Abstract
Objective
Marine actinomycetes from the genus Salinispora have an unexploited biotechnological potential. To accurately estimate their application potential however, data on their cultivation, including biomass growth kinetics, are needed but only incomplete information is currently available.
Results
This work provides some insight into the effect of temperature, salinity, nitrogen source, glucose concentration and oxygen supply on growth rate, biomass productivity and yield of Salinispora tropica CBN-440T. The experiments were carried out in unbaffled shake flasks and agitated laboratory-scale bioreactors. The results show that the optimum growth temperature lies within the range 28–30 °C, salinity is close to sea water and the initial glucose concentration is around 10 g/L. Among tested nitrogen sources, yeast extract and soy peptone proved to be the most suitable. The change from unbaffled to baffled flasks increased the volumetric oxygen transfer coefficient (kLa) as did the use of agitated bioreactors. The highest specific growth rate (0.0986 h−1) and biomass productivity (1.11 g/L/day) were obtained at kLa = 28.3 h−1. A further increase in kLa was achieved by increasing stirrer speed, but this led to a deterioration in kinetic parameters.
Conclusions
Improvement of S. tropica biomass growth kinetics of was achieved mainly by identifying the most suitable nitrogen sources and optimizing kLa in baffled flasks and agitated bioreactors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive marine and ocean ecosystems are unique in their biodiversity (Andryukov et al. 2018). An example of a marine microorganism that has not been fully investigated is the filamentous bacterium Salinispora tropica. In the laboratory, this bacterium was first cultivated in 1989 (Jensen et al. 1991) and was originally classified in the order Actinomycetales (Maldonado et al. 2005). According to the latest research, it actually belongs to the order Micromonosporales, suborder Micromonosporineae and family Micromonosporaceae (Nouioui et al. 2018). These are Gram-positive bacteria producing a vegetative mycelium formed by extensively branched hyphae (diameter of 0.25–0.5 µm); on solid medium they form orange colonies with black spores. They live in marine sediments of tropical and subtropical regions, especially in the Caribbean (Jensen et al. 2015).
Biotechnologically, S. tropica is useful for producing specialized bioactive (secondary) metabolites potentially beneficial to human health. In recent years, this taxon has been studied predominantly in connection with the substance Salinosporamide A (NPI-0052, marizomib). It is a naturally occurring 20S proteasome inhibitor whose use has been proposed for treatment of hematological cancer (Niewerth et al. 2014). Another interesting substance is the sioxanthin pigment. It is a C-40 terpene carotenoid that has a glycosylated bond at one end of the chain and an aryl group at the other end. By this arrangement, the sioxanthin molecule has an amphiphilic character important for its biological effect as an antioxidant (Richter et al. 2015; Jensen et al. 2015). More recently, S. tropica has been developed as the first marine-sourced heterologous host to complement well-established Streptomyces host strains in the expression of biosynthetic gene clusters (Zangh et al. 2018).
The available literature suggests that S. tropica has the ability to adapt its metabolism to a vast array of substrates. Cells have the ability to assimilate at least 21 carbon, 8 nitrogen, and 4 sulfur sources (Contador et al. 2015). A typical laboratory culture medium is based on seawater, or synthetic seawater, containing glucose or starch as a source of carbon and energy, nitrogen sources (yeast extract, peptone) and other nutrients (Tsueng and Lam 2008a; Tsueng et al. 2008). S. tropica was reported to require seawater for growth (Mincer et al. 2002; Maldonado et al. 2005), although a few strains having no such requirement have been isolated (Tsueng and Lam 2008b). Most of the cultivation trials were carried out at the shake flask level and the few papers reporting on bioreactor scale, namely in pilot- (42 L) and large-scale (500 L) fermenters, do not present data on biomass growth kinetics (Tsueng et al. 2008; Manam et al. 2009; Potts and Lam 2010). A recurrent observation is that orange pigment production by S. tropica is associated with vegetative growth (Richter et al. 2015).
For this reason, our study is focused on optimizing culture conditions (medium composition, temperature, salinity) and cultivation methodologies (flask geometry, bioreactor) during vegetative (orange) growth.
Materials and methods
Microorganism
Salinispora tropica CBN-440T is deposited at the culture collection of the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Germany). The strain was obtained in freeze-dried form and maintained in cryovials (T310-2A 2 mL, Simport, Canada) prepared from fresh cultures and stored at − 70 °C.
Media
Nutrient liquid medium (NLM) contained in g/L: 10 glucose (Penta, Czech Republic), 4 yeast extract (Carl Roth, Germany), 2 peptone from meat (Carl Roth, Germany) and 30 artificial sea salt (Royal Nature, Israel). Nutrient solid medium (NSM) contained, in addition, 15 g/L bacteriological agar. Media from M1 to M8 contained in g/L: 10 glucose, 30 artificial sea salt and as a nitrogen source, yeast extract (106 gN/kg, Carl Roth, Germany), or peptone from meat (149 gN/kg, Sigma Aldrich, Czech Republic), or soy peptone (98.8 gN/kg, Carl Roth, Germany), or corn steep extract (16.9 gN/kg, Sigma Aldrich, Czech Republic), or ammonium sulfate (Penta, Czech Republic), or ammonium hydroxide (Penta, Czech Republic), or urea (Penta, Czech Republic) or feather hydrolysate (2.8 gN/kg) in the amounts indicated in Table 1. Feather hydrolysate was prepared according to Stiborova et al. (2016). All media were adjusted to pH 7.0 ± 0.2 with 1 M NaOH or H2SO4. The nitrogen content in media M1 to M8 was set as the same as in reference medium NLM (Contador et al. 2015; Tsueng and Lam 2008b), which was 0.722 gN/L. Analyses of nitrogen content in complex sources were carried out at GEMATEST s.r.o. (www.gematest.cz, Czech Republic).
Shake flask and fermenter cultures
To prepare cryovials, S. tropica colonies grown on plates of NSM were transferred in 30 mL NLM into 100 mL unbaffled Erlenmeyer flasks containing fifty 3 mm solid-glass beads (Sigma Aldrich) and shaken at 150 rpm on a rotary shaker at 23 °C for the time needed to obtain a well dispersed and orange-pigmented biomass. The mycelium morphology was observed using an optical microscope (Primo Star Zeiss, Germany). The cryovials (1 mL aliquots) were used to seed the inoculum cultures, which were then grown in 30 mL NLM in 100 mL unbaffled Erlenmeyer flasks with fifty 3 mm solid-glass beads (Sigma Aldrich) and shaken at 150 rpm on a rotary shaker at 23 °C for the time needed to obtain a well dispersed and orange-pigmented biomass. This was then used to inoculate sterile NLM or M1 to M8 media at 10% v/v of the total volume.
In the first experiments (carried out in triplicate), shaken flask cultures were grown from 7 to 34 days in 100 mL unbaffled Erlenmeyer flasks (bottom diameter 64 mm, height 105 mm, neck diameter 22) containing 50 mL of medium. Flasks were shaken at 150 rpm on a rotary shaker at 23 °C. In the following experiments (also run in triplicate), shake flask cultures were grown from 7 to 12 days in 500 mL unbaffled or baffled Erlenmeyer flasks containing 150 mL of medium, shaken at 150 rpm on a rotary shaker and incubated at a range of temperatures from 23 to 30 °C. The size of both flask types was the following: bottom diameter 100 mm, height 175 mm, neck diameter 29 mm.
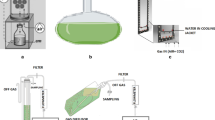
Batch fermenter cultures were carried out in 1.4 L bioreactors (Multifors 1–3, Infors HT, Switzerland) with a 0.7 L working volume. Cultivations were conducted with the following parameters: pH was maintained at 7.0 (H2SO4 or NaOH), stirrer speed 200 or 350 or 450 rpm (2 Rushton impellers, 6 blades, diameter 38 mm), temperature 28 °C and air flow rate 0.7 L/min.
The experimental data were statistically evaluated using the t-test. All statements of significance were based on a probability of p < 0.05. Statistical analyses were performed using MS Excel software.
Analysis
Samples of cell suspension were centrifuged in pre-weighed 2 mL centrifuge tubes at 14,000×g for 10 min. The biomass was then washed with distilled water once and dried at 105 °C for 24 h for determination of biomass concentration (X) expressed as dry cell weight.
The supernatant was filtered through a 0.22 μm nylon filter for glucose analysis. Glucose concentrations were determined by HPLC (PDA Agilent 1100, Agilent Technologies, USA) with a refractive index detector. A Watrex polymer IEX H column (8 µm, 250 × 8 mm) was used with 9 mM H2SO4 acid in de-aerated and deionized water as the mobile phase. The injection volume was 20 µL and the flow rate was 0.5 mL/min. The concentrations of glucose were calculated based on calibration curves created using standard chemicals.
The volumetric mass transfer coefficients (kLa) in shake flasks and bioreactors were determined using the dynamic “gas out-gas in” method with an oxygen electrode (InPro 6950, Mettler Toledo, Switzerland) (Tribe et al. 1995).
Results
Optimization of nitrogen source
In this work, different nitrogen sources (NS) in artificial sea water containing 10 g/L glucose were evaluated in terms of suitability for cultivation of S. tropica CBN-440T in 50 mL unbaffled shake flask cultures. Based on data in Table 1, the media used were divided into 3 groups according to the maximum biomass concentration (Xmax). In the first group were media M1 and M3, in the second NLM and M8 and in the third M2 and M4. Differences in Xmax within each of the three groups were not statistically significant, while between the three groups, differences were statistically significant. In the remaining media M5, M6 and M7, using non-complex NS, no growth of S. tropica was observed. The same group classification, based on the different NS used, was achieved considering the yield coefficients (YX/S) expressed as the weight of biomass growth relative to the weight of glucose consumed. Consequently, the following optimization of growth in shake flasks and in bioreactors was focused on yeast extract (M1) and soy peptone (M3) as the most appropriate NS.
Cultivation in shake flasks
Different cultivation parameters were optimized in the following set of experiments in 150 mL baffled and unbaffled shake flasks. Medium salinity created by 30 g/L sea salt resulted in the highest Xmax and biomass productivity (PX). Both lower (15 g/L) and higher (60 g/L) salt contents in M1 medium statistically significantly decreased Xmax and PX (Table 2).
When evaluating the effect of temperature on biomass growth, it was found that temperatures between 28 and 30 °C were the most suitable from the point of view of PX. The specific biomass growth rate (µ) increased when the cultivation temperatures were switched from 23–25 °C to 28–30 °C, from 0.014 ± 0.0005 to 0.022 ± 0.0012 h−1, respectively.
The initial glucose concentration was tested in the range from 0 to 40 g/L. In the absence of glucose, the Xmax and PX were the lowest. The Xmax was observed at an initial glucose concentration of 10 g/L. Above this, the Xmax gradually decreased. However, the decrease was statistically not significant. The highest PX was also achieved at 10 g/L initial glucose concentration, but glucose concentrations of 5 and 20 g/L resulted in PX values that were not statistically different. At higher glucose levels (40 g/L), the PX decreased significantly, while Xmax and YX/S were lower by a non-significant difference (Table 2 and Fig. 1). The increasing initial glucose concentration from 5 to 40 g/L in baffled shaken flasks resulted in a decrease in µ from 0.052 ± 0.004 to 0.029 ± 0.006 h−1, respectively. In M1 medium cultivations in baffled shake flasks (Fig. 1) containing from 0 to 20 g/L glucose, Xmax was achieved at around 96 h from inoculation, then cells entered into stationary phase. The entrance into stationary phase was delayed to nearly 150 h from inoculation when the initial glucose concentration was 40 g/L. After Xmax was achieved during cultivations in baffled shake flasks, the X gradually decreased due to the occurrence of sporulation (Fig. 1). This was accompanied by a change in culture pigmentation from orange to black.
From the results obtained, it is also evident that the use of baffled shake flasks promoted biomass growth. Comparing the same conditions (10 g/L glucose, 30 g/L sea salt, 23 °C) in baffled and unbaffled flasks, the Xmax and PX in baffled flasks were significantly higher by 46 and 88%, respectively. When comparing µ, in baffled flasks it was almost threefold higher than in unbaffled flasks. The increase of µ, Xmax and PX in baffled flasks was statistically significant. Under these experimental conditions, the volumetric oxygen transfer coefficient (kLa) in baffled shake flasks (9.4 ± 1.2 h−1) was significantly higher than in unbaffled flasks (4.0 ± 0.5 h−1).
Comparing all the results reported in Table 2 and Fig. 1 for the M1 medium, the best conditions were using 10 g/L glucose and 30 g/L sea salt, and incubating baffled flasks at 28 °C. Under these conditions, the Xmax achieved in M1 medium was the highest (5.09 g/L) among all studied conditions by a statistically significant difference. The same significant increase was also observed for PX (0.72 g/L/day). When the same conditions were tested in M3 medium, both Xmax and PX were higher, but not significantly, when compared to M1 medium, whereas YX/S was lower than in M3.
Cultivation in a bioreactor
The cultivation parameters optimized in shake flasks were applied in laboratory scale bioreactors (Fig. 2). The effect of oxygen supply, expressed as kLa, and on biomass growth in M1 medium was studied. The oxygen supply was adjusted in different runs by modulating stirrer speeds to 200, 350 or 450 rpm, which resulted in a kLa equal to 23.7, 28.3 and 39.6 h−1, respectively. The lowest kLa (23.7 h−1) resulted in µ = 0.092 ± 0.006 h−1, which was significantly higher than the maximum µ achieved in baffled shake flasks. The increase in kLa (28.3 h−1) promoted a growth rate of µ = 0.0986 ± 0.011 h−1, although this value was not significantly higher than that at kLa = 23.7 h−1. A further increase in kLa (to 39.6 h−1) lowered µ to 0.074 ± 0.009 h−1. The stirrer speeds of 200, 350 or 450 rpm corresponded to the impeller’s peripheral speeds of 0.398, 0.696 and 0.895 m/s, respectively. In medium M3 at kLa = 28.3 h−1, the µ was equal to 0.079 ± 0.01 h−1, which was significantly lower than in M1 medium under the same conditions.
Under the oxygen supply rate characterized by kLa = 28.3 h−1, the Xmax (5.15 g/L) achieved in M1 medium was not significantly higher than in baffled shake flask cultures (5.15 g/L) with kLa = 9.4 h−1. However, PX (1.11 g/L/day) in the same bioreactor culture increased by 54% when compared to baffled shake flasks (0.72 g/L/day). The bioreactor cultures using M3 medium resulted in Xmax (5.28 g/L) and PX (1.03 g/L/day, calculated for Xmax = 5.06 g/L at 118 h) comparable with M1 medium in a bioreactor (Fig. 2).
Discussion
Marine ecosystems are an unlimited reservoir of unexplored microbial taxa, which include the biotechnologically very attractive group of marine actinomycetes. A number of new bioactive specialized metabolites produced by marine actinomycetes has been identified (Subramani and Aalbersberg 2012). Isolation and screening of marine actinomycetes identified the Salinispora genus as a producer of rare compounds with potent bioactivity (Niewerth et al. 2014; Richter et al. 2015). In natural products, particularly in those of marine origin, a crucial issue is their possible reproducible supply to sustain practical applications of the active principles. Data providing cultivation parameters and growth kinetics of bacteria belonging to the Salinispora genus are absent from the literature. Therefore, this work focusses on cultivation of S. tropica in shake flasks and bioreactors.
The study shows that nitrogen sources such as ammonium sulfate, ammonium hydroxide and urea did not sustain growth of S. tropica (Table 1), while other authors have reported that this bacterium can grow using ammonium ions, urea (both with zero days lag-phase) and other non-complex nitrogen sources. These contradictory results might be ascribed to medium composition, which was either defined and with trace elements or based on artificial sea salt (this work). It is worthy of note that a more detailed comparison is not possible since Contador et al. (2015) did not provide data on cell growth kinetics.
The results from testing complex nitrogen sources show that soy peptone in M3 medium can be used as a suitable alternative to the meat peptone of NLM reference medium, in compliance with the requirements of modern cultivation processes that prefer raw materials of plant rather than animal origin. This is due to the growing public interest in vegetarian sources of food and food supplements (Heenan et al. 2002).
The results of this work confirm the previous findings that S. tropica requires a salinity (ionic strength) close to that of sea water for optimum growth (Mincer et al. 2002). Salinities significantly lower or higher resulted in inferior PX, Xmax and YX/S. It is thus confirmed that S. tropica is an obligate halophile, requiring salinity to grow, and clearly different from most other marine and soil actinomycetes that tend to be only halotolerant.
The data presented on PX, Xmax, YX/S and µ of S. tropica are unique and some comparison is only possible with the work of Tsueng and Lam (2010). These authors reported that S. tropica CNB-440T was capable of achieving Xmax = 6.63 g/L after 5 days of flask cultivation at 28 °C on a rotary shaker (250 rpm). This would correspond to a PX = 1.326 g/L/day, which is significantly higher than the maximum we achieved in baffled shake flasks (0.72 g/L/day) and bioreactors (1.11 g/L/day). However, regarding this result, the authors did not state whether they subtracted the dry weight of the culture blank (biomass and precipitates) from that of the culture at Xmax. In tables, where they provided information on corrected dry weight, the highest Xmax achieved was 5.97 g/L (Tsueng and Lam 2010). However, these tables did not indicate the cultivation time or other culture conditions.
When cultured in liquid medium, S. tropica exhibited freely dispersed filamentous forms, entangled clumps or pellets. Pellets were characteristic for cultivation in unbaffled flasks, while the use of baffled flasks and agitated bioreactors led to an increased fraction of dispersed mycelial forms (Fig. 3). This is the first report on this aspect of S. tropica growth during flask and bioreactor cultivations. Morphological studies during cultivations were previously conducted only on members of the Streptomyces genus, which is the most frequently studied among mycelium-forming actinomycetes. The relationship between Streptomyces morphology and hydrodynamics in a cultivation system was found to be complex. Mechanical stress induced by liquid velocity fluctuations affected cell morphology and growth kinetics, while the morphological forms affected the rheological properties of the culture medium (Olmos et al. 2013). Baffled shake flasks increased both pellet fragmentation and kLa, thus promoting S. tropica growth rates. Until reaching a critical value (350 rpm), an increase in agitation rates in bioreactors also improved PX. Similar observations were made for Streptomyces clavuligerus, where critical values were identified for the agitator’s power input (1.1–2 kW/m3) and impeller’s peripheral speeds (3.77 m/s), at which biomass growth started to be negatively affected (Large et al. 1998; Roubos et al. 2001). These values were significantly higher for S. clavuligerus compared to those calculated for S. tropica in this work (0.243 kW/m3, 0.895 m/s). Future scale-ups of S. tropica cultivations would require a more in-depth understanding of how agitation affects mycelial morphology and growth kinetics (Amanullah et al. 2000).
Conclusions
Estimations of economic profitability for production of microbial metabolites largely depends on the kinetic parameters of biomass production. Interesting bioactive metabolites have been identified in strains belonging to the genus Salinispora, but data on growth rates and cultivation parameters for these marine filamentous actinomycetes are very limited. The data presented in this study extends the publicly available information on biomass productivity, growth rates and biomass yields of S. tropica in different media and cultivation scales, from shake flasks to laboratory bioreactors. These data can help bioengineers with scale-up and sustainability calculations for future biotechnological applications of products produced by members of this genus.
References
Amanullah A, Justen P, Davies A, Paul GC, Nienow AW, Thomas CR (2000) Agitation induced mycelial fragmentation of Aspergillus oryzae and Penicillium chrysogenum. Biochem Eng J 5:109–114
Andryukov BG, Mikhaylov VV, Besednova NN, Zaporozhets TS, Bynina MP, Matosova EV (2018) The bacteriocinogenic potential of marine microorganisms. Russ J Mar Biol 44:433–441
Contador CA, Rodriguez V, Andrews BA, Asenjo JA (2015) Genome-scale reconstruction of Salinispora tropica CNB-440 metabolism to study strain-specific adaptation. Antonie Van Leeuwenhoek 108:1075–1090
Heenan NC, Adams CM, Hosken WR, Fleet HG (2002) Growth medium for culturing probiotic bacteria for applications in vegetarian food products. LWT Food Sci Technol 35:171–176
Jensen RP, Dwight R, Fenical W (1991) Distribution of Actinomycetes in near-shore tropical marine sediments. Appl Environ Microbiol 57:1102–1108
Jensen RP, Moore SB, Ferical W (2015) The marine actinomycete genus Salinispora: a model organism for secondary metabolite discovery. Nat Prod Rep 32:738–751
Large KP, Ison AP, Williams DJ (1998) The effect of agitation rate on lipid utilisation and clavulanic acid production in Streptomyces clavuligerus. J Biotechnol 63:111–119
Maldonado AL, Fenical W, Jensen RP, Kauffman AC, Mincer JT, Ward CA, Bull TA, Goodfellow M (2005) Salinispora arenicola gen. nov., sp. nov. and Salinispora tropica sp. nov., obligate marine actinomycetes belonging to the family Micromonosporaceae. Int J Syst Evol Microbiol 55:1759–1766
Manam RR, Macherla RV, Tsueng G, Dring WX, Weiss J, Neuteboom CTS, Lam SK, Poots CB (2009) Antiprotealide is a natural product. J Nat Prod 72:295–297
Mincer TJ, Jensen PR, Kauffman CA, Fenical W (2002) Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl Environ Microbiol 68:5005–5011
Niewerth D, Jansen G, Riethoff VFL, Meerloo J, Kale JA, Moore SB, Assaraf GY, Anderl LJ, Zweegman S, Kaspers LJG, Cloos J (2014) Antileukemic activity and mechanism of drug resistance to the marine Salinispora tropica proteasome inhibitor Salinosporamide A (Marizomib). Mol Pharmacol 86:12–19
Nouioui I, Carro L, García-López M, Meier-Kolthoff PJ, Woyke T, Kyrpides CN, Pukall R, Klenk PH, Goodfellow M, Göker M (2018) Genome-based taxonomic classification of the phylum actinobacteria. Front Microbiol 9:1–119
Olmos E, Mehmood N, Husein LH, Goergen JL, Fick M, Delaunay S (2013) Effects of bioreactor hydrodynamics on the physiology of Streptomyces. Bioprocess Biosyst Eng 36:259–272
Potts CB, Lam SK (2010) Generating a generation of proteasome inhibitors: from microbial fermentation to total synthesis of salinosporamide A (Marizomib) and other salinosporamides. Mar Drugs 8:835–880
Richter TKS (2014) Discovery, biosynthesis and evolutionary history of sioxanthin, a novel glycosylated carotenoid from marine bacteria Salinispora. UC San Diego. https://escholarship.org/uc/item/7mn416k3. Accessed 27 Oct 2020
Richter SKT, Hughes CC, Moor SB (2015) Sioxanthin, a novel glycosylated carotenoid, reveals an unusual subclustered biosynthetic pathway. Environ Microbiol 17:2158–2171
Roubos JA, Krabben P, Luiten RGM, Verbruggen HB, Heijnen JJ (2001) A quantitative approach to characterizing cell lysis caused by mechanical agitation of Streptomyces clavuligerus. Biotechnol Prog 17:336–347
Stiborova H, Branska B, Vesela T, Lovecka P, Stranska M, Hajslova J, Jiru M, Patakova P, Demnerova K (2016) Transformation of raw feather waste into digestible peptides and amino acids. J Chem Technol Biotechnol 91:1629–1637
Subramani R, Aalbersberg W (2012) Marine actinomycetes: an ongoing source of novel bioactive metabolites. Microbiol Res 167:571–580
Tribe LA, Briens CL, Margaritis A (1995) Determination of the volumetric mass transfer coefficient (kLa) using the dynamic “gas out-gas in” method: analysis of errors caused by dissolved oxygen probes. Biotechnol Bioeng 46:388–392
Tsueng G, Lam KS (2008a) A low-sodium-salt formulation for the fermentation of salinosporamides by Salinispora tropica strain NPS21184. Appl Microbiol Biotechnol 78:821–826
Tsueng G, Lam KS (2008b) Growth of Salinispora tropica strains CNB440, CNB476, and NPS21184 in nonsaline, low-sodium media. Appl Microbiol Biotechnol 80:873–880
Tsueng G, Lam KS (2010) A preliminary investigation on the growth requirement for monovalent cations, divalent cations and medium ionic strength of marine actinomycete Salinispora. Appl Microbiol Biotechnol 86:1525–1534
Tsueng G, Teisan S, Lam KS (2008) Defined salt formulations for the growth of Salinispora tropica strain NPS21184 and the production of salinosporamide A (NPI-0052) and related analogs. Appl Microbiol Biotechnol 78:827–832
Zangh JJ, Moore BS, Tang X (2018) Engineering Salinispora tropica for heterologous expression of natural product biosynthetic gene clusters. Appl Microbiol Biotechnol 102:8437–8446
Funding
This research was supported by the Ministry of Education, Youth and Sports of the Czech Republic, Ministry of Agriculture of the Czech Republic (institutional support MZE-RO1918), and by Insubria University “Fondo di Ateneo per la Ricerca” 2017–2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jezkova, Z., Binda, E., Potocar, T. et al. Laboratory scale cultivation of Salinispora tropica in shake flasks and mechanically stirred bioreactors. Biotechnol Lett 43, 1715–1722 (2021). https://doi.org/10.1007/s10529-021-03121-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03121-1