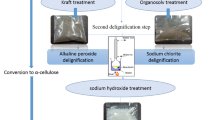
2.2 Extraction of microcrystalline cellulose (MCC)
The desired amount of chopped barkcloth pieces from Ficus natalensis was treated with 1M NaOH at 95 °C for 3 h at a material to liquor ratio of 1:50. Fibres were then thoroughly washed in distilled water to pH 7, and no colour bleeding from the wet sample could be detected in water. The dry scoured fibres were subsequently bleached with 30 ml of 30w/v% hydrogen peroxide in a solution of 0.25M NaOH at 70 °C for 2 h, washed with distilled water to pH 7, then dried and stored at room temperature. Bleached barkcloth fibres were further cut into smaller chips, placed in a three-neck round bottom flask and 2.5 N HCL was dropwise added at a material to liquor ratio of 1:25 under vigorous stirring with a constant speed (900 rpm) for 45 min at 90 °C. The hydrolysed microcrystalline cellulose was then cooled at room temperature and washed with distilled water to pH 7. Centrifugation was performed at 3500 rpm for 5 min on the neutral MCC suspension to remove excess water. The supernatant was poured off, and the precipitate was later oven-dried at 60 °C for 12 h and weighed. The dried MCC was pulverised and stored as a white powder for analysis. Products at each stage are illustrated in electronic supplementary material 1 (ESM 1).
3 Characterization of barkcloth and derived cellulose
The morphology of raw barkcloth (RB), scoured barkcloth fibres (SC), bleached barkcloth fibres (BL) and microcrystalline cellulose (MCC) powder was examined with scanning electron microscope (Quanta 250, Thermo Fisher Scientific, USA).
Infrared spectra were generated with Nicolet® 6700 Fourier Transform InfraRed (Thermo Fisher Scientific, USA), where fine powders of raw barkcloth fibres, scoured barkcloth fibres, bleached barkcloth fibres and microcrystalline cellulose were mixed with potassium bromide (KBr) prior to FTIR analysis.
X-ray diffraction analysis was performed using X-ray diffractometer (Rigaku D/Max 2550/PC, Japan) with Cu-Kα radiation (λ = 1.54 Å) in a 2θ range of 5°–60° at a scanning speed of 1°min−1. The crystallinity indices of raw fibres, scoured fibres, bleached fibres and microcrystalline cellulose samples were calculated based on the empirical method proposed by Segal et al. (1959).
Thermal stability of the samples was studied using a thermogravimetric analyser [Perkins Elmer TGA400- (Thermo Fisher Scientific, USA)]. Approximately 5 mg of each sample were scanned from 30 to 600 °C at a heating rate of 20℃/min under a nitrogen-controlled atmosphere.
4 Results and discussion
From the SEM micrograph in Fig. 1a, it was observed that the barkcloth is composed of individual fibers which are adhered into bundles. After bleaching, as depicted in Fig. 1b, bundles were dismantled leading to fiber exposure and separation.
Each bundle consists of numerous fibres oriented almost parallel to each other with somewhat rough surfaces. Fiber separation and surface roughness were probably due to the removal of adhering materials and other impurities like extractives after the scouring and bleaching. Furthermore, microcrystalline cellulose in Fig. 1c showed fibrous rod-like particles which varied in length between 20 and 240 µm with an average of 90 ± 25 µm. Their average diameter was 17.08 ± 3.3 µm. Details of particle size distribution can also be accessed in ESM 2. The final yield of pulverised MCC compared to the original untreated sample was as low as 30.2 ± 1.5%, which implied that the cellulose content in the barkcloth could be lower than earlier reported (Rwawiire et al. 2017).
While the major peaks identified in the RB spectra are summarized in ESM 7, findings from FTIR analysis in Fig. 1e showed that most amorphous components in the bark were removed during subsequent pretreatments since the peak at 1740 cm−1, related to COO– linkages of carbonyl groups prevalent in hemicellulose, almost disappeared after scouring, bleaching and hydrolysis. Additionally, the peak at 1520 cm−1, common to aromatic rings in lignin structure, was also eliminated from the pretreated samples. Moreover, the peaks at about 1150 cm−1 and 898 cm−1 are associated with β-1,4-glycosidic linkages in pyranose ring of cellulose (Pujiasih et al. 2018). The intensity of these peaks increased gradually after pretreatments of the barkcloth fibres at progressive steps probably because most impurities covering the cellulose microfibrils had been removed.
The X-ray diffraction patterns of the RB, SC, BL, and MCC are shown in Fig. 1d. The four central peaks observed in these diffractograms are representative of 1–10, 110, 200, and 004 crystallographic planes observed at 2θ = 14.91°, 16.4°, 22.66° and 34.4°, respectively, typical of cellulose (Zhang et al. 2018). The crystallinity indices (CI) of RB, SC, BL, and MCC fibres were 34.3%, 69.7% 72.2% and 75.8%, respectively. The gradual increase in CI at each subsequent step was due to the significant reduction in paracrystalline (amorphous) components as demonstrated in the FTIR spectra. Most of such amorphous components were removed during scouring since the crystallinity index almost doubled after this stage from 34.3 to 69.7%. Additionally, acid hydrolysis partially digested β-1,4-glycosidic linkages in the amorphous regions of cellulose microfibrils thereby releasing individual crystallites which further contributed to the high crystallinity degree in MCC.
Thermograms of the analysed materials are presented in Fig. 1f, and a summary of mass losses, onset degradation temperatures (Tonset) and maximum onset degradation temperatures (Tmax) for significant mass regions and char residues during the thermal decomposition process is presented in Table 1. Initially, weight losses of less than 10% were observed in a temperature range of 30–120 °C due to absorbed moisture in the samples. After the weight loss due to the presence of moisture in the RB, SC and BL followed a one-step thermal degradation process with initial decomposition temperatures of 240 °C, 351.8 °C and 312.1 °C, respectively. However, microcrystalline cellulose was thermally degraded between 300–375 °C and 375–525 °C temperature ranges. Moreover, char residues in raw barkcloth fibres were the highest due to the presence of a significant amount of flame retardant components (Mtibe et al. 2015). Therefore, the high thermal decomposition temperature in the range of 300–350 °C could make MCC from barkcloth fibres a potential filler in bio-composites.
5 Conclusion
MCC in the form of rods with approximately 90 ± 25 μm in length and an average diameter of 17.1 ± 3.3 μm was successfully isolated from Ficus natalensis barkcloth. FTIR and SEM analyses revealed that most non-cellulosic components like hemicellulose and lignin were removed during preparation. Approximately 45% of the amorphous components were removed during scouring. The crystallinity index as studied from XRD results increased progressively at subsequent treatment stages. More notably, the degree of crystallinity after scouring increased twice as much as that of raw barkcloth fibres from 34.3 to 69.7%. MCC from Ficus natalensis barkcloth had a higher crystallinity index of 75.8% compared to other studies on microcrystalline cellulose from bast and some wood species. The overall yield of MCC based on the raw material was as low as 30.2 ± 1.5% but had a high thermal decomposition temperature of 315 °C. Thus, microcrystalline cellulose from the barkcloth could act as an excellent filler in biocomposites.
References
Mtibe A, Linganiso LZ, Mathew AP, Oksman K, John MJ, Anandjiwala RD (2015) A comparative study on properties of micro and nanopapers produced from cellulose and cellulose nanofibres. Carbohydr Polym 118: 1–8. https://doi.org/10.1016/j.carbpol.2014.10.007
Nsor-Atindana J, Chen M, Goff HD, Zhong F, Sharif HR, Li Y (2017) Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr Polym 172: 159–174. https://doi.org/10.1016/j.carbpol.2017.04.021
Pujiasih S, Kurnia, Masykur A, Kusumaningsih T, Saputra OA (2018) Silylation and characterization of microcrystalline cellulose isolated from indonesian native oil palm empty fruit bunch. Carbohydr Polym 184: 74–81. https://doi.org/10.1016/j.carbpol.2017.12.060
Rwawiire S, Tomkova B, Militky J, Hes L, Kale BD (2017) Acoustic and thermal properties of a cellulose nonwoven natural fabric (barkcloth). Appl Acoust 116(Supplement C):177–183. https://doi.org/10.1016/j.apacoust.2016.09.027
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29(10):786–794. https://doi.org/10.1177/004051755902901003
Zhang Z, Zhu M, Zhang D (2018) A thermogravimetric study of the characteristics of pyrolysis of cellulose isolated from selected biomass. Appl Energy 220: 87–93. https://doi.org/10.1016/j.apenergy.2018.03.057
Acknowledgements
Special thanks to Mr. Bakidde Matovu for supplying the barkcloth, and Bao Jian Kun for assistance in accessing characterization equipment.
Funding
No funding was received from any agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mugaanire, I.T., Wang, H. & Sun, J. Fibrous microcrystalline cellulose from Ficus natalensis barkcloth. Eur. J. Wood Prod. 77, 483–486 (2019). https://doi.org/10.1007/s00107-019-01382-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-019-01382-2