Abstract
Depolymerization of polyethylene terephthalate (PET) is a promising technology for producing recycled monomers. Using a deep eutectic solvent (DES)-based catalyst, the PET glycolysis process produces bis-(2-hydroxyethylene terephthalate) (BHET). This recycled monomer reacts with isocyanate and forms polyurethane foam (PUF). The DES-based one-pot reaction is advantageous because it is a low-energy process that requires relatively lower temperatures and reduced reaction times. In this study, choline chloride/urea, zinc chloride/urea, and zinc acetate/urea based DESs were adopted as DES catalysts for glycolysis. Subsequently, the conversion of PET, BHET yield, and OH values were evaluated. Both filtered and unfiltered reaction mixtures were used as polyols for PUF polymerization after characterization of the acid and hydroxyl values of the polyols, as well as the NCO (–N=C=O) value of isocyanate. In the case of unfiltered reaction mixtures, PUF was obtained via a one-pot reaction, which exhibited higher thermal stability than PUF made from the filtered polyols. This outcome indicated that oligomeric BHET containing many aromatic moieties in unfiltered polyols contributes to the thermal stability of PUF. This environmentally friendly and relatively simple process is an economical approach for upcycling waste PET.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last decade, polyethylene terephthalate (PET) has been extensively used for various applications owing to its excellent chemical and mechanical properties, such as transparency, non-toxicity, light weight, thermal stability, and ease of processing. However, the slow degradation of PET waste is harmful for the environment, ecosystems, and circular economic principles [1, 2]. Waste recycling, waste landfilling, waste incineration, and other treatment technologies are among the possible disposal mechanisms. Many studies have been conducted on various approaches of depolymerization, such as hydrolysis, glycolysis, aminolysis, ammonolysis, and methanolysis [3,4,5,6]. Among these, it is relatively easier to obtain bis-(2-hydroxyethylene terephthalate) (BHET) monomers under mild reaction conditions, including the use of ethylene glycol (EG) and a catalyst [7, 8].
Deep eutectic solvents (DESs) comprise a combination of quaternary ammonium salts or Lewis acids as hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs), such as urea [9, 10]. DESs have interesting features, such as low cost, easy processing, environmental friendliness, and biological compatibility [11, 12]. Ser et al. [13] investigated the DES reaction parameters for the glycolysis reaction between EG and waste PET.
DESs comprise two to three components that are capable of hydrogen bonding to form a eutectic mixture with a melting point lower than that of each component [14]. Choline chloride/urea and ZnCl2/urea based DESs have been adapted for PET glycolysis [11, 15]. Guanidine hydrochloride/urea-based DES has been used as a recyclable and reusable catalyst in synthetic chemistry [16, 17]. The green characteristics of DESs are based on the non-volatility and benign nature of HBAs and donors. Various catalysts, such as metal acetates, oxides, carbonates, sulfates, titanium phosphate, zeolites, ionic liquids, metal–organic frameworks, and melamine, have been applied for the glycolysis of PET [18,19,20].
As polyurethane (PU) is produced by reacting diisocyanate or tri-isocyanate with a polyol, recycled monomers such as BHET can function as polyols to produce polyurethane foam (PUF). PUFs commonly consist of hard and soft segments, of which blocks are mainly influenced by components such as polyols, isocyanates, chain extenders, blowing agents, surfactants, catalysts, and defoaming agents [21, 22]. Therefore, DES-based glycolysis induced by EG intrinsically produces a large number of short chains, which leads to the rigid structure of PUFs. The chemical structure of polyol has a significant influence on the thermal and hydrolytic degradation of PUFs, as well as the hydrophobic characteristics [23]. The presence of aromatic ring structures on BHET increases the resistance to thermal degradation as thermal energy tends to dissipate along the aromatic chain [24]. This distinct advantage of the BHET bespeaks the potential for a one-pot reaction.
In this study, glycolysis of waste PET directly to flame retardant PUF was performed in the presence of several DESs. The acid and hydroxyl values of the polyols and NCO (–N=C=O) values of isocyanate were controlled for the polymerization process. Dual catalytic systems such as tertiary amine and organotin catalysts were adopted for PUF synthesis. This one-pot method was preferred because of its simplicity. Moreover, this environmentally benign approach can contribute toward minimizing the depletion rate of non-renewable energy sources, such as fossil fuels. Analytical methods, namely scanning electron microscopy (SEM), Fourier transform-infrared spectroscopy (FT-IR), X-ray diffraction (XRD), micro-combustion calorimetry (MCC), gel permeation chromatography (GPC), and thermogravimetric analysis (TGA) were used to check the morphology of the product, verify completion of polymerization, analyze the effect of BHET monomers on DESs, and validate the enhanced thermal stability, respectively, for DES-based PUF from waste PET.
Experimental Section
Materials
1,4-Diazabicyclo[2.2.2]octane (DABCO 33-LV), dibutyltin dilaurate (DBTDL), potassium acetate, tetrahydrofuran (THF), zinc chloride (ZnCl2), zinc acetate (ZnC4H6O4), choline chloride (C5H14ClNO), sodium hydroxide (NaOH), potassium hydroxide (KOH), EG, and polyethylene terephthalate (PET) were purchased from Sigma Aldrich. Tris (2‐chloropropyl) phosphate (TCPP), thiourea, and urea were purchased from Samchun Pure Chemical Co., Ltd. (Korea). Silicon surfactant (DC 193) was purchased from Dow Inc. (USA). 4,4-Diphenylmethane diisocyanate (MDI) and guanidine hydrochloride (GHCL) were purchased from Junsei Chemical Co. Ltd. (Japan).
Synthesis
A schematic diagram of the total synthesis route starting from PET glycolysis to PUF polymerization is shown in Fig. 1.
Glycolysis of PET
Seven DES systems were prepared, as shown in Table 1, and heated for 1 h [11, 13, 17, 25, 26].
In the case of DES 1-based glycolysis, 12 g of DES was mixed with 3 g of PET and 15 g of EG. For DES 2 and 6, 0.15 g of the respective DES catalyst was added to 3 g of PET and 15 g of EG [11, 13]. The DES-based glycolysis reactions were carried out at 180 °C for 2 h under reflux in a nitrogen atmosphere. After glycolysis, the unreacted PET was manually removed. The mixture was vacuum-filtered to separate out the precipitated BHET oligomers. Thus, the as-made BHET (a-BHET) comprised BHET, DES catalyst, EG, and BHET oligomeric compounds. In contrast, the filtered BHET (f-BHET) contained BHET, DES catalyst, and EG.
Urethane Polymerization
As listed in Table 2, a-BHET and f-BHET were pre-mixed with TCPP, DABCO 33-LV, DC 193, DBTDL, and H2O. These polyol compounds were mixed with MDI to produce PUF samples in consideration of cream, gel, and tack-free time.
Urethane Polymerization from Commercial PET Bottles
Coca Cola bottles were purchased from a market and washed with deionized (DI) water after the drink had been consumed. The PET was chopped into small pieces and used with glycolysis in the presence of the DES 2 catalyst, as shown in Table 1. A one-pot reaction for the polymerization of the PUF was followed by the synthesis of the same recipes, as shown in Table 2. Finally, IR and TGA analyses were carried out on the PUF sample made from the Coca Cola bottle waste.
Characterization
ATR-FT-IR Analysis
An FT-IR spectrometer (ALPHA-II, Bruker, Billerica, US) with an attenuated total reflectance (ATR) module was employed to verify the completion of polymerization reactions upon PUF synthesis. The IR spectrum of EG-PUF was recorded using an FT-IR run with the OPUS software. The conditions for FT-IR measurements were a scan range of 350–8000 cm−1 with 24 scans, and a resolution of 4 cm−1.
Viscosity Measurement
The viscosity of polyols prepared from DES 1, DES 2, and DES 6 was measured at 25 °C using a Brookfield viscometer (Model DV3T) and spindle (SC4-31). The rotational speed was maintained at 250 rpm.
Gel Permeation Chromatography
A gel permeation chromatograph (GPC, Waters e2695 Alliance) was used with a differential refractometer (DRI) connected to Styragel HR-0.5, 1, 4E, 4 (7.8 mm × 300 mm) columns. The polyols were diluted with tetrahydrofuran (THF) and filtered before being injected into the GPC. The THF eluent flow rate was set to 0.6 mL/min with an injection volume of 100 μL. As EG-PUFs were not easily solubilized in THF, it took 24 h with magnetic stirring to dissolve.
Thermogravimetric Analysis
The thermal degradation behavior of PUFs was investigated using TGA (EVO II TG8120 series, Rigaku) in a nitrogen atmosphere to analyze the effect of DES components on the thermal stability of the synthesized PUFs.
Micro-combustion Calorimetry (MCC) Analysis
Micro-combustion calorimetry (MCC) was performed using an FTT micro calorimeter (Fire Testing Technology Ltd., United Kingdom). Samples (5 mg) were heated from 100 to 600 °C at a heating rate of 1 °C/s under a stream of nitrogen. After pyrolysis, the gas stream with oxygen at the rate of 80:20 (v/v) entered the combustor at 900 °C, where the samples were completely oxidized. The oxygen consumption and heat release rates were determined using the oxygen concentrations and flow rates of the combustion gases.
SEM and XRD Analysis
In terms of morphology, DES-based PUFs from waste PET were examined using SEM (XL30S FEG, Philips) with Schottky field emission as an electron gun at an acceleration voltage of 10 kV after approximately 5 nm-thick platinum coatings.
XRD patterns of the DES-based PUFs were also employed to further characterize the polymer properties of a-BHET and f-BHET based PUFs.
Calculation of Hydroxyl Value (OH Values) of Polyols
Based on the BHET obtained from DES-based glycolysis of waste PET, the hydroxyl and acid values of the polyols were calculated as follows. The acid value is the number of milligrams of potassium hydroxide, which is required to neutralize the acid function in 1 g of polyol. The sample was dissolved in a mixture of THF (20 mL) and water (4 mL). This solution was titrated with a solution of potassium hydroxide (0.83 N KOH/ethanol solution). Bromothymol blue was used as an indicator. The acid value was calculated using the following equation:
where V1 is the volume (mL) of the KOH solution used for the test sample, V0 is the volume (mL) of the KOH solution for the blank sample, m (g) is the mass of the sample, and T is the normality of the KOH solution (mol/L).
The hydroxyl value was determined using the standard method, NF T 52-113. Briefly, 0.5 g of the sample was added to 20 mL of an acetylating solution containing a 1000:127(v/v) pyridine and acetic anhydride mixture in a 50 mL round-bottomed flask fitted with a condenser and a magnetic stirrer. The solution was heated at 100 °C for approximately 2 h. Thereafter, it was cooled to room temperature and diluted using 100 mL of water. Under vigorous stirring, the resulting solution was titrated with a 0.5 N NaOH standard solution using phenolphthalein as an indicator [27, 28].
where V1 is volume (mL) of NaOH solution used for the test sample [29], V0 is the volume (mL) of NaOH solution for the blank sample, m (g) is the mass of the sample, and T is the normality of the NaOH solution (mol/L).
NCO value of MDI was also obtained as follows.
The NCO value of MDI was 33.19, and the equivalent weight of isocyanate was 126.56.
Calculation of the Reaction Conversion of PET to BHET
PET (3 g) was degraded using ethylene glycol at a predetermined ratio of PET and EG. The mixture was heated at 180 °C for 2 h. After the reactants reached the desired temperature, DES was added as a catalyst. After the glycolysis reaction, the unreacted PET pellets were separated from the reaction mixture, dried, and weighed to calculate the conversion of PET. The glycolysis reaction mixture was subsequently stirred, 10 mL of boiling water was added, and the sludge was mixed for 10 min. The resulting sludge was filtered through filter paper. The filtrate was stored in a refrigerator, and the filter cake was dried in an oven at 65 °C. The unreacted PET was weighed to calculate the conversion, and the filter cake was weighed to calculate the yield of BHET [29, 30].
Results and Discussion
As illustrated in Fig. 1, according to the schematic of synthesis routes to PUF from PET, glycolysis of waste PET was first carried out. The reaction products of glycolysis contained BHET, DES catalyst, EG, BHET oligomers, and unreacted PET. After the unreacted PET was manually removed, vacuum filtration was performed to separate the BHET oligomers from the as-made BHET mixture (a-BHET). Both a-BHET and f-BHET were adopted as polyols for PUF, and the physicochemical properties of the resulting PUF samples were compared and evaluated.
Seven DESs reported in the literature were prepared, and their corresponding properties were studied, as shown in Table 1. DESs 1, 2, 3, and 6 resulted in a homogeneous and transparent liquid. Despite its formation as a transparent liquid, DES 3 was unable to glycolyze PET. In contrast, gray opaque liquid and slurry mixtures from DESs 4 and 5 were obtained, respectively, while DES 7 formed a white solid. DESs 1, 2, and 6 were found to be the most efficient catalysts for PET depolymerization.
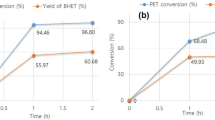
When DES-based glycolysis of waste PET was completed, it looked like a white milky liquid, as shown in Fig. 2a. DES 2 based glycolysis reactions exhibited fast PET conversion; > 75% of PET was converted to BHET species and the conversion maximum reached 90%, as shown in Fig. 2b. The DES 6-based reaction showed slightly lower PET conversion than that of the DES 2 reaction, and > 85% of PET conversion was obtained after 2 h of glycolysis. The lowest reaction rate was observed for the DES 1 reaction, where the conversion was only ~ 20% after 1 h. It is understood that Zn2+ ions and Cl− ions in DES 2 would have functioned as the main catalyst and co-catalyst, respectively, which resulted in high PET conversion. The similar roles of Zn2+ and Cl− through coordination bonding in the PET glycolysis reaction have been reported in the literature.[11]. On the other hand, because only Cl− ions and Zn2+ ions were in DES 1 and DES 6, respectively, these reactions would lead to lower PET conversion [31]. As DES 1 is a nonmetal DES system, the recycled monomer is also free of potential metal poisoning. However, the activity of the DES 1 catalyst in the conversion of PET was relatively lower than that of metal based DES catalysts. By vacuum filtration, the a-BHET was segregated into a filter cake and f-BHET isolated. As shown in Fig. 2c, the yield of BHET in the filtered phases was linked to PET conversion, where the reaction with DES 2 gave rise to a higher BHET yield, and the lowest yield was obtained with DES 1. This result further showed that the PET chains were efficiently cleaved by transesterification during the DES 2-based catalysis. The viscosities of the polyols obtained from the DES 1, DES 2, and DES 6 catalyst systems were 83, 28, and 32 cP, respectively. The molecular weight of polyols obtained from DES 2 and DES 6 exhibited a similar molecular weight of 602.6 g/mol, and the polyols from DES 1 were slightly lower at 562.3 g/mol (Fig. 2d).
Subsequently, the hydroxyl value of f-BHET was characterized to determine the amount of isocyanate required for PUF polymerization. The number average functionality of the polyols was 2. In the case of the f-BHET mixture produced by DES 2 (DES 2-f-BHET), a highest hydroxyl value of 2297 was obtained. Interestingly, the hydroxyl value of DES 1-BHET (1881) was higher than that of DES 6-BHET (1585), even though the PET conversion by DES 1 was lower than that of DES 6. This was because the fraction of EG in DES 1-BHET was high due to the low conversion of PET into BHET species. With regard to filter cakes, another phase separated from a-BHET and the presence of a hydroxyl group was not detected by the titration method. Thus, the PUF reaction using a-BHET, including filter cake components, could have fewer hydroxyl values than f-BHET. Based on the hydroxyl value, the relative amount of isocyanate to f-BHET was determined, and the detailed compositions for PUF polymerization are summarized in Table 2. In the one-pot reaction using a-BHET, the isocyanate value was compared with that of f-BHET produced by the same DES. Thus, the isocyanate/hydroxyl group rate in a-BHET based polymerization was slightly higher than that in f-BHET based polymerization.
Figure 3 shows the SEM images of six representative PUF samples after polymerization reactions using the recipes listed in Table 2. In the polymerization, the morphology varied because of differences in the hydroxyl values according to the types of BHET and the presence of BHET oligomers in a-BHET. However, similar anisotropic cell morphologies with spherical structures were observed in all samples, regardless of the reaction composition, as shown in Fig. 3. Furthermore, the cell sizes reflecting the speed of gelation and crosslink density both appeared to be approximately 200 μm. Thus, a significant difference in PUF according to the type of BHET was not identified by SEM.
To further explore the microstructure of the synthesized PUF samples, XRD data were obtained from the six samples, as shown in Fig. 4. A broad spectrum at 2θ = 21° was observed for all the samples, indicating the amorphous nature of the PUFs. When the peaks of PUF prepared from f-BHET were compared, a slight peak shifting to lower 2θ angles was observed. PUF prepared from f-BHET using the DES 2 catalyst (PUF-f-DES 2) was located at the lowest 2θ, whereas PUF-f-DES 1 was observed at a higher 2θ position. This peak shift is believed to be associated with the hydroxyl values in f-BHET, which eventually led to tighter polymer chain spacing of PUF-f-DES 2. Peak broadening was also observed in PUF samples prepared from a-BHET, compared with PUFs prepared using f-BHET. This implied that the orientation of the PU chains were significantly disturbed by the presence of oligomeric BHET in a-BHET. Thus, it was evident that the PUF prepared in this study comprised different microstructures depending on the synthesis composition.
Figure 5 illustrates the FT-IR analysis of PUF prepared from f-BHET and a-BHET. Characteristic peaks of polyurethane can be seen at approximately 3250 cm−1 (N–H stretching), 1715 cm−1 (hydrogen-bonded urethane), and 1600–1200 cm−1 (N–H bending and C–N stretching). The peak at ~ 2260 cm−1 is attributed to isocyanate. As shown in Fig. 5a, clear IR absorption at ~ 3300 cm−1 was observed for all PUF samples made from f-BHET, while the N–H stretching peak appeared to be weak for PUF-f-DES 1. The hydrogen bonds of urethane and N–H bending/C–N stretching bands attributed to the interaction between the polyurethane chains were clearly seen in Fig. 5a. The isocyanate peak at ~ 2260 cm−1 was observed for PUF-f-DES 1, indicating the incomplete consumption of isocyanates. In the case of PUF-f-DES 2 and PUF-f-DES 6, the isocyanate peak was barely observed. Similar trends were also observed for the PUF samples made from a-BHET, as shown in Fig. 5b. Both PUF-a-DES 2 and PUF-a-DES 6 exhibited higher degrees of PUF chemical structures with a minimum presence of isocyanates. From the data, it can be said that PUF polymerization effectively occurred from both filtered and unfiltered BHET, whereas the degree of polymerization did not significantly change depending on the a-BHET and f-BHET compositions. Thus, the presence of oligomeric BHET in a-BEHT did not inhibit the polymerization of PUF, and PUF could be prepared by a one-pot reaction with no additional filtration processes. As shown in Fig. 5b, unreacted isocyanates remained in the order of DES 1 > DES 2 > DES 6-based PUF. As previously discussed, the fraction of EG in DES 1-BHET was high because of the low conversion of PET into BHET species, which could lead to overestimation of the hydroxyl value in the DES 1 polyol batch. In the case of the DES 6-BHET batch, shown in Table 3, the hydroxyl value was far lower than that of the other two polyol batches, although the depolymerization degree for the DES 6 batch was comparable to that of the DES 2 batch. We believe that this discrepancy originated from the buffering action of acetates in the DES 6 batch, which consumed the OH− moiety in the polyol, and led to a lower loading of isocyanates for polymerization. However, further investigation is required to elucidate this phenomenon.
TGA analysis was performed on the PUF samples to evaluate their thermal stability. Figure 6a–c show the TGA thermograms of PUF samples prepared with a-BHET or f-BHET, obtained from reactions using the same DES catalysts. The TGA thermograms of PUF samples prepared by a one-pot reaction using a-BHET are compared in Fig. 6d. In general, all the PUF samples exhibited similar TGA curves. The first weight loss started at temperatures above 200–300 °C, which occurred mainly by the dissociation of urethane bonds into isocyanates and alcohol. There were a series of gaseous quenching reactions and radical scavenging from zinc, chloride, phosphorus, and polymeric urethane chains. The phosphorus and chloride ions reacted with the excited OH· and H· radicals to produce less reactive free radicals [32, 33]. Weight loss above 300 °C was attributed to the intensified loss of urethane bonds, generation of amines and oxidized carbons, and degradation of BHET and EG structures. Charring barrier degradation started from the condensed phase [34,35,36].
As shown in Fig. 6a, there was no significant change in the TGA curve. This was due to the low conversion of PET and choline chloride contents such that most of the hydroxyl groups reacted with isocyanate as polyols, and the effect of chloride on flame retardancy was minimized. On the other hand, using DES 2 and DES 6 catalysts displayed distinct differences where PUF samples from a-BHET showed less weight loss over the entire temperature, as shown in Fig. 6b, c. This indicated that the PUF samples prepared through a one-pot reaction had better thermal stability and flame retardancy. These improved thermal properties originated from the aromatic contents of the oligomeric BHET in a-BHET [37]. Among the PUF samples from a-BHET in Fig. 6d, PUF-a-DES 2 showed the lowest weight loss over the temperature range examined. The gaseous radical quenching by zinc and chloride ions, and the accelerated formation of charred layers by zinc salts in the condensed phase led to the highest thermal stability of PUF-a-DES 2 [38]. Based on TGA, thermally stable PUF can be prepared using a one-pot reaction using BHET prepared from the glycolysis of PET. The selection of the DES catalyst and the presence of oligomeric BHET can be engineering factors to yield thermally stable PUFs. The FT-IR spectra indicated a marked difference in the remaining unreacted isocyanate after urethane polymerization, which was in good agreement with the TGA curves, showing the enhanced thermal resistance of DES 2 and 6 based PUFs.
Micro-combustion calorimetry (MCC) was performed to study the flame retardancy of the PUF samples. Figure 7 shows the HRR and DGA data of PUF-EG samples, and the associated heat release rate (HRR), total heat release (THR), and heat release capacity (HRC) are reported in Table 4. MCC experiments were performed three times, and the average values are listed in Table 4. As shown in Fig. 7 and Table 4, PUF-a-DES 2 exhibited lower heat release and weight loss, demonstrating higher flame retardancy than the other PUF samples. However, the degree of improvement in flame retardancy was not substantial, and the PUF-a-DES 6 sample showed lower flame retardant properties than that of PUF-a-DES 1, where the MCC data were not well correlated with the TGA data shown in Fig. 6. This outcome indicated that there should be further additions of flame retardants to synthesize PUF with high flame retardancy, although the presence of BHET could contribute to flame retardancy.
Figure 8 shows the one-pot synthesis of PUF obtained from waste Coca Cola PET bottles. As shown in Fig. 8a, the PET bottle were washed with DI water and chopped into small pieces. After the glycolysis reaction using DES 2 catalyst under the reaction conditions shown in Table 1, a slightly gray colored solution was obtained, which differed from the PET purchased from Sigma Aldrich (Fig. 2a). This indicated that some impurities were present in the Coca Cola bottles. Following PUF polymerization, a yellowish solid was isolated. In addition, the glycolysis reaction proceeded well with no bulk PET observed after the reaction. As shown in Fig. 8b and c, the corresponding IR and TGA data of PUFs from the Coca Cola PET were similar to those of PUF-a-DES 2. Therefore, PUF preparation from waste PET material through the one-pot reaction was successful.
Conclusion
The depolymerization of waste PET was carried out to produce high-value products such as flame retardant PUFs. Based on the DES, the glycolysis of waste PET was applied to produce the recycled monomer BHET, which was then polymerized to PUF. In this study, among the seven tested DES systems, DES 1, 2, and 6 were best suited for glycolysis and produced DES-based polyols. The polyol was reacted with isocyanate to produce PUFs. The thermal stability and flame retardancy of the PUF-prepared recycled polyol were evaluated using TGA and MCC. In the case of unfiltered reaction mixtures, PUF could be obtained through a one-pot reaction, which exhibited higher thermal stability than PUF made from the filtered polyols. Among the PUF samples prepared using the one-pot reaction, PUF-a-DES 2 exhibited higher thermal stability and flame retardancy than the other samples because of the higher degree of depolymerization. However, the enhancement in the flame retardancy of the PUF, as measured using MCC, was not significant. This outcome indicated that further addition of flame retardants, such as expandable graphite and silica, is needed to obtain PUF with high flame retardancy. This one-pot reaction is an environmentally friendly and relatively simple process; therefore, it is a promising pathway for waste PET upcycling.
Data Availability
The datasets used and/or analyzed are available from the corresponding author upon reasonable request.
References
Chamas A, Moon H, Zheng J, Qiu Y, Tabassum T, Jang JH, Abu-Omar M, Scott SL, Suh S (2020) Degradation rates of plastics in the environment. ACS Sustain Chem Eng 8:3494–3511
Keijer T, Bakker V, Slootweg JC (2019) Circular chemistry to enable a circular economy. Nat Chem 11:190–195
Shojaei B, Abtahi M, Najafi M (2020) Chemical recycling of PET: a stepping-stone toward sustainability. Polym Adv Technol 31:2912–2938
Kárpáti L, Fogarassy F, Kovácsik D, Vargha V (2019) One-pot depolymerization and polycondensation of pet based random oligo- and polyesters. J Polym Environ 27:2167–2181
Lipik VT, Abadie MJM (2007) Polyethylene Terephthalate Chemical Recycling in the Melted State. Polym-Plast Technol Eng 46:695–701
Ahmad I, Mei TM (2009) Mechanical and morphological studies of rubber wood sawdust-filled UPR composite based on recycled PET. Polym-Plast Technol Eng 48:1262–1268
Guo Z, Lindqvist K, de la Motte H (2018) An efficient recycling process of glycolysis of PET in the presence of a sustainable nanocatalyst. J Appl Polym Sci 135:46285
Kawkumpa S, Saisema T, Seoob O, Trakankit C, Atorngitjawat P, Sakulsaknimitr W (2019) Synthesis of polyurethane from glycolysis product of PET using ZnO as catalyst. RMUTSB Acad J 7:29–39
Ünlü AE, Arıkaya A, Takaç S (2019) Use of deep eutectic solvents as catalyst: a mini-review. Green Process Synth 8:355
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082
Wang Q, Yao X, Geng Y, Zhou Q, Lu X, Zhang S (2015) Deep eutectic solvents as highly active catalysts for the fast and mild glycolysis of poly(ethylene terephthalate) (PET). Green Chem 17:2473–2479
Yunita I, Putisompon S, Chumkaeo P, Poonsawat T, Somsook E (2019) Effective catalysts derived from waste ostrich eggshells for glycolysis of post-consumer PET bottles. Chem Pap 73:1547–1560
Sert E, Yılmaz E, Atalay FS (2019) Chemical recycling of polyethlylene terephthalate by glycolysis using deep eutectic solvents. J Polym Environ 27:2956–2962
Zhang Q, De Oliveira Vigier K, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108–7146
Musale RM, Shukla SR (2016) Deep eutectic solvent as effective catalyst for aminolysis of polyethylene terephthalate (PET) waste. Int J Plast Technol 20:106–120
Rushell E, Tailor YK, Khandewal S, Verma K, Agarwal M, Kumar M (2019) Deep eutectic solvent promoted synthesis of structurally diverse hybrid molecules with privileged heterocyclic substructures. New J Chem 43:12462–12467
Parnica J, Antalik M (2014) Urea and guanidine salts as novel components for deep eutectic solvents. J Mol Liq 197:23–26
Esquer R, García JJ (2019) Metal-catalysed Poly(Ethylene) terephthalate and polyurethane degradations by glycolysis. J Organometall Chem 902:120972
Ertas K, Güçlü G (2005) Alkyd resins synthesized from glycolysis products of waste PET. Polym-Plast Technol Eng 44:783–794
Chee T-M, Tin Sin L, Bee S-T, Tee T-T, Kadhum AAH, Rahmat AR (2015) Roles of calcium, zinc, copper and titanium compounds on the degradation of polymers. Polym-Plast Technol Eng 54:441–461
Akdogan E, Erdem M, Ureyen ME, Kaya M (2019) Rigid polyurethane foams with halogen‐free flame retardants: thermal insulation, mechanical, and flame retardant properties. J Appl Polym Sci 47611
Ivdre A, Abolins A, Sevastyanova I, Kirpluks M, Cabulis U, Merijs-Meri R (2020) Rigid polyurethane foams with various isocyanate indices based on polyols from rapeseed oil and waste PET. Polymers (Basel) 12:738
Gunatillake PA, Adhikari R (2011) Biodegradable polyurethanes: design, synthesis, properties and potential applications. In: Felton GP (ed) Biodegradable polymers: processing, degradation and applications. Nova Science Publishers, Hauppauge, NY, pp 431–470
Borowicz M, Paciorek-Sadowska J, Lubczak J, Czupryński B (2019) Biodegradable, flame-retardant, and bio-based rigid polyurethane/polyisocyanurate foams for thermal insulation application. Polymers (Basel) 11:1816
Mukesh C, Mondal D, Sharma M, Prasad K (2014) Choline chloride–thiourea, a deep eutectic solvent for the production of chitin nanofibers. Carbohyd Polym 103:466–471
Liu B, Fu W, Lu X, Zhou Q, Zhang S (2018) Lewis acid-base synergistic catalysis for polyethylene terephthalate degradation by 1, 3-dimethylurea/Zn (OAc) 2 deep eutectic solvent. ACS Sustain Chem Eng 7:3292–3300
Auvergne R, Colomines G, Robin J-J, Boutevin B (2007) Synthesis and characterization of UV-curable resins from the glycolysis of PET: vinyl ether/maleate UV-curing system. Macromol Chem Phys 208:690–701
Colomines G, Robin J-J, Tersac G (2005) Study of the glycolysis of PET by oligoesters. Polymer 46:3230–3247
Hu Y, Wang Y, Zhang X, Qian J, Xing X, Wang X (2020) Synthesis of poly(ethylene terephthalate) based on glycolysis of waste PET fiber. J Macromol Sci A 57:430–438
Sert E, Yılmaz E, F.S.J.J.o.P. Atalay, t. Environment (2019) Chemical recycling of polyethlylene terephthalate by glycolysis using deep eutectic solvents. 27:2956–2962
Zhou L, Lu X, Ju Z, Liu B, Yao H, Xu J, Zhou Q, Hu Y, Zhang S (2019) Alcoholysis of polyethylene terephthalate to produce dioctyl terephthalate using choline chloride-based deep eutectic solvents as efficient catalysts. Green Chem 21:897–906
Wang Y-W, Shen R, Wang Q, Vasquez Y (2018) ZnO Microstructures as flame-retardant coatings on cotton fabrics. ACS Omega 3:6330–6338
Ning Y, Guo S (2000) Flame-retardant and smoke-suppressant properties of zinc borate and aluminum trihydrate-filled rigid PVC. J Appl Polym Sci 77:3119–3127
Schartel B (2010) Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials (Basel) 3:4710–4745
Neisius M, Liang S, Mispreuve H, Gaan S (2013) Phosphoramidate-containing flame-retardant flexible polyurethane foams. Ind Eng Chem Res 52:9752–9762
Duquesne S, Le Bras M, Bourbigot S, Delobel R, Camino G, Eling B, Lindsay C, Roels T, Vezin H (2001) Mechanism of fire retardancy of polyurethanes using ammonium polyphosphate. J Appl Polym Sci 82:3262–3274
Li M, Luo J, Huang Y, Li X, Yu T, Ge M (2014) Recycling of waste poly(ethylene terephthalate) into flame-retardant rigid polyurethane foams. J Appl Polym Sci. https://doi.org/10.1002/app.40857
Ye L, Zhang Y, Wang S, Gao G, Liu J, Zhou Y, Liu H (2014) Synergistic effects and mechanism of ZnCl2 on intumescent flame-retardant polypropylene. J Therm Anal Calorim 115:1065–1071
Funding
This research was funded by the Korea Research Institute of Chemical Technology and the Chung-Ang University Research Grant in 2020.
Author information
Authors and Affiliations
Contributions
PS analyzed and interpreted the data. SC and Elsa created the diagram and performed the experiments. SMG analyzed and interpreted the data, especially the kinetics. PS was a major contributor in writing the manuscript, and SMG supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, P.S., Kim, SC., Tikue, E.T. et al. One-Pot Reaction of Waste PET to Flame Retardant Polyurethane Foam, via Deep Eutectic Solvents-Based Conversion Technology. J Polym Environ 30, 333–343 (2022). https://doi.org/10.1007/s10924-021-02202-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02202-6