Abstract
The concept of using glutaraldehyde (GTA) to crosslink natural rubber (NR) particles bearing diacetone acrylamide (DAAM) functional groups during film formation was investigated in the present work. The primary advantage of this curing system is that it is feasible under ambient conditions, which can lower operating costs of the curing process. Graft copolymers of NR and poly(diacetone acrylamide) prepared with 5 wt% of DAAM (NR–g–PDAAM5) were synthesized by seeded emulsion polymerization at 50 °C. Then, the tensile properties were measured for cast films formed from NR–g–PDAAM5 latex in the absence and presence of GTA. The results revealed increased tensile strength of the NR–g–PDAAM5 film, when GTA was added into the latex prior to film casting. The crosslinking of NR–g–PDAAM5 latex film by reaction with GTA, after film casting, was also investigated using attenuated total reflection Fourier transform infrared (ATR–FTIR) and dynamic mechanical thermal analysis (DMTA). ATR–FTIR analysis demonstrated that crosslinking reactions formed conjugated C=C double bonds between the active carbonyl groups of DAAM and GTA. The complementary use of DMTA also corroborated that crosslinking reactions took place involving the grafted PDAAM chains on the NR particles. This was evidenced by a clear shift towards higher temperatures of the tan δ peak, corresponding to the Tg of NR–g–PDAAM phase, when GTA was incorporated into the NR–g–PDAAM5 latex before film formation. Additionally, a noticeable increase in thermal stability of the NR–g–PDAAM5 film was also observed with added GTA. Hence, it can be concluded that GTA is an efficient room-temperature crosslinker for NR particles functionalized with DAAM. This curing system can also be considered an alternative, simple, and inexpensive method for curing NR latex compounds, as only one component (GTA) is required in the curing process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
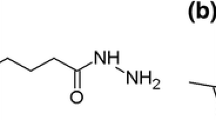
Over the last decade, many significant advances have been made in the field of latex film formation. One notable achievement in this field is the synthesis of reactive latex particles capable of forming crosslinkable films at ambient or slightly elevated temperatures. Latex particles bearing different functional groups are typically synthesized by copolymerizingthe base monomers with crosslinkable monomers (e.g., methacrylic acid and 2-hydroxyethyl methacrylate) via emulsion polymerization [1]. Crosslinking between functionalized latex particles during film formation can typically be achieved by reacting with a post-added crosslinker. Among the various functionalizations, ketone groups are frequently introduced onto the surfaces of acrylic latex particles, by using monomers with ketone groups. Diacetone acrylamide (DAAM, see Fig. 1a) is the most frequently used monomer for this purpose, in the context of room-temperature-curable acrylic coatings and adhesives [2,3,4,5]. Polymer latex particles bearing pendant DAAM groups are known to undergo interfacial crosslinking by reacting with a hydrazine. As the crosslinking reactions occur between the active ketone groups present in DAAM molecules and a hydrazine, these are formally known as keto-hydrazide crosslinking reactions [2]. Adipic acid dihydrazide (ADH, see Fig. 1b) is the most commonly used crosslinker in this type of systems [2,3,4]. Although ADH has gained importance as a crosslinking agent for epoxy coatings for decades, it has recently been used for crosslinking acrylic latexs containing DAAM [6]. The crosslinking reaction between DAAM groups by reaction with ADH is not only feasible at room temperature, but it can also be accelerated by reduction in pH (i.e., an acid-catalyzed reaction). The rate of crosslinking reaction increases as the pH of the latex decreases [2]. In addition, a stoichiometric amount of ADH (i.e., a 1:2 molar ratio of ADH:DAAM) is commonly used in crosslinking core–shell latex particles, containing DAAM groups in the shell. The amount of DAAM incorporated into the shell layer is normally in the range of 2–10 wt% [3, 4]. It has also been reported that imine linkage is formed on the reaction of DAAM groups with ADH [2].
Grafting of DAAM functional groups onto natural rubber (NR) latex particles also offers the benefits of ambient temperature crosslinking to NR film, when ADH is employed as crosslinker [7]. Although ADH has been shown to be effective in creating interfacial crosslinking between NR particles bearing DAAM functional groups, this crosslinking system is unlikely to be cost-effective for production on a large scale due to the relatively high cost of ADH.
Glutaraldehyde (GTA, see Fig. 1c) is a multifunctional aldehyde because it can exist in various forms in an aqueous solution (i.e., monomeric, dimeric, trimeric, and polymeric forms) [8]. It has been widely used to crosslink proteins, with main applications in enzyme and protein immobilization, because it can react with many reactive groups in proteins, especially amino groups [8,9,10]. Additionally, GTA has also been employed to crosslink polymer molecules containing hydroxyl or amide groups. Several publications have described the crosslinking of poly(vinyl alcohol) with GTA [11,12,13,14]. This crosslinking reaction occurs when the aldehyde groups of GTA react with the hydroxyl groups of poly(vinyl alcohol) to from acetal linkage. Use of GTA for crosslinking hydrogel based on polyacrylamide (PAA) has been reported by Kumbar et al. [15] and Dmitriev et al. [16]. An imine linkage is formed by crosslinking reaction between the amide group of PAA and aldehyde group of GTA.
As crosslinking of these functionalized polymers with GTA is feasible at ambient and low temperatures, GTA has gained increasing attention as a crosslinking agent in recent years.
GTA is not only less expensive than ADH, but also commercially available in aqueous solutions of various concentrations ranging from < 2 to 70% (w/v). However, ADH has already been proven an effective crosslinker for latex films formed from graft copolymers of NR and poly(diacetone acrylamide), NR–g–PDAAM, latex [7], whereas corresponding crosslinking with GTA has not been reported yet. This paper is the first report on crosslinking NR particles bearing DAAM groups with GTA. The aim of the work reported herein was to investigate the mechanical, dynamic mechanical and thermal properties of NR–g–PDAAM latex films cured with GTA under ambient conditions.
The chemical structure and morphology of NR–g–PDAAM were evaluated by 1H NMR and TEM analyses, respectively. Effect of the molar ratios of GTA to DAAM groups present in NR–g–PDAAM on tensile properties of the latex films was first examined. Crosslinking reaction of NR–g–PDAAM film with GTA was monitored using ATR–FTIR spectroscopy. It is known that crosslinking reduces the mobility of the polymer chains and tends to increase modulus of latex films. Therefore, the extent of crosslinking in latex films was examined by dynamic mechanical thermal analysis (DMTA) technique. The crosslink density measurement was also performed. Moreover, the effects of crosslinking on the thermal stability of NR–g–PDAAM latex films were studied using thermogravimetric analysis (TGA).
Experimental
Materials
High ammonia concentrated natural rubber (HANR) latex, manufactured by Yala Latex Co., Ltd. (Yala, Thailand), was used for preparation of NR–g–PDAAM. The dry rubber content (DRC) of the HANR latex was 60.6%. DAAM with purity 99%, cumene hydroperoxide (CHP, 80%) and tetraethylene pentamine (TEPA, 85%) were manufactured by Sigma-Aldrich Chemicals (Steinheim, Germany). GTA (25% aqueous solution), manufactured by Loba Chemie Pvt. Ltd. (Mumbai, India), was used as the crosslinker for latex films. All reagents were used as received without further purification.
The Preparation of NR–g–PDAAM5 Latex
Graft copolymerization of DAAM onto NR latex particles was carried out under a flowing nitrogen atmosphere in a thermostatic water bath. An aqueous solution of sodium dodecyl sulfate (SDS) was first prepared by adding 1 g of SDS into 70 cm3 of 2.5% (v/v) ammonium hydroxide. The resulting solution was then charged into a reaction vessel containing 156.3 g of NR latex (60.6% DRC). The mixture was stirred at room temperature for 30 min with bubbling nitrogen before CHP (0.49 g) was added. The resulting mixture was stirred for a further 1 h to allow the partitioning of CHP onto the NR particles. After that, the mixture was heated to 50 °C before an aqueous solution containing 5 g of DAAM monomer and 0.48 g of TEPA was added into the reaction vessel at 10 min intervals over a period of 2 h. After completing the addition, the reaction was allowed to proceed for a period of 4 h at 50 °C under stirring.
It is important to note that the removal of the unreacted monomer from the graft copolymer latex usually requires a special apparatus or thermal energy. Thus, it is more practical to convert unreacted DAAM to its derivatives of low toxicity. Elimination of unreacted DAAM monomer remaining in NR–g–PDAAM latex was carried out by treating the NR–g–PDAAM latex with an aqueous solution of sodium sulphite at a temperature of 80 °C. The reaction was allowed to proceed at this temperature for 30 min under stirring [17, 18].
Morphological Studies
The crude NR–g–PDAAM5 latex was diluted with deionized water to a total solid content of ~0.05%. Specimens for TEM analysis were prepared by drop casting the diluted latex on a 400-mesh copper TEM grid. The gird was then allowed to air-dry at room temperature in a dust-free area before it was exposed to vapors of osmium tetroxide for 1 h. The morphology of NR–g–PDAAM latex was examined using a JEOL JEM-2010 transmission electron microscope (TEM).
1H NMR Spectroscopy
Soxhlet extraction technique was employed to separate free NR and free homopolymer from the crude NR–g–PDAAM5 before it was characterized by 1H NMR. The initial extraction of NR–g–PDAAM5 was by light petroleum ether and then a further extraction was conducted using acetone. Each extraction step was performed for a period of 24 h. After purification by Soxhlet extraction, the chemical structure of NR–g–PDAAM5 was then analyzed. The 1H NMR spectra were recorded on a Bruker Avance III 400 MHz spectrometer in deuterochloroform, using tetramethysilane as the reference compound.
The mole and weight percentages of grafted poly(diacetone acrylamide), PDAAM, in the NR–g–PDAAM was estimated by ratio of integrated area of the signal at 2.17 ppm (i.e., the methyl-ketone protons in PDAAM repeat units) to that of the olefinic protons of NR units at 5.13 ppm, according to the following equations:
where I2.17 and I5.13 are the integrated peak areas corresponding to the methyl-ketone protons in PDAAM repeat units at 2.17 ppm and the olefinic proton of NR at 5.13 ppm; mol%PDAAM and mol%NR are the mol% of PDAAM and NR in the NR–g–PDAAM; MDAAM and MNR are the molecular masses of the repeating units for DAAM (169 g mol−1) and NR (68 g mol−1), respectively.
Study of Crosslinking Reaction
Preparation of NR–g–PDAAM Latex Films
The pH of the NR–g–PDAAM5 latex was first adjusted to 10 by addition of 25 wt% ammonium hydroxide solution. After that, a stoichiometric amount (0.5:1), a two-fold excess (1:1), or a four-fold excess (2:1) of GTA over DAAM groups present in the NR–g–PDAAM5 was added into the latex by mechanical stirring for 2 min. The amount of GTA required for reaction of GTA with DAAM groups was calculated based on the moles of polymerized DAAM in the grafting reaction (see Table 1). Latex films were then made by casting the resulting latex, in the presence and absence of GTA, into a silicon mould (15 × 15 × 2 cm3). The casting was allowed to dry in a hot air oven at 30 °C for 7 days before being subjected to further analysis.
Fourier-Transform Infrared
ATR–FTIR spectroscopy was employed to study the changes in chemical functional groups of NR–g–PDAAM molecules after crosslinking with GTA. The measurements were conducted using a Bruker Tensor 27 Infrared Spectrometer fitted with an ATR accessory, covering the range 4000–400 cm−1 at a resolution of 4 cm−1.
Tensile Testing
The latex films were tested for tensile strength and elongation at break according to ASTM D412, using Hounsfield Tensometer model H 10 KS. Dog-bone shaped specimens (51 × 254 mm2) with 38 mm wide and 51 mm long gauge section were used in the tensile testing. The crosshead speed was set at 500 mm min−1, and a minimum of five replicate specimens were tested for each case.
Crosslink Density
Equilibrium swelling test was carried out to estimate the crosslink densities of the NR–g–PDAAM latex films. A circular specimen of the latex film with 2 cm diameter was weighed to an accuracy of 0.1 mg with an analytical balance before being placed in a screw-cap bottle containing toluene (30 mL). The latex film was allowed to absorb the swelling solvent at room temperature until equilibrium was attained. The swelling equilibrium was determined by measuring the weight gain of the specimen at various immersion times. When no further weight increase was detected, the measured weight was recorded as equilibrium state. After that, the specimen was dried in a vacuum at 40 °C to a constant weight in order to determine the amount of toluene absorbed by the latex film. This enables calculating the volume fraction of rubber in the swollen network (Vr) by the method of Ellis and Welding [19]:
where D is the de-swollen weight of latex film, F is the weight fraction of insoluble components (i.e., other than the rubber) in the latex film, T is the initial weight of the latex film before swelling, As is the weight of solvent in the swollen film, and ρs is the density of solvent (toluene = 0.865 g cm−3).
The crosslink density (ν) in latex film can then be estimated from the value of Vr according to the Flory–Rehner equation [20, 21]:
where Vs is the molar volume of solvent (toluene = 106.28 cm3 mol−1) and χ is the interaction parameter for a specific polymer–solvent pair. It is important to note that the value of χ for NR-toluene system (0.38) was used in this calculation.
Dynamic Mechanical Thermal Analysis (DMTA)
The dynamic mechanical properties of latex films cast from the crude NR–g–PDAAM5 latex in the absence or presence of GTA were investigated using a dynamic mechanical thermal analyzer (DMTA–V, Rheometric Scientific). DMTA analyses were performed in a tension mode with a strain amplitude of 0.01% and the fixed frequency of 1.0 Hz. The viscoelastic properties of latex films (i.e., storage modulus, E′, loss modulus, E″, and their ratio, tan δ) were measured in a range of temperature between − 80 and 100 °C, using a heating rate of 5 °C min−1. The sample size was 5 mm (W) × 50 mm (L) with varying thicknesses from 1.0 to 1.5 mm.
Thermogravimetric Analysis
TGA and derivative thermogravimetric analysis (DTG) curves of NR–g–PDAAM latex films were recorded using a TGA Q100 TA Instrument-Walters. For each test, about 5 mg of the sample was used. The analysis was performed from ambient temperature to 700 °C at five constant rates of heating: 5, 10, 20, and 40°C min−1. The purge gas was nitrogen with a flow rate of 20 mL min−1.
Results and Discussion
Morphological and Structural Characterization
The morphology of NR–g–PDAAM particles, which were synthesized using 5 wt% of DAAM (NR–g–PDAAM5), is shown in Fig. 2. It can be clearly seen from Fig. 2a that the unmodified NR latex particle had a relatively spherical shape with smooth surface. As the residual carbon–carbon double bonds (C=C) in NR molecules were positively stained with osmium tetroxide (OsO4), the NR phase appears dark in the TEM image. The presence of many nodules of PDAAM particles on the surfaces of NR particles confirms the successful grafting of PDAAM onto NR backbone as the unstained PDAAM phase appears brighter (Fig. 2b).
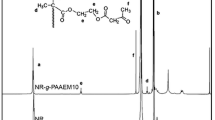
The chemical structure of the graft copolymers was confirmed by 1H-NMR analysis. The 1H-NMR spectrum of NR–g–PDAAM5 is shown in Fig. 3 and the peaks in the corresponding spectrum were assigned as follows. The 1H-NMR peaks at 5.13, 2.03, and 1.67 ppm were due to the methyne proton (–C=CH–), methylene proton (–CH 2 –C(CH3)=CH–CH 2 –), and methyl proton (–C(CH 3 )=CH–) in cis-1,4-polyisoprene, respectively. It can also be seen that these chemical shifts were similar to those observed in the unmodified NR. The peak at 1.35 ppm was due to the methyl groups connected to the methine carbon in grafted PDAAM chains. Finally, the peak at 2.17 ppm was assigned to the methyl protons (CH 3 –(C=O)–) adjacent to the keto-carbonyl group of DAAM. The content of PDAAM units in the NR–g–PDAAM5 was estimated from a ratio of the integrated peak area of the methyl-ketone protons in PDAAM at 2.17 to olefinic protons in NR at 5.13 ppm. The estimated content of PDAAM units was found to be about 2.25 wt%.
Tensile Properties of Latex Films
Theoretically, the crosslinking in this system is thought to occur between the active ketone groups of grafted PDAAM chains and the aldehyde groups of GTA molecules, as shown in Scheme 1.
From Scheme 1, the stoichiometry of this particular crosslinking reaction is an 0.5:1 molar ratio of GTA:DAAM. The effect of different molar ratios of GTA:DAAM (i.e., 0.5:1, 1:1 and 2:1 molar ratios of GTA:DAAM) on the tensile properties of NR–g–PDAAM5 latex film was examined.
Figure 4 shows that the tensile strength of NR–g–PDAAM5 film increased from 2.85 to 9.38 MPa when the stoichiometric amount of GTA (i.e., a GTA:DAAM molar ratio of 0.5:1) was added into NR–g–PDAAM5 latex prior to film casting. A noticeable increase in tensile strength was also observed with increasing the molar ratio GTA:DAAM from 0.5:1 to 1:1. This is probably a result of increased crosslinking in the latex film with excess GTA. As the degree of crosslinking increases, the elongation at break of the NR–g–PDAAM5 film is reduced (see Fig. 4). This is mainly because the crosslinking of NR–g–PDAAM5 chains restricts their mobility.
Additionally, a further increase in a molar ratio of GTA:DAAM to 2:1 (i.e., a four-fold stoichiometric excess of GTA) led to a significant increase in modulus, but dramatic drops in both tensile strength and elongation at break. This is probably caused by the excessive crosslink formation in the latex film. It is well accepted that the tensile properties of crosslinked NR are related to its ability to undergo crystallization on extension, which is commonly known as strain-induced crystallization (SIC) [22,23,24]. However, this phenomenon tends to decrease as crosslink density increases. This is likely to be a result of the reduction in chain length between crosslink points with an increase in the crosslink density [25, 26]. When the chain length between the rubber networks becomes relatively short, the ability of stretched rubber chains to orient along the stretching direction and crystallize during stretching is reduced. This would limit the extent of SIC that occurs in NR, resulting in a reduction in its tensile properties. Thus, these observations provide good evidence supporting interfacial crosslinking between NR–g–PDAAM particles by reactions with GTA, during film casting.
GTA has been extensively used as a protein crosslinking agent in various applications, including food products, textiles, cosmetics, as well as medical applications [27]. Although GTA can exist in different forms in aqueous solution, crosslinking of proteins with GTA is generally assumed to occur through the reaction between the aldehyde groups of GTA and the amino groups (NH2) of proteins, as presented in Scheme 2 [27, 28].
NR latex contains about 2% (w/w) of proteins, which can be broadly classified into two types, i.e., water-soluble proteins, and water-insoluble proteins that are tightly attached to rubber particles [29]. Therefore, it is speculated that the marked increase in tensile strength of NR–g–PDAAM5 films with added GTA may be associated with crosslinking proteins present in the NR latex particles, by their reactions with GTA. The hypothesis is that GTA was not only able to create crosslinking between grafting PDAAM chains on the NR molecules, but it was also able to produce crosslinking between proteins contained in NR latex. This speculation was consistent with the observations of Johns et al. [30], who reported that the addition of appropriate amount of GTA (a 10 wt% aqueous solution) into NR latex prior to film casting increased the tensile strength of NR latex films from 0.88 MPa to a maximum of 3.91 MPa. In those experiments, the tensile testing of NR films was performed after drying them in a hot-air oven at 45 °C for 48 h.
Based on the above discussion, the effect of proteins on the tensile properties of NR–g–PDAAM5 films was also investigated. In the present study, low-protein natural rubber (LPNR) latex was prepared by treating NR latex with aluminium hydroxide, Al(OH)3, according to methods described by Honeycutt [31,32,33,34]. The protein content of the resulting LPNR was in a range of 1.45–0.63 wt%, which was measured by a Kjeldahl method. It should be noted that LPNR latex films, which were not subjected to any water-leaching, were used for the determination of total protein in the films.
Hence, the LPNR latex contained the lowest levels of proteins (0.63%) was selected for further preparation of graft copolymers. The graft copolymers were prepared via emulsion polymerization, using a LPNR/DAAM weight ratio of 95/5 (abbreviated as LPNR–g–PDAAM5). The chemical structure of LPNR–g–PDAAM5 was confirmed by 1H NMR analysis after separating ungrafted NR (free NR) and ungrafted homopolymer (free PDAAM) from the graft copolymers, as shown in Fig. 3. It can be seen from Fig. 3 that there are no significant differences between the 1H NMR spectra of NR–g–PDAAM5 and LPNR–g–PDAAM5. This finding was consistent with the published results of Nakason et al. [35] who reported that the removal of proteins from the NR latex slightly increased the grafting efficiency of methyl methacrylate onto NR particles. This is because proteins are expected to be involved in the termination of active radical species during the grafting.
Tensile testing of films formed from LPNR–g–PDAAM5 latex, with or without the addition of GTA crosslinker, was conducted in order to evaluate the effects of proteins on the tensile properties of the films. Figure 5 shows that the latex films of LPNR–g–PDAAM5 latex with added GTA had higher tensile strengths than the film without GTA. This indicates that crosslinking took place in the LPNR–g–PDAAM5 latex film by reactions with GTA, during film formation under ambient conditions.
However, the tensile strengths of LPNR–g–PDAAM5 films with GTA were found to be lower than those of the NR–g–PDAAM5 films when using 0.5:1 and 1:1 molar ratios of GTA:DAAM. Moreover, it was also observed that the tensile strength and elongation at break of LPNR–g–PDAAM5 films dropped dramatically when GTA was used in a four-fold molar excess to DAAM as a crosslinker. This observation is similar to that obtained from the NR–g–PDAAM5 film, which can be attributed to the formation of excessive crosslink in the latex films.
The results also reveal that, in all cases, the crosslink densities for the LPNR–g–PDAAM5 films were lower than those for the NR–g–PDAAM5 films, as proved by the data obtained from the crosslink density measurement (see Table 2). Hence, these results indicate that the level of proteins in NR latex is one of important factors affecting the crosslink density in the NR–g–PDAAM films formed in the presence of GTA crosslinker. It can also be seen that the optimal tensile properties of the corresponding films were achieved with the addition of a two-fold excess of GTA (i.e., a GTA:DAAM molar ratio of 1:1). Therefore, the NR–g–PDAAM5 latex films crosslinked with an equimolar (i.e., a 0.5:1 of GTA:DAAM) or two-fold molar excess of GTA were selected for further study.
ATR–FTIR Analysis
Figure 6 shows the FTIR spectra in the region from 3200 to 1200 cm−1 for the NR–g–PDAAM5 latex films, in absence and presence of GTA. It is observed that, in all cases, the cast films exhibited absorption peaks at about 2919 and 1670 cm−1, corresponding to –CH2 stretching and C=C stretching of the cis-1,4-polyisoprene, respectively. FTIR analysis also reveals that the ketone carbonyl groups in the grafted PDAAM at 1722 cm−1 disappeared upon reaction with GTA.
The crosslinking reactions in this system are thought to mainly take place between the DAAM groups present in the NR–g–PDAAM and the GTA crosslinker. When the active aldehyde groups of GTA react with ketone groups in DAAM, a new absorption peak of C=C would be expected in the FTIR spectra of NR–g–PDAAM. According to the proposed crosslinking reaction (see Scheme 1), it is expected that the absorption peaks from the newly formed C=C would appear at wavenumbers lower than 1670 cm−1 (i.e., the stretching vibrations of C=C in cis-1,4-polyisoprene). This is because the conjugation of the carbonyl group in PDAAM units with the new C=C double bond shifts this absorption peak to lower frequency [36]. As expected, a new absorption peak at about 1584 cm−1 was observed when GTA was mixed into the latex prior film formation. This peak is in the correct region for the stretching vibrations of C=C double-bonds with a conjugated system. Additionally, clearly the intensity of this peak increased with the molar ratio of GTA:DAAM (i.e., from 0.5:1 to 1:1). Therefore, these results support the hypothesis that interfacial crosslinking between NR–g–PDAAM particles took place through the reactions of DAAM groups with GTA during film casting.
Dynamic Mechanical Properties
DTMA is a popular thermal analysis technique for measuring viscoelastic properties of polymers at various temperatures. The storage modulus (E′) and tan δ as a function of temperature for NR–g–PDAAM5 cast films, with or without added GTA crosslinker, were investigated using DMTA. It is noted that, in the present study, the glass transition temperature (Tg) of a polymer was identified from the temperature corresponding to the maximal tan δ, (tan δ)max.
It can be seen from Fig. 7 that two large drops in E′ were observed for the NR–g–PDAAM5 film without GTA, upon increasing the temperature. The first drop in E′ by about two orders of magnitude started at − 68 °C, signifiying the temperature at which the NR phase began to change from glassy to rubbery state. The second drop began at − 31 °C, corresponding to transition of the NR–g–PDAAM phase [37]. When GTA was incorporated into the NR–g–PDAAM5 latex prior film casting, two sharp drops in the storage modulus were also observed. However, the storage modulus profiles of NR–g–PDAAM5 films with the addition of GTA noticeably shifted toward higher temperatures. It was also observed that the NR–g–PDAAM5 films with added GTA had lower E′ than the films without GTA in the glassy region (i.e., in the temperature range of − 80 to − 62 °C) and this trend was reversed beyond the corresponding region. It has been widely accepted that, in the glassy region, crosslinking between polymer chains has a relatively small effect on modulus [38] since only localized molecular motions can occur [39]. Thus, this observation is thought to be a result of the difference in the glass-forming tendency between the NR–g–PDAAM5 films with and without addition of GTA when they were quenched to temperatures below the Tg of NR phase. It is expected that the uncrosslinked NR–g–PDAAM5 chains have a better glass forming characteristic than the crosslinked NR–g–PDAAM5 chains. This leads to the formation of more homogeneous glassy polymer, which is reflected in a higher degree of modulus value in a glassy state [40, 41].
However, the cast films of NR–g–PDAAM5 with added GTA had a higher E′ in the rubbery plateau region than the films without GTA. This is attributed to the crosslink structure of the NR–g–PDAAM5 films from reactions with post-added GTA during film formation. Hence, it is evident from Fig. 7a that the stiffness of NR–g–PDAAM5 films increased when GTA was added into the NR–g–PDAAM5 latex prior to film casting.
A comparison of tan δ at 1.0 Hz as a function of temperature for the cast films of NRg-PDAAM5 formed in the absence and presence of GTA is presented in Fig. 7b. Two peaks in tan δ versus temperature are observed for the NR–g–PDAAM5 film without GTA. The first peak appearing at − 56 °C corresponds to Tg of the NR phase in NR–g–PDAAM5, and the second peak appearing at − 13 °C is characteristic to the NR–g–PDAAM phase [37]. It can clearly be seen from Fig. 7b that the intensity (height) of the tan δ peak, corresponding to the NR phase, in the NR–g–PDAAM5 film with added GTA is less than that observed for the cast film without GTA. Moreover, the corresponding tan δ peak also shifted slightly toward higher temperatures. The intensity of the (tan δ)max peak reflects the mobility of polymer chains at that temperature: reduced (tan δ)max peak intensity is expected when the mobility of the polymer chains decreases. Hence, these results indicate that the mobility of NR–g–PDAAM chains in the cast films was more restricted when GTA was mixed into the NR–g–PDAAM latex before film formation. This is because of the crosslinked structure in the latex films, restricting mobility of the polymer chains. The intensity of (tan δ)max peak also decreased with the concentration of GTA.
In addition, broadening and shift to higher temperatures of the tan δ peak that corresponds to the NR–g–PDAAM phase can be seen in Fig. 7b, when GTA was added into the latex prior to film casting. As the temperature corresponding to (tan δ)max peak was taken as the value for Tg, these results reveal that the Tg of NR–g–PDAAM phase increased from the initial about − 13 to 2.8 and 51 °C for the films with a stoichiometric amount and a two-fold excess of GTA, respectively. The shifts are caused by molecular mobility restrictions due to crosslinking. Therefore, a larger shift was observed for the cast film with more GTA (the 1:1 molar ratio of GTA:DAAM), as more crosslinking is expected with more GTA crosslinker. When the polymer chains are linked together in a crosslinked structure, more thermal energy is required to induce mobility of the polymer chains. This is seen as increased Tg. Hence, this result provides solid evidence that crosslinking between the DAAM functional groups took place in the NR–g–PDAAM5 film when reacted with GTA during film formation.
Thermal Stability of Latex Films
The thermal stability of the NR–g–PDAAM5 films was studied by TGA analysis under nitrogen atmosphere. Figure 8 shows the residual mass percentage versus temperature (TGA curve) for the NR–g–PDAAM5 films in the absence and presence of GTA crosslinker.
In all cases, two main steps of weight loss were observed for NR–g–PDAAM films with and without addition of GTA, occurring in the temperature ranges 220–320 and 364–440 °C. The weight loss in the first step (i.e., ~6% weight loss) is mainly attributed to the release of ammonia from the amide groups of DAAM [42]. The second weight loss step is where the main decomposition of NR–g–PDAAM occurs, starting at ~334 °C. The weight loss associated with this decomposition is ~91%, which is mainly attributed to random scission of bonds along the NR–g–PDAAM chains. This thermal decomposition breaks the polymer chains into shorter fragments. When the resulting fragments are small enough to be volatile, they exit from the solid phase.
In addition, it can be noticed from Table 3 that at 400 °C the mass loss for NR–g–PDAAM5 film without GTA was 78.06%, while that of the films with GTA was in the range of 66–68%. This indicates that the thermal stability of the NR–g–PDAAM5 films increased with added GTA, reflecting the formation of a three-dimensional network during film formation. Additionally, it was also found that the carbonaceous residue left after pyrolysis of the NR–g–PDAAM5 films with the addition of GTA at 700 °C was higher than that left by the film without GTA (see Table 3). This suggests that the degradation products obtained from the former film were more thermally stable than those generated from the latter film.
The derivative of the residual mass percentage versus temperature (DTG curve) forthe NR–g–PDAAM films in the absence and presence of the GTA crosslinker is presented in Fig. 9. Each DTG peak generally signifies the temperature at which the highest rate of thermal degradation occurs (Tmax) for each degradation step. It can be seen from Fig. 9 that, in all cases, the thermal decomposition of NR–g–PDAAM5 films occured in two main steps. The main DTG peak for both types of NR–g–PDAAM5 films with and without GTA appeared at 384 °C, indicating that the major mass loss occurred in this step.
However, the intensity of the corresponding DTG peak for the films with GTA was lower than that of the film without GTA. This indicates that the NR–g–PDAAM5 films with GTA had a lower rate of degradation than the film without GTA. Moreover, the DTG curves of NR–g–PDAAM5 films with GTA also exhibited a larger shoulder peak on the left side of the DTG peak (i.e., in the 410–490 °C temperature range) when compared to that of the film without GTA. It was also observed that the corresponding shoulder peak became more pronounced when a molar ratio of GTA:DAAM was increased from 0.5 to1:1. This result agrees well with the observation that, in the temperature range from 400 to 500 °C, the TGA curve of NR–g–PDAAM films with GTA was noticeably different from that of the film without GTA (see Fig. 8). The different degradation behavior is probably caused by the crosslinking in the NR–g–PDAAM film formed in the presence of GTA, which is reflected by its increased thermal stability. These experimental data indicate that the thermal stability of NR–g–PDAAM film improves with the addition of GTA. The increase in thermal stability is mainly because of crosslinking between NR–g–PDAAM5 latex particles during film formation, by reactions with GTA.
The activation energy (Ea) for the thermal degradation of the films can be estimated from the TGA curves obtained at different heating rates (β), according to Ozawa’s method [43]. Therefore, in order to determine the Ea, the thermal degradation of the NR–g–PDAAM5 films with and without the addition of GTA was carried out under nitrogen (20 mL min−1) at four heating rates: 5, 10, 20, and 40 °C min−1.
The TGA curves are presented in Fig. 10 for the NR–g–PDAAM5 film formed in the presence of GTA at 1:1 molar ratio of GTA:DAAM, with various heating rates under nitrogen atmosphere. It can clearly be seen that the TGA curves obtained at different heating rates were almost similar (Fig. 10a). However, an increase in the heating rate shifted the TGA curves towards higher temperatures. Additionally, the DTG peak locations also shifted towards higher temperatures as the heating rate increased, as presented in Fig. 10b. This indicates that the maximum rate of thermal degradation occurred at different reaction temperatures for different heating rates.
As the heating rate increases, the retention time required for the NR–g–PDAAM5 film to reach a given temperature is shorter. This would typically lead to a lower fractional conversion (α) in the decomposition reactions, which can be calculated according to Eq. (5) [44, 45].
where w o and w f represent values at the starting and the end of the mass loss region of interest, and w t is the weight of the sample at a time t.
In the present work, TGA measurements were conducted under non-isothermal conditions, in which the sample was heated at a constant heating rate (β = dT/dt = constant) while changes in the mass of the sample were measured. Therefore, the rate of conversion can be rewritten into a non-isothermal rate expression as given in Eq. (6), which describes the reaction rate as a function of temperature at a constant heating rate.
where A is the pre-exponential factor (min−1), T is the reaction temperature (K), and R is the gas constant (8.314 J mol−1 K−1).
Integrating Eq. (6) and rearranging it according to the Flynn–Wall–Ozawa method using Doyle’s approximation gives [46, 47]
where g(α) is constant for a given degree of conversion.
In accordance with Eq. (7), the Ea for the thermal degradation of the NR–g–PDAAM5 film was estimated by plotting log β against 1/T for different degrees of conversion (i.e., α = 0.2–0.8). It can be seen from Fig. 11 that the plots of log β versus 1/T for a fixed degreeof conversion produced a straight line, and the slope of each such line was equal to − 0.4567Ea/R. This allows the value of Ea to be estimated from the slopes in the plot.
In all cases, it was observed that the Ea for thermal degradation changed with the degree of conversion (see Fig. 12). The lowest Ea was found at the lowest degree of conversion,α = 0.2. As the conversion proceeded from 0.2 to 0.6, the value of Ea tended to increase gradually. When the extent of degradation reached conversion 0.6, an abrupt increase in the Ea value was observed. These results indicate that the early stages of thermal degradation required less energy than at higher degrees of conversion. Thermal decomposition of polymers typically starts at the weakest links in the polymer chains, which have relatively low Ea and are damaged already at low temperatures. As the size of the volatile fragments increases, more thermal energy is required to convert them to volatile species. This is reflected in the increase of Ea as the thermal degradation proceeds to higher degrees of conversion.
The results also reveal that the NR–g–PDAAM films with added GTA had a higher value of Ea than the film without the crosslinker, at equal degrees of conversion. The values of Ea are in the ranges 205–289 kJ mol−1 for the NR–g–PDAAM film with a two-fold excess of GTA, and 189–248 kJ mol−1 for the film without GTA. This is attributed to the crosslinking in the latex films with added GTA, which makes them more thermally stable. Thus, the results provide further evidence of crosslinking in the NR–g–PDAAM films with GTA, during film formation under ambient conditions.
Conclusions
This initial study has demonstrated the feasibility of creating interfacial crosslinking between NR–g–PDAAM5 particles under ambient conditions by reactions with GTA. NR–g–PDAAM5 was synthesized by seeded emulsion polymerization at 50 °C. Interfacial crosslinking of NR–g–PDAAM particles during film formation was achieved by post-adding an aqueous solution of GTA into the NR–g–PDAAM5 latex prior to film casting. The tensile properties of the NR–g–PDAAM latex films were then determined. An increase in the tensile strength of the film was found when GTA was incorporated into the NR–g–PDAAM5 latex before film casting. The FTIR spectra also provided evidence for the formation of crosslinking through the reaction between the ketone carbonyl groups of grafted PDAAM with GTA crosslinker.The crosslinking of NR–g–PDAAM5 latex films by reaction with GTA was also studied using DMTA. A clear shift in the tan δ peak, corresponding to the Tg of the NR–g–PDAAM phase, to a higher temperature was observed for the films with added GTA. The thermal degradation behavior of NR–g–PDAAM5 films in the absence and presence of GTA was also studied.It was observed that, in all cases, the thermal degradation of NR–g–PDAAM5 films was improved upon crosslinking with GTA. The improvement was further obtained when the concentration of GTA was increased. Furthermore, the NR–g–PDAAM5 films with GTA also showed higher values of Ea for thermal degradation than those of the film without GTA. This indicates that the thermal stability of the NR–g–PDAAM5 films with GTA is better than that of the film without GTA. Thus, these results demonstrates that crosslinking between NR–g–PDAAM particles during film formation can be achieved under ambient conditions by reacting GTA.
References
Feng J, Pham H, Macdonald P, Winnik MA, Geurts JM, Zirkzee H, van Es S, German AL(1998) J Coat Technol 70:57
Kessel N, Illsley DR, Keddie JL (2008) J Coat Technol Res 3:285
Foster AB, Lovell PA, Rabjohns MA (2009) Polymer 50:654
Tale NV, Jagtap RN (2010) Iran Polym J 19:801
Zhang X, Liu Y, Huang H, Li Y, Chen H (2012) J Appl Polym Sci 123:1822
Jones FN, Nichols ME, Pappas SP (2017) Organic coatings: science and technology, 4th edn. Wiley, Hoboken
Thongnuanchan B, Ninjan R, Kaesaman A, Nakason C (2015) Polym Bull 72:135
Olde Damink LHH, Dijkstra PJ, Van Luyn MJA, Van Wachem PB, Nieuwenhuis P, Feijen J (1995) J Mater Sci Mater Med 6:460
Farris S, Song J, Huang Q (2010) J Agric Food Chem 58:998
Kiernan JA (2000) Microsc Today 1:8
Gebben B, van den Berg HWA, Bargeman D, Smolders CA (1985) Polymer 26:1737
Dai S, Barbari TA (1999) J Membr Sci 156:67
Alemzadeh I, Vossoughi M (2002) Chem Eng Process 41:707
Figueiredo KCS, Alves TLM, Borges CP (2009) J Appl Polym Sci 111:3074
Kumbar SG, Soppimath KS, Aminabhavi TM (2003) J Appl Polym Sci 87:1525
Dmitriev I, Kuryndin I, Bobrova N, Smirnov M (2015) Mater Today Commun 4:93
Pye DJ (1960) Polymer composition and method. US Patent 2,960,486
Zweigle ML (1973) Removal of monomer from acrylamide polymers with sulfur dioxide. US Patent 3,780,006
Ellis B, Welding GN (1964) Rubber Chem Technol 37:563
Flory PJ, Rehener J (1943) J Chem Phys 11:521
Hagen R, Salmen L, Stenberg B (1996) J Polym Sci 34:1997
Gent AN, Kawahara S, Zhao J (1998) Rubber Chem Technol 71:668
Trabelsi S, Albouy P-A, Rault J (2002) Macromolecules 35:10054
Tosaka M, Kawakami D, Senoo K, Kohjiya S, Ikeda Y, Toki S, Hsiao BS (2006) Macromolecules 39:5100
Chenal J-M, Chazeau L, Guy L, Bomal Y, Gauthier C (2007) Polymer 48:1042
Huneau B (2011) Rubber Chem Technol 84:425
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) BioTechniques 37:790
Wang Y, Mo X, Sun XS, Wang D (2007) J Appl Polym Sci 104:130
Eng AH, Ong EL (2001) In: Bhowmick AK, S Howard (ed) Handbook of elastomers, 2nd edn. Marcel Dekker, Inc., New York, pp 29–60
Johns J, Nakason C, Thitithammawong A, Klinpituksa P (2012) Rubber Chem Technol 85:565
Honeycutt T, Flowery B (2005) Decreasing allergenicity of natural latex rubber prior to vulcanization. US Patent 20050277722 A1
Honeycutt T (2006) Decreasing allergenicity of natural latex rubber prior to vulcanization. US Patent 7056970 B2
Honeycutt T, William D, Matthew C, Russell C, Mark S (2007) Rubber World 237:32
Honeycutt T, Sharivker V, Sharivker S, Blinov V, Doyle W (2005) In international latex conference papers, Charlotte
Nakason C, Kaesaman A, Yimwan N (2003) J Appl Polym Sci 87:68
Smith MB (2015) Organic chemistry: an acid–base approach, 2nd edn. CRC Press, Boca Raton
Thongnuanchan B, Ninjan R, Kaesaman A, Nakason C (2015) J Polym Res 22:115
Ebewele RO (2000) Polymer science and technology. CRC Press LLC, Boca Raton
Hutchinson JM (1997) In: Haward RN, Young RJ (eds) The physics of glassy polymers, 2nd edn. Springer, London, pp 128–138
Phillips JC (1979) J Non-Cryst Solids 34:153
George S, Neelakantan NR, Varughese KT, Thomas S (1997) J Polym Sci B 35:2309
Burrows HD, Ellis HA, Utah SI (1981) Polymer 22:1740
Ozawa T (1965) Bull Chem Soc Jpn 38:1881
Park JW, Oh SC, Lee HP, Kim HT, Yoo KO (2000) Polym Degrad Stab 67:535
Ceamanos J, Mastral JF, Millera A, Aldea ME (2002) J Anal Appl Pyrol 65:93
Popescu C (1996), Thermochim Acta 285:309
Kim W, Kim SD, Lee SB, Hong IN (2000) J Ind Eng Chem 6:348
Acknowledgements
This work was supported by the Research Fund of Prince of Songkla University, SAT581267S. The authors would like to thank the Research and Development Office (RDO) and Assoc. Prof. Seppo Karrila for editing this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thongnuanchan, B., Ninjan, R., Kalkornsurapranee, E. et al. Glutaraldehyde as Ambient Temperature Crosslinking Agent of Latex Films from Natural Rubber Grafted with Poly(diacetone acrylamide). J Polym Environ 26, 3069–3085 (2018). https://doi.org/10.1007/s10924-018-1193-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1193-8