Abstract
In this paper the modifications induced in butyl rubbers (pristine, chlorinated and brominated sorts) by γ-irradiation are investigated by swelling, chemiluminescence and FT-IR. The susceptibility of butyl rubbers for the generation of radicals orders their stabilities in the following sequence: IIR > IIR—Cl > IIR—Br. The incorporation of butyl rubbers into ethylene-propylene terpolymer matrix brings about increased densities of radicals initiating modifications in the oxidation state in respect with recombination, which are intensified as the processing dose increases. Based on the variation of carbonyl and hydroxyl indices the favorable route for the recycling EPDM based formulations would be suggested in this study. The chemiluminescence spectra proving the formation of peroxyl radicals at about 100 °C prove their availability as reclaiming solutions. IIR—Br is the recommendable butyl rubber for the recovery procedure by association with EPDM. The suitability of IIRs for recycling purposes is analyzed by the variation in their crosslink densities, free volumes and swelling degrees. The crosslinking behavior of stabilized EPDM/IIR blends that runs to the improvement of durability is depicted by Charlesby–Pinner representation, which involves the different simultaneous contribution of scission and crosslinking processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dumping of polymer wastes raises the problems of their environmental depollution and valorization by various procedures including radiation processing [1–3]. The rubber industry would be a main beneficiary of this kind of technologies, because the intimate processes occurring during conversion contribute to the modifications of a large spectrum of industrial and municipal wastes maintaining their functional performances on acceptable limits. From the environmental standpoint the radiation treatment is applicable even to incompatible polymers due to the radical mechanisms of recombination [4].
The increase of about 600 times up to the million pounds of collected plastics over the period between 1990 and 2013 [5] is a reality. An example for the imperative necessity of waste collection is Europe where the amount of waste plastics attains annually 15.6 Mtonne. A large diversity of plastics subjected to reclaiming [6] and the proportion of 19% in the recycled materials stream structure [7] demonstrate that the recovery sector is a pillar for the depollution of environment and it sustains economic growth.
The durability of plastics wastes, longer than 100 years especially in the case of resistant and/or stabilized materials [8], does not recommend the landfill disposal for their long term storage. The other alternative is the radiation processing which is intensively promoted by International Atomic Energy Agency [9, 10]. The availability of radiation treatment applied to polymers is clearly revealed by several papers [for example 11–13]. This procedure has considerable advantages in comparison with chemical methods: further wastes are not generated, strictly controlled and suddenly stopped operations can be easily applied, a large diversity of polymer materials can be processed without special energy requirements, the presence of solvents is not usually needed, the addition of peroxides is not demanded because they are radiolytically generated and their excess would initiate premature material ageing, several pairs of polymers that are thermodynamically incompatible can be intimately structured in engineering products.
The accumulating elastic rubber wastes (used tires, pipes, gaskets and seals, wire and cable insulations included in the large category of recoverable materials by radiation processing) ask peremptorily their decay. The basic information on the alterations of butyl rubbers (IIR) induced by high energy radiation were earlier reported [14–18]. This bearing was also confirmed by thermal treatment [19] and photodegradation [20]. In addition, the more resistant polymer under γ-irradiation, ethylene-propylene terpolymer – EPDM [21–24] may be associated with different elastomers for the production of versatile blends [25–27]. The restriction conditions concerning the radiation damaging in EPDM blends are related to the achievement of foreseen formation rate for free radicals, which initiate compatibilization by crosslinking [28].
The growing interest on the potential applications of irradiated blends [29] generates the problem of blends durability, which is strictly correlated with material stability. The endurance behavior of blending components is essential for the convenient preservation of their oxidation resistance.
The investigations on radiation treatment of butyl rubbers were reported for their applications in tire industry. While the low doses applied to butyl rubber cause a sharp decrease in molecular weight initiating degradation [30], ethylene propylene terpolymer can be crosslinked with convenient increase in gel fraction [31, 32]. The use of ionizing radiation for the compatibilization of EPDM and IIRs eliminates the addition of peroxide as initiator, whose nonconsumed fraction remains captive in the polymer bulk and its spots become the start points of degradation. The EPDM-based blends can be taken into consideration as the systems in which one component, in our case—IIR, is the main donor radical source, while EPDM disposes macromolecular chains for the grafting process of generated radicals.
This work presents the contributions of three sorts of butyl rubbers: pristine material (IIR), chlorinated (IIR-Cl) and brominated (IIR-Br) elastomers to the evolution of radiochemical oxidation in their blends with ethylene-propylene diene monomer (EPDM). The variation in the IIR loading offers the evidence for the degradation trend, when EPDM/IIR mixtures are exposed to low dose rate of γ-radiation.
Experimental
Materials and Sample Preparation
Butyl rubbers (IIRs) as Butyl 268 (pristine IIR) whose unsaturation degree is initially 1.70 mol%, Bromobutyl 2222 (brominated IIR, IIR-Br) containing 1.03 mol% unsaturation and Chlorobutyl HT 1066 (chlorinated IIR, IIR-Cl)—raw material with 1.26 mol% unsaturation were delivered by Exxon Mobil Chemicals. All polymers were used as received. The molecular structures of studied polymers are presented in Fig. 1. In Table 1 the formulations of pre-prepared rubbery samples are listed.
The host polymer, ethylene-propylene terpolymer (EPDM) was manufactured by APRCHIM Piteşti (Romania) as Terpit C®. It consists of ethylene (64.5 wt%), propylene (32 wt%) and ethylidene 2-norbornene, ENB (3.5 wt%). The main initial properties were: number of CH3 for 100 carbon atoms: 0.983, unsaturation (C–C/1000 carbon atoms): 0.184, melting index (dl/g): 1.38.
The films with the same thickness (100 µm) for spectrometric investigation and sheets for chemiluminescence records were obtained by the solvent (CCl3) evaporation from polymer solutions previously mixed in proper ratios according with their concentrations. The IIRs loadings are 5, 10 and 20 phr.
Irradiation
γ-Exposure was performed in air, at room temperature inside an irradiation machinery provided with 137Cs source. Dose rate was 0.4 kGy h−1. The dose accumulation procedure was preferred, because it conserves the identity of sample characteristics. The radiation treatment was applied to unstabilized material formulation and to the blends previously modified with 0.5 phr antioxidant (Igranox 1010, Ciba Geigy®).
Characterization
Chemiluminescence (CL) determinations were carried out by nonisothermal measurements over the temperature range 30–250 °C at heating rate of 5 °C min−1. The equipment, chemiluminograph LUMIPOL 3 produced by Slovak Academy of Sciences (Bratislava), monitored the evolution of testing temperature with a good error (±0.5 °C). The samples were prepared by purring solutions on aluminum trays followed by solvent evaporation producing films at room temperature. Each dry polymer sample had weight around 3 mg. The CL intensities are expressed in Hz g−1, because the recorded count numbers must be normalized to the sample mass for the accurate comparison of results obtained on different mass samples. The inertness of aluminum to oxidation assures the lack of counting background.
FT-IR spectra were recorded on JASCO 4200 A (Japan) spectrophotometer at 50 scans and 4 cm−1 resolution. Carbonyl and hydroxyl indexes were calculated as the ratios between the absorbances measured at 1718 and 3390 cm−1, respectively and the absorbance at 2920 cm−1 [33]. The molar extinction coefficients, 310 and 90 l mol−1 cm−1 [34] were used for the calculation of carbonyl and hydroxyl concentrations, respectively.
The measurements of swelling degrees (Q), free volumes (Vr) and crosslink densities (ν) were performed by the immersion of samples (15 × 15 × 1 mm) in toluene (analytical grade) at room temperature and weighing after 5 days of stabilization, according to ASTM D-3616-5 standard. During this operation testing samples were covered avoiding solvent evaporation. For the calculation of swelling degree we used the equation:
where Q is swelling degree (%), m and m0 are the sample weights after and before swelling.
The values of free volumes were calculated by the division of mass variation with material density for each component (p means polymer and s means solvent):
where mass fraction of polymer is
and mass fraction of solvent is
The equation Flory–Rehner Eq. [35] was applied for the evaluation of crosslink density:
where n is crosslink density (cm−3), Vr is free volume, V is molar volume of toluene (106.5 cm3 mol−1), χ is parameter of polymer/solvent interaction. For the pair EPDM (main component)/toluene 0.58 was applied.
Three similar samples per point were processed and the average values had the error of ±0.5%.
Results
The radiation processing applications for the manufacture of new polymeric products are based on the bond scission, whose energy values present lower values. According with the figures listed in Table 2 and the nonspecificity energetic transfer from incidental radiation and polymer macromolecules, the highest probability for scission is presented by π bond of C=C, C–Cl and C–Br [36]. They are the sources of carbon-centered radicals involved in further reactions.
These places become radical sources by the further processes especially oxidation. Under high energy irradiation the two main initial processes takes place, scission and crosslinking. Their radiochemical yields of scission and crosslinking characterized by the number of events occurred for 100 eV absorbed energy, G(S) and G(X) respectively, define the radiation strength. Butyl rubbers have G(X) less than 0.5 and G(S) 2.9–3.7 [32, 37]. The large difference between the values of G(S)/G(X) for butyl rubber (~6) and ethylene-propylene-diene terpolymer (~0.62) [38] emphasizes the role of butyl rubbers as free radical providers in the mixtures with EPDM.
Solubility Tests
In the solubility tests defining the molecular cohesion the penetration rate for solvent molecules depends on many factors, through which the free space between molecules, the size of molecules and the crosslinking degree are relevant. The γ-exposure of IIRs causes a diminution in the polymer molecular weight and an increase in the dispersion indices.
The swelling investigations underline the differences between butyl rubbers when halogen atoms exist in their configurations. While the IIR-Br presents slight differences between the swelling degrees for different doses and immersion times due to the obvious high degree of scissions still on the early stage of irradiation, neat and chlorinated butyl rubber present significant increasing in the absorbed amounts of toluene as the dose enhances for all swelling times (Fig. 2). In Fig. 2a the swelling degrees corresponding to the immersion times longer than 72 for irradiated butyl rubber at 200 kGy could not be evaluated because samples were completely dissolved. This bearing can be explained by the high degree of fragmentation. In Fig. 2b the samples of chlorinated butyl rubber shows an increase in swelling at 5 kGy, then decreases up to 100 kGy and further increases significantly at 200 kGy, because this rubber is simultaneously degraded at two positions: double bonds (unsaturation degree: 1.26 mol%) and chloride substitutions. Their contributions determine the changes in swelling degree as the irradiation dose is augmented.
The radiation stability of these butyl rubbers is tightly correlated with the diffusion of foreign molecules, because the energy transfer during irradiation is characterized by fragmentation and by a positive variation in free volumes (Fig. 3). The presence of double bond in the backbone of butyl rubber (unsaturation degree is 1.70 mol%) determines a continuous reduction in vr figures for low irradiation doses. After γ-exposure the other two halogenated butyl rubbers (unsaturation degrees are 1.03 and 1.26 for brominated and chlorinated rubbers, respectively) the values of free volume are somewhat constantly maintained (Fig. 3b, c).
Modification in the free volumes of γ -exposed IIR (a), IIR-Cl (b) and IIR-Br (c). The color meanings are similar to Fig. 2. (Color figure online)
The action of γ-radiation concerns simultaneous scission and crosslinking. Figure 4 illustrates the variation of crosslinking in γ-irradiated butyl rubbers. The strike diminution of crosslinking density happened in γ-exposed butyl rubber is the effect of high insoluble fraction in raw material. While the swelling degree of this material is maintained constant (around 142%, Fig. 2a) on the early stage of irradiation, the crosslinking density decreases monotonically (Fig. 4a). Meanwhile the butyl rubber exhibits a sharp dropping in crosslinking density, the gelation of the other two halogenated materials progresses unlikely. The increasing dose affects IIR-Cl by the slight diminution of intermolecular crossing bridges with an evident effect at the doses exceeding 100 kGy. For IIR-Br the deterioration of material integrity could not be proved. It can be assumed that the deposited energy from incidental radiation is preferentially consumed by the scission of C–Cl and C–Br bonds, whose energies are less than the energy of C–C bonds forming intermolecular network.
Modification in the crosslinking density of γ-irradiated IIR (a), IIR-Cl (b) and IIR-Br (c). The color meanings are similar to Fig. 2. (Color figure online)
The inclination of γ-irradiated butyl rubbers to the diffusion of foreign entities, either molecules or free radicals, is demonstrated by the adequate swelling properties, which can be considered basic information for their radiation processing. The reclaiming of polymer wastes is realistically justified by the inherent potential of radiolysis transformations.
Oxidation Investigation by FT-IR Spectroscopy
FT-IR spectra reveal the changes in the oxidation states. The new created free radicals are involved in the competitive recombination and oxidation. Due to IIR components the increase in the oxidation level can be noticed at greater doses correlated with the effects of the chemical nature and concentration. In Fig. 5 the comparison between FT-IR spectra recorded on the irradiated samples at 220 kGy shows the differences that exist when EPDM is compounded with butyl rubbers. As it is known [17] the ethylene-propylene terpolymer is the most stable component. The halogenated IIR plays the opposite role of radical provider according with the easiness in the bond scission. The most vulnerable units, the isoprene configurations where halogen atoms are linked, become carbon centered sites where oxygen would be attached to form peroxyl radicals. The chains of the both components, EPDM and IIR, are simultaneously fragmented during γ-irradiation because double bonds exist in ethylidene 2-norbornene and isoprene moieties, respectively. Due to the molecular scission the new born radicals initiate further oxidation, if the recombination does not preferentially occur [39]. The oxidation is propagated by the attack of peroxyl radicals onto the both types of materials or by disproportionation reactions (classical Bolland and Gee mechanism) followed by the oxidation of new formed double bonds decreasing the quality of final product. The oxidation progresses by the accumulation of oxidation products, namely carbonyl and hydroxyl containing structures, whose concentrations are well characterized by carbonyl and hydroxyl indexes. In Fig. 6 the contribution of IIR-Br component to the increasing the oxidation degree is shown. The value of 100 kGy is the representing dose point, when the vibration carbonyl band centered on the 1715 cm−1 peak becomes meaningful. On the lower dose range the intensities on the FT-IR region 1586–1851 cm−1 grow similarly. It means that the oxidation is not a selective process because of the secondary reactions of peroxyl radicals and hydroperoxides [40], while at doses exceeding 100 kGy up to 300 kGy. The rise in carbonyl products is fed by the decomposition of high concentration of hydroperoxides.
The faster accumulation of carbonyl compounds in respect with the increasing amounts of hydroxyl structures (Fig. 7) is happened either by the linking of molecular oxygen on main chain and the decay of peroxyl units into C=O function, or by the decomposition of hydroperoxides. The loading of 5% of butyl rubber is the most convenient concentration in respect with the lowest oxidation state in γ-processed EPDM/IIR blends. This assertion can be extended to the other two studied formulations because the number of scissions increases proportionally with received doses.
The contributions of aldehydes and ketones to the oxidation state are defined by the competition between chain scission reactions and decomposition of hydroperoxides in which water is produced. The formation of water is suggested by the twin peaks at 2331 and 2360 cm−1 recorded in the spectra of irradiated blends containing IIR. The concomitant growth of these bands and the 1715 cm−1 band is the proof for water springing from the reactions of hydroperoxides. It may be assumed that the accumulated water is subjected to radiolysis and their degradation intermediates, protons and hydroxyls participate in some extent to the radiolytic oxidative degradation of polymers.
The comparison between the radiation resistances of butyl rubbers is shown in Fig. 8, where the presence of halogen atoms affects the rate of oxidation. The material behavior reflects the increasing in the ratios of radiochemical scission to crosslinking yields, G(X)/G(S), defined by Charlesby-Pinner equation, which become more and more far from unit by changes in the molecular structures from pristine IIR onto halogen substituted IIR. The expelling halogen atoms from the molecules of chlorinated and brominated butyl rubbers is demonstrated by the diminishing of their vibration bands at 615 and 536 cm−1 ascribed to C–Cl and C–Br, respectively [41].
The decay in the diene could be observed by the modification in the transmittance intensities at 808 cm−1 [42, 43]. The double bonds from butyl rubbers and the unsaturation from ethylene propylene terpolymer (trans-vinylene at 965 cm−1, vinyl at 909 cm−1 and vinylene at 889 cm−1 [34]) are also formed during radiolysis, but the recorded intensities are the results of their simultaneous formation and consumption (Table 3 ).
Oxidation Investigation by Chemiluminescence
The experimental technique of chemiluminescence allows the developing oxidation profiles in a good connection with the temperature effects on polymeric structures. Chemiluminescence spectra are recorded by the decay process of excited-state carbonyl groups formed during the termination step in the autocatalytic degradation process [44]. The CL emission intensities are considerable different in the function of intermediate populations [45]. In the cases EPDM/IIR blends, the presence of any butyl rubber creates oxidation centers from where oxidation is spread [46]. The increase in degradation temperature brings about an augmentation in the scission probability and the acceleration of oxygen diffusion. The oxidation takes place faster in the rubber phase, where weaker bonds are placed and where the penetration of oxygen is more facile. The CL spectra of studied systems subjected to 100 kGy γ dose are presented in Fig. 9. The great difference between them is the presence of the emission peak recorded at 100 °C in ethylene-propylene terpolymer/pristine butyl rubber. It disappears on the spectra obtained with halogenated butyl rubbers. The explanation of this behavior would be the screening action of halogen atoms in respect with double bond of isoprene units against the attack of oxygen, because the both atoms (oxygen and bromine) are electronegative elements. On the other side, the rate of oxidation is higher when halogenated molecules are present in the degrading samples, because their thermal and radiochemical instabilities are deficient. Even the both pristine polymers show the second peak at 210 °C, it does not exist in the cases of halogenated butyl rubbers, because they are faster oxidized. The greater CL intensities recorded at higher temperatures on halogenated IIR samples prove that the rate of excited intermediates as carbonyl groups is dominant. The macroradicals that result from the scission of polymeric chains are easily oxidized at higher temperatures because they can facilely move in less viscous environment to collide oxygen molecules. The mechanism of oxidative degradation in EPDM and IIR-X was previously reported [46, 47]. In fact, the efficient processing of the studied systems by γ-irradiation must be accomplished at exposure doses less 100 kGy, because the oxidation of materials reaches convenient degree. This dose range is a proper option for the post-processing operations of EPDM/IIR-X because during rolling, extrusion, compression molding, injection molding or thermoforming occurred at higher temperatures the degradation is accelerated (Fig. 9).
Crosslinking
The reinforcement of polymer materials is based on radical recombination, which is in competition with oxidation. At low dose rate like it was applied in this study the free radicals react preferentially with diffused oxygen. Consequently, the accumulation of gel fraction is smooth as it was previously reported [32].
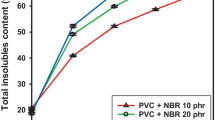
The addition of oxidation protector brought about a minimization of oxidation in the favor of radical recombination. The successful method for hardening polymer wastes foreseen in their reclaiming as resistant products is based on the scavenging capacity of additive. The variation of gel content (Fig. 10) describes the evolution of gelation in EPDM/IIR blends principally based on the crosslinking of EPDM. The correlation between the changes in swelling degrees, free volumes and crosslink densities in irradiated butyl rubbers (Figs. 2, 3, 4) with the increase in the gel content of γ-exposed EPDM-based blends points out the specific contributions of the second component to the homolytic crosslinked fractions. The sharp decrease in the crosslink density of IIR suggests the formation of network in EPDM/IIR blend about exclusively due to elastomer component. The difference between the oxidation propagation in halogenated butyl rubbers explains the competition effect involving the preferential mechanism of radical decay: recombination and reactions with oxygen. The evidences of IIR-Br for oxidation are described by two investigation methods, FTIR (Fig. 5) or gel content measurements (Fig. 10). The Charlesby–Pinner representation (Fig. 11) and the equations followed by the persisting sol amounts (Table 2) prove that γ-irradiation of EPDM/IIRs mixtures is a praxis solution for the conversion of butyl rubbers into useful products. The slope values in the liner dependencies of S + S1/2 (where S is soluble fraction) on reciprocal dose indicate the tendency to destroy the new formed networks. By this processing alternative the product life time and diversity in material formulation can be optimized.
Charlesby–Pinner representation for radiation treated EPDM/IIR blends. The composition details and the color meanings are similar to Fig. 10. (Color figure online)
It must emphasize that the notable effect of this “clean” recovery of rubbers is the convenient resistance of final products relative to the diversity of foreseen applications.
Conclusion
In this paper the radiation processing of butyl rubbers demonstrates the availability of radiochemical recycling of EPDM/IIR blends in respect with landfill, incineration and pyrolysis procedures based on the molecular fragmentation without any other negative concern. The blends consisting of ethylene-propylene terpolymer and butyl rubbers under different halogen-substitution type in isoprene moieties show oxidation and crosslinking, but the recyclability depends on the effects of the competition between recombination and oxidation occurred to the processing conditions. The oxidative degradation accomplished under γ-irradiation progresses much faster, because the concentration of halogenated component provides free radicals available for oxidation at low dose rate.
The use of these blends in the technological applications recommends EPDM/neat IIR as the most resistant material because, at least, the increases in the carbonyl and hydroxyl indexes are smooth. In economical practice it is expected that the conversion of elastomer wastes assisted by γ-irradiation at the doses below 100 kGy would be efficient. Because the diffusion of oxygen from working environment depends on the crosslinking degree the higher dose rates and low rubber loading would be preferred for the proper management of processing. So far this kind of investigation can be successfully extended to any other end-of-life material options. It must not disregard that new product which has low crosslinking degree could be further recycled.
The irradiation option for recyclable polymer materials with different origins like EPDM and IIR is self-recommended accomplishment as a background operation in the economical loops, because the improved performances in respect with further employment can be achieved by the design of proper formulation. The change of virgin material into composite systems creates the encouraging input sources for several technologies essentially defined as saving procedures. The presumable applications of post-consumer plastics recovered by γ-irradiation will be the start-points in the reconsideration of polymer wastes.
The stability analysis by chemiluminescence simultaneously done with spectroscopic investigation and swelling evaluation provides a realistic description of technological potential of radiation processing for saving natural resources which generate long life polymers.
References
Burillo G, Clough RL, Czvikovszky T, Guven O, Le Moel A, Liu WW, Singh A, Yang JT, Zaharescu T (2002) Radiat Phys Chem 64:41
Sinha V, Patel MR, Patel JV (2010) J Polym Environ 18:8
Khan WS, Asmatulu R, Davuluri S, Dandin VK (2014) J Mater Sci Technol 30:854
Enomoto I, Katsumura Y, Kube H, Sekiguchi M (2010) Radiat Phys Chem 7:718
Johnson J (2014) Post-consumer plastic recycling rates continue strong growth. Plastics News Report
Marsh K, Bugusu B (2007) Food J Food Sci 72:R39
EEA—European Environment Agency Report (2016) Most recent data: Further Eurostat Information. Main tables and database
Pritchard G (1999) Reinforced plastics durability, ch. 2. CRC, Boca Raton
IAEA—International Atomic Energy Agency (2004) Advances in radiation chemistry of polymers. TECDOC 1420
IAEA—International Atomic Energy Agency (2009) Controlling of degradation effects in radiation processing of polymers. TECDOC 1617
Zaharescu T, Jipa S, Setnescu R, Setnescu T (2000) J Appl Polym Sci 77:982
A. G. Chmielewski, M. Haji-Saeid and Ahmed S (2005) Nucl. Instrum. Meth. Phys. Res. B236 44
Martínez-López M, Martínez-Barrera G, Barrera-Díaz CE, Ureña-Nuñez F, Loredo dos Reis JM (2016) Constr Build Mater 121:1
Wang BL, Xu ZY, Zeng XM, Ma SM, Zang YX, Sun DM (1993) Radiat Phys Chem 42:215
Barttacharya A (2000) Prog Polym Sci 25:371
Teinov AV, Zavyalov NV, Khokhlov YA, Sitnikov NP, Smetanin ML, Tarantasov VP, Shadrin DN, Shorikov IV, Liakumovici A. L., F. K. Miryasova (2002) Radiat Phys Chem 63:245
Karaağaç B, Şen M, Deniz V, Güven O (2007) Nucl Instrum Meth Phys Res B265:290
Smith M, Berlioz S, Chailan JF (2013) Polym Degrad Stab 98:682
Botros SH (1998) Polym Degrad Stab 62:471
Singh RP, Chandra R (1982) Polym Photochem 2:257
Davenas J, Stevenson I, Celette N, Vigier N, David L (2003) Nucl Instrum Meth Phys Res B208:461
Abou Zeid MM, Rabie ST, Nada AA, Khalil AM, Hilal RH (2008) Nucl Instrum Meth Phys Res B266&:p 111t;/bib>
Özdemir T (2008) Radiat Phys Chem 77:787
Hacioğlu F, Özdemir T, Çavdar S, Usanmaz A (2013) Radiat Phys Chem 83:122
Zaharescu T, Jipa S, Giurginca M, Podină C (1998) Polym Degrad Stab 62:569
Chipară MD, Grecu VV, Chipară MI, C. Ponta, J. Reyes Romero (1999) Nucl Instrum Meth Phys Res B151:444
El-Sabbagh SH (2003) J Appl Polym Sci 90:1
Tostar S, Stenvall E, M. R. S. J. Foreman, Boldizar A (2016) Recycling 1&:p 101t;/bib>
M. H. Haji-Saeid, M. E. Sampa, N. Ramamoorty, A. Chmielewski, O. Güven (2007) Nucl Instrum Meth Phys Res B265&:p 51t;/bib>
Zaharescu T, Cazac C, Jipa S, Setnescu R (2001) Nucl Instrum Meth Phys Res 185&:p 360t;/bib>
Manaila E, Stelescu D, Craciun G (2012) In: Boczkowska A (ed) Polymer series—advanced elastomers. Technology, properties and applications, ch. 1. INTECH, Rijeka
Zaharescu T, L. I. P. Kayan, Lungulescu ME, Parra DF, Lugão AB (2016) Iranian Polym J 25:725
Allen NS, Hoang E, Liauw CM, Edge M, Fontan E (2001) Polym Degrad Stab 72&:p 367t;/bib>
Carlsson DJ, Čhmela S, Weiss DM (1989) Makromol Chem Macromol Symp 27&:p 139t;/bib>
Barton AFM (1991) In: Handbook of solubility parameters and other cohesion parameters, 2nd edition. CRC Press, Boca Raton
Luo Y-R (2007) Comprehensive handbook of chemical bond energies. Taylor & Francis, Boca Raton
Makuuchi K, Cheng S (2012) Radiation processing of polymer materials and its industrial applications. (Wiley, New York
Wood RJ, Pikaev AK (1993) Applied Radiation Chemistry. Wiley, New York
Zaharescu T, Jipa S, Giurginca M (1998) J Macromol Sci Pure Appl Chem A35:1093
Šećerov B, Marino-Cincović M, Popović S, Nedić Z, Kačarević-Popović Z (2008) Polym Bull 60:313
Mateescu G (1982) In: FTIR Spectroscopy. Romanian Academy Printing House, Bucharest, p.<background-color:#96C864;> </background-color:#96C864;>235
Rivaton A, Cambon S, J–L. Gardette (2006) Polym Degrad Stab 91:136
Zaharescu T, Zen HA, Marinescu M, Scagliusi SR, E. C. L. Cardoso, Lugão AB, Chem (2016) Papers 70&:p 459t;/bib>
Fearon PK, Whiteman DJ, Billingham NC, Bigger SW (2001) J Appl Polym Sci 79:1986
Ahlblad G, Reitberger T, Terselius B, Sternberg B (1999) Polym Degrad Stab 65:169
Zaharescu T, Postolache C, Giurginca M (1996) J Appl Polym Sci 59:969
Zaharescu T, Giurginca M, Jipa S (2009) Polym Degrad Stab 63:245
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaharescu, T., Scagliusi, S.R., Luchian, A.M. et al. Degradability Characterization of EPDM/IIR Blends by γ-irradiation. J Polym Environ 26, 616–625 (2018). https://doi.org/10.1007/s10924-017-0966-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-0966-9





 ) 0 kGy; (
) 0 kGy; ( ) 5 kGy; (
) 5 kGy; ( ) 50 kGy; (
) 50 kGy; ( ) 100 kGy; (
) 100 kGy; ( ) 200 kGy. (Color figure online)
) 200 kGy. (Color figure online)



 ) 0 kGy; (
) 0 kGy; ( ) 40 kGy; (
) 40 kGy; ( ) 100 kGy; (
) 100 kGy; ( ) 180 kGy; (
) 180 kGy; ( ) 280 kGy; (
) 280 kGy; ( ) 300 kGy. (Color figure online)
) 300 kGy. (Color figure online)



