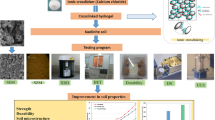
Abstract
A novel carrageenan-based hydrogel (CBH) was developed via cross-linking and ion interaction methods to obtain a coating for NPK fertilizer grains. Attenuated total reflectance–Fourier transform infrared analysis (ATR–FTIR), scanning electron microscopy (SEM), texture profile analysis (TPA), rheological study, and swelling kinetics were used to characterize the physicochemical properties of the CBH. To test the slow-release behavior of the coated fertilizer and the adequate provision of nutrients for plants, the leaching rates of N, P, and K were determined through soil column experiments, and plant growth indicators were measured in greenhouse experiments in a yellow potato crop (Solanum phureja). The results showed that CBH is a material with homogeneous appearance, porous structure, similar chemical structure to κ-carrageenan, and resistance and low deformation at the fracture point. The swelling behavior experiments showed a maximum increase in thickness of 74% by water absorption, with osmolality being an important factor controlling the entry of water into the CBH. Wash experiments in soil columns showed that the noncoated granules released 12 and 18% less N–NH4+ and K+ than the noncoated fertilizer, both after 90 mm of accumulated precipitation. Less than 0.1% of P–PO4−3 was lost in both treatments. No significant differences were observed in any of the growth plant and quality indicator variables between the plants grown with coated and noncoated fertilizer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the increasing yields per unit area achieved since the onset of chemical fertilization after World War II [1,2,3], traditional fertilization practices in agriculture have proven inefficient. Between 50 and 80% of the nitrogen (N), 50% of the phosphorous (P) and up to 63% of the potassium (K) applied to the soil are not taken up by the crops [4,5,6]. Some of the nonassimilated nutrients are lost through leachates, especially in high-rainfall areas [4, 7], with serious environmental consequences. Generally, leached N and P go to ground and surface water, causing problems associated with eutrophication, decreasing biodiversity and producing water pollution [8, 9]. On the other hand, N can be released into the atmosphere in the form of N oxides [10], which in the long term can exacerbate global warming and contribute to the destruction of the ozone layer [11, 12]. It is necessary to reduce the environmental impact of fertilizers. However, unless there is a rise in fertilizer efficiency, an increase in the use of NPK fertilizers from 200.5 to 324 million tones (Mt) from 2018 to 2050 has been foreseen [13, 14] due to the accelerated world growth rate of 80 million people per year [15, 16]. This increases food demand and, consequently, the need for improved crop yields. Therefore, it is highly relevant to generate alternative strategies to buffer the environmental impact of fertilizers.
Controlled-release fertilizers (CRF) using mineral and organic polymers as coating agents have been implemented as an interesting alternative to reduce the environmental impact, given their slower rates of nutrient release compared to those of noncoated fertilizers [17]. One of the greatest issues to overcome is that most of the materials used to prepare these nutrient carriers are nondegradable, costly, toxic and produce inconsistent release patterns that do not meet the nutritional needs of plants [17, 18]. The demand for new eco-friendly materials with better controlled-release properties has motivated the research of alternative polymers [19]. Several polysaccharides from different sources (algae, microbes, plants and animals), such as alginate, cellulose, starch, pectin and chitosan, have been proposed as nontoxic and biodegradable coating alternatives [20, 21]. Although polysaccharides in their native form are not useful for producing stable nutrient carriers [21], hydrogels from polysaccharides, prepared by the chemical cross-linking method, produce a stable 3D matrix that slowly releases nutrients by diffusion to the soil when used as a fertilizer grain coater [17]. Additionally, hydrogels have a high water absorption capacity attributed to their interconnected superpore structures with diameters of several hundred microns, creating open channels that allow for capillary action [22]. When added to the soil, the hydrogel acts as a water reservoir and improves physical soil properties [21, 23, 24].
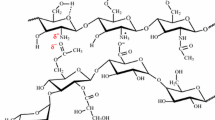
Carrageenan is the name given to a family of sulfated polysaccharides found in the wall and matrix of red algae in the Rhodophyceae class. These polysaccharides are highly anionic; with high molecular weight; a content of 15–40% ester-sulfate; and a chemical structure of alternate units of d-galactose and 3,6-anhydrogalactose (3,6 AG), which are bound through α-1,3- and β-1,4-glucosidic bonds (Fig. 1). The presence of a cross-linking agent allows carrageenan to form hydrogels in which polysaccharides physically cross-link and form three-dimensional networks that swell in water [25, 26]. These gels can absorb up to ten thousand times their weight in water, without dissolving or losing their integrity. The addition of thermoplastic products, such as glycerin, allows the hydrogel polysaccharide mobility in its chains and delays degradation, acting as a plasticizer to form smooth and uniform gels [27]. Although there are advances in covered urea fertilizer that improve the water-holding capacity of soil, the use of carrageen-based coating agents in agriculture has been limited [26].
The present study concerns the synthesis and characterization of a κ-carrageen-based hydrogel (CBH) with a high water retention capacity that reduces nutrient leaching from the fertilizer to the environment when used to coat NPK fertilizer granules with no negative effects on plant growth. To test the slow-release behavior of the coated fertilizer and to determine the adequate provision of nutrients for plants, the leaching rates of N–NH4+, P–PO4−3, and K+ were determined through soil column experiments, and plant growth indicators were measured through greenhouse experiments. Potato plants were selected to evaluate the effect of the coated fertilizer given the worldwide importance of this crop, with close to 19.3 million hectares planted [28] and more than 246.000 tonnes of NPK fertilizers used in this crop annually in Latin America, in addition to potato being one of the crops with the highest fertilizer application rates around the world [29]. Therefore, it is important to find strategies to help mitigate the contributions of pollutants to the environment caused by agricultural fertilization.
Methods
Synthesis of the CBH Polymer and Preparation of the Coated Fertilizer
An aqueous κ-carrageenan powder solution was prepared by dissolving a commercial κ-carrageenan powder (Cimpa MCH 5722) in distilled water to obtain a final concentration of 3% (w/v). Next, 1.5 mL of glycerol (Chemi Laboratory) was added as a plasticizing agent, and the mixture was stirred continuously until a clear solution was obtained. This mixture was poured into silicone molds and allowed to cool slowly until the gelation was complete to obtain 1.5 cm3 capsules that were used to analyze the physicochemical and mechanical properties of the CBH polymer.
The fertilizer was coated with CBH following this same procedure, except that before gelation was completed, NPK fertilizer granules [15-15-15-11 (S), Vecol] were added to the silicone mold at a final concentration of 14% (p/p).
Physicochemical Properties of the CBH
Attenuated Total Reflectance–Fourier Transform Infrared Analysis (ATR–FTIR)
The chemical structure of the CBH polymer was characterized by interpreting the infrared spectra (IR) of the CBH polymer samples. The IR was recorded using a Fourier transform infrared (FTIR) spectrometer (Thermo Scientific Nicolet iS10 with an ATR module, verified with 1.5 mm polystyrene standards traceable to NIST standards) in the wavenumber range of 4000–600 cm−1 with 64 scans per sample on average. The resolution and signal-to-noise ratio (SN) were 0.4 cm−1 and 15,000:1, respectively. A total of 0.1 g of the CBH polymer sample was used for each test. Each sample was vacuum-sealed in a water-resistant polystyrene pack and stored in a container with silica gel desiccant before the IR tests.
Surface Morphology
The surface morphology of the prepared film samples was studied using a scanning electron microscope (JEOL, model JSM 6490-LV, Tokyo, Japan). Prepared film samples were directly deposited on aluminum stubs using double-sided adhesive carbon conductive tape and coated with a thin gold layer with the help of gold sputter. An accelerating potential of 12 kV was used during the experiments.
Instrumental Texture Profile Analysis (TPA)
A texture analyzer TAXT2i (stable micro systems) and its accompanying computer software (SAS) were used to measure the force–time curve. In all experiments, samples were compressed under a cylindrical probe (7 mm thick) at a test speed of 1 mm s−1 and a control force of 5 g. The deformation level was 25% of the original sample height, and the gels were compressed twice to avoid the invalid parameters that were obtained when the sample collapsed under higher deformation levels [30]. A cross-head speed (50 mm min−1) was chosen to prevent the total destruction of the gel structure in the first compression. This speed was also recommended to obtain values highly correlated to the sensory responses [30]. From the first deformation curve, the point where the peak force occurred was recorded as the deformation peak force and was used as an indicator for the structure strength of the samples. Three replicate samples were tested.
Rheological Study
The rheological studies were carried out with a viscometer RV (Brookfield DV-11+pro) at 70 °C. The viscosity of hydrogels was measured at shear rates ranging from 1 to 60 s.
Thickness and Melting Point of the CBH Polymer
A hand-held digital Vernier caliper was used to measure the capsule thickness to an accuracy of 0.000127 cm. Five measurements were made on each test sample, and a mean thickness was calculated. The increase in thickness by water absorption was measured every 5 min for 60 min [31].
The CBH with a glass sphere on it, contained in a test tube inside a water bath, was used to measure the hydrogel melting point [31].
Swelling Kinetics
The water absorption capacity of the CBH polymer was evaluated in distilled water as well as in 10 mmol L−1 of NaCl, KCl, and CaCl2 saline solutions to test the effects of various ions on swelling performance. The absorption was measured by the gravimetric method. CBH capsules of 1.5 cm3 were weighed before and after immersion at 25 °C in 50 mL of the evaluated solution. At 5-min intervals, the swollen capsules were removed from the medium and weighed immediately after being blotted with a piece of filter paper to remove any surface water. The measurements were continued until an equilibrium swelling state was achieved. The same steps were taken to study the behaviors of the coated fertilizer in distilled water. All experiments were performed at least three times.
The degree of swelling (W) for each sample at time t was calculated using Eq. 1
The swelling kinetics were described by a second-order model, as indicated in Eq. 2 [32], where k is the swelling rate constant, W∞ is the equilibrium swelling, the maximum amount of solution that penetrates into the hydrogel until equilibrium is reached, and W is the degree of swelling at any time t.
By integrating Eq. 2 and applying the initial conditions, Eq. 3 is obtained [33]. When the swelling kinetics correspond to second-order kinetics, Eq. 3 is a linear relationship where the equilibrium swelling rate constant is observed to be directly proportional to the square of W∞. The slope and the intercept obtained when graphing t W−1 versus t correspond to W∞ and k, respectively.
The W, W∞ and k average values for the hydrogel in each of the evaluated solutions were compared by Tukey’s minimum significant difference test. The values were considered different with a level of significance of P < 0.05. The data were analyzed with the statistical software R 3.0.3 [34].
Leaching Experiments in Soil Columns
Polyvinyl chloride (PVC) columns (internal diameter 7.5 cm, height 15 cm), sealed at the bottom end with a geotextile mesh, were packed with 35 g of sand previously washed with HCl. On top of the sand, 433 g of sandy loam textured soil was added (Table 1). The soil was formerly dried at 40 °C for 24 h and sieved with a 2 mm mesh. Then, the soil packed in the column was saturated with 60 mL of distilled water, allowing it to drain for a period of 24 h. Next, 3 cm of surface soil was removed, and the NPK fertilizer [15-15-15-11 (S), Vecol] was placed according to each treatment. Finally, the fertilizer was covered with the same 3 cm of soil that was previously removed. Fertilization treatments included 2.27 g of uncoated fertilizer granules (treatment 1 = T1), 2.27 g of CBH-coated fertilizer granules (treatment 2 = T2) and no fertilizer (treatment 3 = T3). The 2.27 g of fertilizer used in T2 was placed in a single CBH capsule as described in the preparation of the coated fertilizer. Each column was irrigated daily for 16 days with 25 mL of water, corresponding to a daily precipitation of 5.6 mm. The leachate produced after irrigation was collected throughout the day. All treatments were repeated in triplicate.
The percentage of leached nutrients (%LN) was calculated by Eq. 4
where L is the nutrient concentration in the lixiviate and Co is the initial concentration of the nutrient in the column, that is, 334.2 mg for N–NH4+, 348.3 mg for K+ and 186.4 mg for P–PO4−3. Finally, %LT3 is the percentage of the leached nutrient in the no fertilizer treatment. The T3 results were subtracted from T1 and T2 to correct the fraction of nutrients lixiviated from T1 and T2. The leachates were analyzed for N–NH4+ and P–PO4−3 by the automated spectrophotometry method using a Skalar Sanplus Analyzer SA 3000/5000 Mode 5000-01. Atomic absorption spectrophotometry was used to analyze K+ using a Unicam Solar 969 spectrophotometer.
Effect of the Coated Fertilizer on Plant Growth
In this experiment, we worked with the potato species of the Andes, Solanum phureja, variety Colombia. Growth characteristics related to crop production (tuber weight and size) and potato quality indicators such as specific gravity and the number of tubers in three different size classes (a. diameter > 4 cm, b. diameter between 2 and 4 cm, and c. diameter < 2 cm) were measured. Chlorophyll concentration in leaves was measured as an indicator of the presence of nitrogen during the productive cycle.
A complete randomized design was used, with three treatments, five replications per treatment and four plants per replicate. The experiment had a total of 20 plants per treatment. The treatments correspond to uncoated fertilizer (T1), coated fertilizer with CBH (T2), and control plants with no addition of fertilizer (T3). In general, in Colombia, 25 g of NPK fertilizer are applied to each plant in S. phureja culture at the beginning of the productive cycle. Hence, a total of 25 g of a commercial NPK fertilizer was added to each plant, except for T3. In T2, 25 g of fertilizer was distributed in 11 hydrogel capsules of approximately 15.4 cm3 each. This fertilization distribution was made so that each capsule had a final concentration of 14% (2.27 g of fertilizer capsule−1). Potatoes were cultivated in soil in 0.13 m3 pots in the greenhouse facilities of the National University of Colombia. The type of soil was the same as that used in the leaching test. The experiment was carried out between September 29, 2017 and January 8, 2018.
At the end of the productive cycles, 104 days after the seedling stage, destructive samplings were taken to register the tuber weight and size. Chlorophyll was determined using the fourth youngest true leaf and estimating the mean of four measurements from different areas with an AtLEAF PLUS meter. Specific gravity was measured by the weight in air/weight in water method [35]. Regarding data analysis, the assumptions of normality were verified by the Shapiro–Wilk and Kolmogorov–Smirnov tests. The homogeneity of variance was verified by the Bartlett test. If any variable did not meet the assumptions, a Box-Cox transformation was performed, and the analysis of variance was performed again. A one-way analysis of variance (ANOVA) was employed to determine the differences among the treatments. The average values were compared by Tukey’s post hoc analysis. The results were expressed as the mean ± standard deviation and were considered significantly different at P ≤ 0.05. ANOVA analyses were performed using R 3.0.3 [34].
Results and Discussion
Physicochemical Properties
The IR spectra of the CBH polymer show several typical absorptions of κ-carrageenan (Fig. 1a), for example, the stretching vibrations of the ester–sulfate bonds assigned to bands 1422 and 1226 cm−1 as reported for pure carrageenan samples [36], and a moderately strong band at approximately 844 cm−1 showing the presence of C–O–SO4 at the C4 carbon of d-galactose-4-sulfate (Fig. 1a, b). Similarly, the presence of a strong band assigned by the absorption to the C–O bonds of κ-carrageenan at approximately 912 cm−1 indicates that the chemical structure has a 3,6-anhydro-d-galactose group (Fig. 1a, b). Characteristic bands of the structure of the κ-carrageenan are also shown in 1158 cm−1, which have been displaced from 1034 cm−1 due to the aqueous formation of the hydrogel. This band can be assigned to the C–O bonds of the ring 3,6-anhydro-l-galactopyranose. On the other hand, the presence of a band at 1226 cm−1 corresponds to the sulfate ester. This band is stronger for dry solid carrageen than for the hydrated gel [37]. The band at 697 cm−1 in the spectrum is assigned to the skeletal flexure of the galactose ring, especially in the anomeric region, which has been previously reported between 700 and 900 cm−1 [38]. Finally, the signal at 3340 cm−1 corresponds to the OH− group absorptions, concluding that the glycerol was physically bound and integrated with the carrageenan as the hydrogel formed.
Surface Morphology of the CBH
SEM images of the CBH polymer show a material with a homogeneous appearance with pore structure and “interstices”, spaces where a predominantly aqueous proportion was maintained in the polymer networks (Fig. 2a). This structure is similar to the interconnected pores reported in previous studies for different hydrogels [39]. SEM images of the CBH polymer with the fertilizer inside show crystals of different lengths corresponding to NPK nutrients homogeneously deposited on the surface of the hydrogel (Fig. 2b). Similar images have been presented before for a superabsorbent hydrogel containing fertilizer [40].
Mechanical Properties of the CBH
The results for the physical tests of the CBH are shown in Table 2. The melting point of the synthesized hydrogel (65.3 °C) corresponds to the temperature at which the network structure disappears upon heating. The CBH polymer solution shows the formation of thermoreversible gels by cooling, with gelation temperatures of 45 °C. These results coincide with other studies [41] showing-modified κ-carrageenan/gelatin hydrogels with gel temperatures of up to 45 °C. When the gel absorbs water, it increases its size by 74%, as shown by the thickness measurements. The texture analysis shows a maximum elasticity value at 12,293 N. These values indicate the maximum resistance that the hydrogel endured before breaking. Additionally, the measured adhesive strength and adhesiveness were − 0.121 N and − 0.044 N s−1, respectively. These forces are products of the attraction between the hydrogel surface and the intermolecular forces of the hydrogel network. The hydrogel showed resistance and low deformation at the fracture point. This phenomenon may be due to the amount of plasticizer that allowed the reduction in intermolecular forces, such as hydrogen bridges. This behavior is comparable with that reported previously for a biodegradable polymer of starch, modified with glycerin and water as plasticizers [42], where the intermolecular forces were reduced in the hydrogen bridges and, therefore, the material presented greater flexibility and resistance.
The adhesiveness strength shows that the hydrogel does not have a sticky texture. Additionally, the Young’s modulus value of 0.0772244 MPa indicates that the CBH is a soft and malleable hydrogel, given that this value is lower than 0.1 MPa [U1]. The measured Young’s value for the CBH is 100 times higher than those reported for alginate gels modified with carrageenan (0.001216 MPa). The adhesiveness strength points out that CBH is suitable for the encapsulation of fertilizer grains.
With a Young’s modulus from 45 to 83 MPa, the CBH presents a similar behavior as reported in a previous study [43], where there is a strong interaction between polymer-plasticizer, formed by the hydrogen bonds of the chains of the carrageenan matrix, giving flexibility and stretching ability. With this work, it was evidenced that the addition of plasticizers improves mechanical properties in polymers of κ-carrageenan.
Swelling Kinetics
The water absorption of the CBH was carried out quickly during the first 10 min, then the speed decreased, and after 20 min, the equilibrium was reached (Fig. 3a). The correlation coefficients (0.999–1) for the curves t wt−1 versus t (Table 3) indicate that the absorption kinetics of the hydrogel in both the water and saline solutions and the hydrogel encapsulating fertilizer conform to a second-order model.
Differences in the maximum weight (W∞) reached by the CBH polymer submerged in different solutions are due to the ionic strength of the medium, which regulates the swelling of the hydrogels. First, as a result of the negative charges of the polymer, the free volume of the hydrogel matrix depends on the presence of ionized groups in the solution to absorb water. In this case, K+, Na+ and Ca+2, which, upon entering the structure of the gel, stabilize the negative charges of the polymer and organize the three-dimensional polymer structure. In Fig. 3a, b, it is observed that W∞ is lower for solutions with calcium ions (Ca+2) than with sodium ions (Na+) due to the hydrogel networks becoming densely occupied by Ca+2, leaving little space for water to enter. On the other hand, the salts in the solutions are completely ionized, increasing their osmotic pressure. Solute ions in solution solvate the water molecules, decreasing the amount of these molecules that diffuse into the polymer hydrogel network. Thus, the hydrogel in the CaCl2 solution, containing more dissolved ions, has the lowest value for W∞ (Fig. 3b). NaCl and KCl are salts with the same number of ions; therefore, higher W∞ for the hydrogel in the KCl solution is due to the smaller potassium hydration radius, which causes a lower retention of water molecules in the solution compared to the retention in the NaCl solution (Fig. 3b). Similar results have been reported for cellulose [44] and polyvinyl alcohol (PVA) gels [45]. At higher concentrations of KCl, a gel-to-sol transition has been found for the κ-carrageenan structure due to large changes in osmotic pressure [46], which would produce a very soft and unmanageable capsule for agricultural management. As expected, the hydrogel containing fertilizer also showed a lower water absorption compared to the pure hydrogel capsule (Table 3). This phenomenon is due to the space reduction inside the polymeric network, limiting the amount of water molecules inside the hydrogel.
The results also show that the speed at which water molecules enter into the CBH polymer (k) is strongly dependent on the ions present inside and outside the polymer matrix (Fig. 3c). The hydrogels rapidly reached equilibrium in the presence of fertilizer and Ca+2 ions. These results are comparable with the theoretical calculations published previously [47]. Differences in k among treatments may be due to the viscosity and density of the hydrogel once submerged in the solution [48].
Column and Field Experiments
Leachates from columns with the CBH-coated fertilizer showed lower concentrations of N–NH4+ and K+ compared to those in T1 (Fig. 4a, c). At 90 mm of precipitation, 2.02% (± 0.25) of the N–NH4+ and 6.8% (± 0.81) of the K+ were leached from T2, while 14.1% (± 2.38) of the N–NH4+ and 25% (± 5.2) of the K+ were leached from T1. In the case of P–PO4, minimum amounts of this ion were detected in the leachates from both treatments. Only 0.08% (± 0.006) of P–PO4− from T2 and 0.06% (± 0.01) from T1 had been lost (Fig. 4b) by the end of the experiment. The small amount of P–PO4− lost through leaching must be the result of phosphate fixation to the soil due to its low pH and high iron content (Table 1). The column experiments also show that the CBH coat not only decreases the amount of nutrients in the leachates but also decreases the variation in nutrient release. T2 shows a more homogeneous release of N–NH4+, P–PO4− and K+ among the three replicate column experiments than T1.
It is difficult to make comparisons between the performance of the CBH and other organic polymers in their ability to slow down the release of NH4+ and K+ because most of them are evaluated under different experimental conditions. In one of the few cases where comparisons can be made, the CBH demonstrates a better performance, limiting the release of NH4+. The hydrogel derived from the cellulose acetate and ethylenediaminetetraacetic dianhydride (HEDTA) [49], whose release behavior was evaluated under conditions similar to those in the present work, retained approximately 0.6% more NH4+ and 75% more K+ than a conventional fertilizer after 90 mm of precipitation was applied. In comparison to the HEDTA the CBH retained 12.1% and 18.2% more NH4+ and K+, respectively, than the uncoated fertilizer after the same 90 mm of precipitation. The CBH is not as efficient at slowing down the release of K+, but it is more efficient at decreasing the amount of NH4+ that is released into the soil.
The greenhouse experiment results show no differences between T1 and T2 in any of the plant growth and quality indicator variables. Both the plants treated with the noncoated and the coated fertilizer showed equal tuber weight, size distribution and frying index values (P > 0.05) (Fig. 5a–d). The concentration of chlorophyll also remained the same in T1 and T2 on all sampling dates (P > 0.05) (Fig. 5d). A reduction in chlorophyll was observed in the two treatments towards the end of the productive cycle, when the leaves senesce, which is a normal process indicating the remobilization of nutrients [50]. Plants with no fertilizer added (T3) showed significantly lower tuber weight and number of tubers in the C2 category (P < 0.05) when compared to T1 and T2 (Fig. 5a–d) as a consequence of the limited nutrient supply [51], as has been observed in other studies [49, 52,53,54].
The delay in the release of NH4+ and K+ caused by the CBH coating does not affect the production of potato plants. Nonetheless, unlike the observed results in other studies using synthetic polymers or minerals as coating materials [54,55,56], productivity did not improve when the coated fertilizer was applied to the potato plants. However, it is difficult to compare these results with data from other studies proposing organic polysaccharides as coating materials. Most of the published literature focuses on the characterization of the organic polymer and does not evaluate the effect of the organic polymer on plant growth. In one of the few works published on this topic [57], effects of each of the natural polymers, sodium alginate, cellulose acetate and ethyl cellulose, mixed with biochar, were evaluated on wheat growth (Triticum aestivum), finding that all tested organic polymeric materials had a negative effect on grain yield. These results contrast with what was observed for the HEDTA hydrogel [49], which promoted the performance of eucalyptus seedlings in terms of growth in height and stem diameter. Based on published literature, it seems that the effect of organic polymers used as coating material in controlled-released fertilizers on plant growth varies depending on the type of plant, soil and management regimen [58].
The results in the present study indicate that the CBH has potential as a coating that is worth exploring. In Colombia only, the area dedicated to potato crops is approximately 132.161 ha [59], and currently, 86 kg ha−1 of NH4+ and 68 kg ha−1 of K+ are applied at the beginning of the production cycle. However, plants do not begin to assimilate nutrients until 30–45 days after being sown [60]. In the intermediate time, a significant percentage of NH4+ and K+ are lost, especially through leachates. The use of a CBH as a coating could reduce the amount of nutrients that are being lost until nutrient assimilation by the plants begins and could even make it possible to reduce the dose of 25 g of fertilizer applied per plant and, thus, the production cost.
Conclusions
The CBH is a manipulable material that has the potential to be used as a coating of NPK fertilizer granules to reduce the amount of nitrogen and potassium that are released into the soil solution, with no effects on tuber quality or production of Solanum phureja plants. It is worth evaluating the behavior of this coating in field experiments with the aim of finding new strategies to reduce the amount of fertilizer applied to the crop and to reduce the environmental impact of fertilizers without affecting plant production.
References
Russel DA, Williams GG (1977) Soil Sci Soc Am J 41:260–265
FAO (1981) Crop production levels and fertilizer use. FAO Fertilizer and Plant Nutrition Bulletin 2, Rome
Tilman D, Cassman KG, Matson PA (2002) Nature 418:671–677
Smil V (2000) Annu Rev Energ Environ 25:53–88
Cassman KG, Dobermann A, Walters DT (2002) AMBIO 31:132–140
Alfaro MA, Jarvis SC, Gregory PJ (2004) Soil Use Manag 20:182–189
Zhao BQ, Li XY, Liu H, Wang BR, Zhu P, Huang SM, Bao DJ, Li YT, So HB (2011) NJAS Wagen J Life Sci 58:177–183
Ongley OD (1996) Control of water pollution from agriculture. FAO Irrigation and Drainage Paper No. 55. Food and Agriculture Organization of the United Nations, Rome
Withers PJA, Neal C, Jarvie HP, Doody DG (2014) Sustainability 6:5853–5875
Bouwman AF, Boumans LJM, Batjes NH (2002) Global Biogeochem Cycles 16:6–13
Bhatia A, Pathak H, Aggarwal PK (2004) Curr Sci 87:317–324
Molina-Herrera S, Haas E, Klatt S, Kraus D, Augustin J, Magliulo V, Tallec T, Ceschia E, Amman C, Loubet B, Skiba U, Jones S, Brümmer C, Butterbach-Bahl K, Kiese R (2016) Sci Total Environ 553:128–140
FAO. Food and Agriculture Organization of United Nations (2015) World fertilizer trends and outlook to 2018. Food and Agriculture Organization of United Nations, Rome
Drescher A, Glaser R, Richert C, Nippes KR (2011) Demand for key nutrients (NPK) in the year 2050. University of Freiburg Department of Geography. Draft report
UN DESA (2017) World population prospects: the 2017 revision. United Nations, New York
Calabi-Floody M, Medina J, Rumpel C (2018) Adv Agron 147:119–157
Azeem B, KuShaari K, Man ZB, Basit A, Thanh TH (2014) J Control Release 181:11–21
Naz MY, Sulaiman SA (2016) J Control Release 225:109–120
Jing W, Song LIU, Yukun QIN (2017) Chin J Oceanol Limnol 35:1086–1093
Campos EVR, de Oliveira JL, Fraceto LF, Singh B (2014) Agron Sustain Dev 35:47–66
Guilherme MR, Aouada FA, Fajardo AR, Martins AF, Paulino AT, Davi MF, Rubira AF, Muniz EC (2015) Eur Polym J 72:365–385
Kuang J, Yuk KY, Huh KM (2011) Carbohydr Polym 83:284–290
Bai W, Song J, Zhang H (2013) Acta Agric Scand Sect B Soil Plant Sci 63:433–441
Hou X, Li R, He W (2017) J Soils Sediments 1–11
Rhim J, Wang L (2013)) Carbohydr Polym 96:71–81
Wang Y, Liu M, Ni B, Xie L (2012) Ind Eng Chem Res 51:1413–1422
Avella M, Emilia DP, Immirzi B, Impallomeni G, Malinconico M, Santagata G (2007) Carbohydr Polym 69:503–511
FAO (2008) International year of the potato. http://www.fao.org/potato-2008/en/world/. Accessed 1 Nov 2018
FAO (2002) Fertilizer use by crop. FAO Fertilizer and Plant Nutrition Bulletin No. 16. Rome
Pons M, Fiszman SM (1996) J Texture Stud 27:597–624
Senff H. Richtering W (1999) J Chem Phys 111:1705–1711
Macleod GS, Collett JH, Fell JT (1999) J Control Release 58:303–310
Qiao D, Liu H, Yu L, Bao X, Simon GP. Petinakis E, Chen L (2016) Carbohydr Polym 147:146–154
R Core Team (2016) A language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria
Dean BB, Thornton RE (1992) The specific gravity of Potatoe. Extension bulletin 1541. Washington State University, Cooperative Extension, Pullman
Manuhara GJ, Praseptiangga D, Riyanto RA (2016) Aquat Procedia 7:106–111
Torres MD, Chenlo F, Moreira R (2018) Carbohydr Polym 180:72–80
Rhein-Knudsen N, Ale MT, Ajalloueian F, Yu L, Meyer AS (2017) Food Hydrocolloids 63:50–58
Chen J, Park K (2000) J Control Release 65:73–82
Rashidzadeh A, Olad A (2014) Carbohydr Poly 114:269–278
Derkach SR, Voron’ko NG, Kuchin YA, Kolotova DS, Gordeeva AM, Faizullin DA, Makshakova ON (2018) Carbohydr Polym 197:66–74
Ruiz Aviles G (2006) Ingeniería y Ciencia 2:5–28
Farhan A, Hani NM (2017) Food Hydrocolloids 64:48–58
Li X, Li Q, Xu X, Su Y, Yue Q, Gao B (2016) J Taiwan Inst Chem Eng 60:564–572
Shi Y, Xiong D, Liu Y, Wang N, Zhao X (2016) Mater Sci Eng C 65:172–180
Ako K (2017) Carbohydr Polym 169:376–384
Drozdov AD, Christiansen JDC, Sanporean CG (2016) Int J Solids Struct 87:11–25
Daza Agudelo JI, Badano JM, Rintoul I (2018) Mater Chem Chem Phys 216:14–21
Senna AM, Botaro VR (2017) J Control Release 260:194–201
Have M, Marmagne A, Chardon F, Masclaux-Daubresse C (2017) J Exp Bot 68:2513–2529
Hopkins WG, Hüner NPA (2008) Introduction to plant physiology, 4th edn. Wiley, USA
Li X, He JZ, Liu YR, Zheng YM (2013) J Soils Sediments 13:711–719
Li Y, Sun Y, Liao S (2017) Agric Water Manag 186:139–146
Li P, Lu J, Wang Y (2018) Agric Ecosyst Environ 251:78–87
Zhu Q, Zhang M, Ma Q (2012) Sci Hortic 143:109–114
Zhao B, Dong S, Zhang J, Liu P (2013) PLoS ONE 8:1–8
González ME, Cea M, Medina J, González A, Diez MC, Cartes P, Monreal C, Navia R (2015) Sci Total Environ 505:446–453
Chen D, Suter H, Islam A, Edis R, Freney JR, Walker CN (2008) Aust J Soil Res 46:289–301
Fedepapa (2018) El agricultor y su papel en el pais. Bogotá, Colombia
Harmunt K, Stephan-Beckmann S (1997) Potato Res 40:135–153
Acknowledgements
The authors would like to thank Dr. Carlos Nústez for providing access to the greenhouse facility at National University. This work was carried out with the support of the Departamento Administrativo de Ciencia, Tecnología e Innovación de la República de Colombia (Grant No. 1202-669-45888) and Jorge Tadeo Lozano University. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. All data analyzed during this study are included in this article supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santamaría Vanegas, J., Rozo Torres, G. & Barreto Campos, B. Characterization of a κ-Carrageenan Hydrogel and its Evaluation as a Coating Material for Fertilizers. J Polym Environ 27, 774–783 (2019). https://doi.org/10.1007/s10924-019-01384-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01384-4